Abstract
Damaged proteins containing abnormal isoaspartyl (isoAsp) accumulate as seeds age and the abnormality is thought to undermine seed vigor. Protein-l-isoaspartyl methyltransferase (PIMT) is involved in isoAsp-containing protein repair. Two PIMT genes from rice (Oryza sativa L.), designated as OsPIMT1 and OsPIMT2, were isolated and investigated for their roles. The results indicated that OsPIMT2 was mainly present in green tissues, but OsPIMT1 largely accumulated in embryos. Confocal visualization of the transient expression of OsPIMTs showed that OsPIMT2 was localized in the chloroplast and nucleus, whereas OsPIMT1 was predominately found in the cytosol. Artificial aging results highlighted the sensitivity of the seeds of OsPIMT1 mutant line when subjected to accelerated aging. Overexpression of OsPIMT1 in transgenic seeds reduced the accumulation of isoAsp-containing protein in embryos, and increased embryo viability. The germination percentage of transgenic seeds overexpressing OsPIMT1 increased 9–15 % compared to the WT seeds after 21-day of artificial aging, whereas seeds from the OsPIMT1 RNAi lines overaccumulated isoAsp in embryos and experienced rapid loss of seed germinability. Taken together, these data strongly indicated that OsPIMT1-related seed longevity improvement is probably due to the repair of detrimental isoAsp-containing proteins that over accumulate in embryos when subjected to accelerated aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteins are generally subject to spontaneous covalent damage in cells, which is thought to be a root cause of aging (Clarke 2003; Li and Clarke 1992). Aging can be considered as a battle of in vivo protective system combating the accumulation of damage caused by chemical and biological processes. It is generally accepted that altered proteins can be repaired by replacement through de-novo synthesis. However, organisms sometimes have to repair protein damage where protein synthesis is strictly limited (Clarke 2003; Mudgett and Clarke 1993; Oge et al. 2008; Rajjou and Debeaujon 2008). Plant seeds have a very low metabolic activity and resource use capability, which means that protein de-novo synthesis almost ceases (Rajjou et al. 2012). To achieve homeostasis when exposed to aging, seeds use repair systems that have a low resource and energy cost to alleviate and repair damage without de-novo synthesis (Chatelain et al. 2013; Oge et al. 2008; Verma et al. 2013). Spontaneous formation of abnormal isoaspartyl (isoAsp) residues from l-aspartyl or l-asparaginyl residues in proteins is a common form of protein covalent damage in cells and tissues. Damaged proteins containing abnormal isoAsp accumulate as the seed ages, and this is thought to undermine seed vigor and longevity (Chen et al. 2010; Mudgett and Clarke 1993).
Protein-l-isoaspartyl methyltransferase (EC2.1.1.77; PIMT), which is involved in the first step of the isoAsp repair process, catalyzes the S-adenosyl-l-methionine (AdoMet)-dependent reaction that transfers a methyl group to the α-carboxyl group of isoAsp (or in some cases, the β-carboxyl group of d-aspartyl residues; Clarke 2003). PIMT is highly conserved and documented in a broad range of organisms, from gram-negative bacteria to plants and animals (Kagan et al. 1997a; Kim et al. 1997; Li and Clarke 1992; Villa et al. 2006). The protective role of PIMT against age-related stress has been widely recorded and described in previous studies. For example, Escherichia coli that overexpressed PIMT had resistance to heat-shock and an extended life span under high temperature stress (Li and Clarke 1992). In contrast, PIMT-deficient mutants showed detrimental phenotypes, e.g. E. coli mutants showed higher sensitivity to secondary environmental stresses in the stationary phase (Li and Clarke 1992; Visick et al. 1998), Caenorhabditis elegans had a reduced survival rate in the dauer phase (Kagan et al. 1997b), and PIMT-deficient mice suffered epileptic seizures and had a shorter life span (Kim et al. 1997). Taken together, in PIMT-deficient mutants, adverse phenotypes were observed at life stages and in tissues where resources were limited, which suggests that PIMT plays an important role in reducing isoAsp damage in proteins in vivo, and can help resist aging in cells and tissues with low metabolic activities.
PIMT has also been shown to be involved in overcoming aging and stress-related conditions in plants. In plants, PIMT activity has been mainly detected in seeds, and is thought to be related to seed vigor and longevity (Kagan et al. 1997a; Mudgett and Clarke 1993, 1994; Thapar et al. 2001). Centuries old Sacred Lotus (Nelumbo nucifera) seeds still maintained a PIMT activity that was equivalent to new seeds (Shen-Miller 2002), and low vigor stored barley (Hordeum vulgare cv Himalaya) seeds overaccumulated isoAsp and had a low PIMT enzyme activity (Mudgett et al. 1997). Recently, two other studies have provided substantive evidence that PIMT improved vigor and longevity in Arabidopsis thaliana seeds by repairing the damage to isoAsp-containing protein during aging (Oge et al. 2008; Verma et al. 2013). These results highlighted an underlying relationship between the detrimental effect of isoAsp on seed vigor during the aging process and the role of the PIMT repair enzyme in delaying this trend.
Most of cereal crop seeds are typical albuminous seeds, which mainly contain a starch-rich endosperm for resource and energy consumption during seed germination. Furthermore, the endosperm content has been found to affect seed longevity in rice (Oryza sativa L.) and wheat (Triticum aestivum; Bernal-Lugo et al. 1999; Petruzzelli and Taranto 1989; Zhou et al. 2002). Although PIMT has been shown to exist in monocot seeds, no substantive evidence linking its activity to seed vigor and viability in the seeds from this class has yet been presented.
In this study, two PIMT genes were isolated from rice and designated as OsPIMT1 and OsPIMT2, respectively. In plants, PIMT1 and PIMT2 have distinctive expression patterns (Mudgett and Clarke 1996; Verma et al. 2013; Xu et al. 2004), so we investigated the specific distribution of these two genes in different tissues and cell compartments. The expression pattern for OsPIMT1 suggested that it might play a role in rice seeds. Subsequently, we acquired an OsPIMT1 loss-of-function T-DNA insertion line, pimt1-1, which had shorter shoot lengths and a reduced germination percentage compared with wild type (WT) after artificial aging treatment. OsPIMT1 overexpression and RNAi lines were generated to confirm OsPIMT1’s role in maintaining rice seed vigor and longevity. Germination assays of isolated embryos and whole seeds from the transgenic lines indicated that the repair enzyme encoded by OsPIMT1 preserved embryo vitality by reducing the accumulation of detrimental isoAsp-containing protein, which, in turn, enhanced seed longevity and vigor.
Materials and methods
Plant materials, growth conditions and treatments
Nipponbare and ZH11 rice plants, and their transgenic lines were grown at the Experimentation Station of Fujian Academy of Agriculture Sciences, Fuzhou, Fujian, China. The seeds were immersed in a water bath at room temperature overnight and then germinated in a petri dish containing two layers of moist filter paper at 35 °C for 48 h. After germination, the seedlings were supplied with Yoshida solution (Yoshida et al. 1976) for 4 days, and hygromycin was added to the transgenic seedling at the final concentration of 25 mg ml−1 to screen out positive plants. After 3 days of screening, the seedlings were allowed to recover in Yoshida solution for an additional 14 days. Finally, the seedlings were transplanted and grown on in a paddy field.
For the hormone and stress treatment experiments, 14-days-old Nipponbare seedlings were grown in Yoshida solution at 28 °C/75 % RH and supplied with continuous light (100 μmol m−2 s−1). For the hormone treatment, the seedlings were treated with 200 μM ABA, 5 mM salicylic acid (SA), or 200 μM jasmonic acid (JA) for 24 h, and the sampling time points were 0, 1, 3, 7, 12 and 24 h. For the cold or heat treatments, 14-days-old Nipponbare seedlings were incubated at 4 or 45 °C for 5 h, respectively. For the salt or oxidative stress treatment, 14-days-old seedlings were grown in Yoshida solution containing 200 mM NaCl or 20 μM methyl viologen for 5 h, respectively. For the drought treatment, 14-days-old seedlings were placed on filter paper and air-dried for 3 h. The aerial tissues were immediately harvested, frozen in liquid nitrogen and stored at −80 °C until needed.
RNA isolation and qRT-PCR
Total RNA was isolated from rice tissues using Trizol (Invitrogen, USA) following the manufacturer’s instructions. Total RNA (0.5 mg) was reverse-transcribed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Japan) following the manufacturer’s protocol.
For the qRT-PCR analysis, a 1:20 dilution of the first strand mixture was used in a 20 μl solution and the reaction conditions and program followed the manufacturer’s instructions (FastStart Universal SYBR Green Master (ROX), Roche, USA). The results were quantified and normalized relative to the endogenous control (UBQ5 or β-actin) using the comparative CT method (Schmittgen and Livak 2008) and plate-to-plant error was corrected using a 1:10 dilution of the first strand mixture from rice leaf cDNA as an inter-run calibrator when different batches of experiments were carried out. The values shown in the figures were calculated from at least three biological replicates.
For RT-PCR, 15–35 reaction cycles were performed to determine the logarithmic amplification phase. Around 2.5 µl PCR products per lane were separated by 3 % agarose gel electrophoresis. The results shown represent one of three replicates that had similar results.
The primers used in this section are listed in Supplemental Table S1.
Isolation and molecular cloning of the OsPIMT full-length cDNA sequences from rice
Total RNA was isolated from immature seeds using Trizol (Invitrogen, USA), following the manufacturer’s instructions. The 3′ RACE and 5′ RACE cDNA templates were prepared using a Smart RACE kit (Clontech, USA). Gene specific primer (GSPs) designs were based on the Os08g0557000 and Os04g0481400 coding regions. For 5′ RACE, nest PCR was carried out to enrich the 5′ cDNA ends using a dilution of PCR products from the first round PCR as templates. Subsequently, RACE products were cloned into the pJET1.2/blunt cloning vector (Fermentas, USA) for sequencing. The full-length Os08g0557000 and Os04g0481400 cDNA sequences were generated by fusing the 5′ RACE and 3′ RACE products using an overlap PCR strategy. The primers used in this section are listed in Supplemental Table S1.
Mutant screening and verification of the T-DNA insert line
The OsPIMT1 gene sequence was blasted for mutant screening in the RMD (Rice mutant database, http://rmd.ncpgr.cn). The candidate T-DNA lines were screened by their insertion site using the records found in the RMD. The potential loss-of-function pimt1-1 line was obtained and verified by a combination of two pairs of primers: a genomic specific primer pair flanking the insertion site (MT1-tDNA-S and MT1-tDNA-A) and an insertion verifying primer pair (MT1-tDNA-S and MT1-tDNA-T). Subsequently, RT-PCR was performed to verify the mutagenesis in OsPIMT1 using two pair of primers: a pair of primers upstream from the T-DNA insertion site (MT1qPCR-F and MT1-qPCR-R) and a genomic specific primer pair (MT1-tDNA-S and MT1-tDNA-A). The homozygous plants were screened using a multiple PCR primer mixture (MT1-tDNA-A, MT1-tDNA-S and MT1-tDNA-T) to identify the homozygous T-DNA insertion lines. The primer sequences used in the mutant screening and verification process are listed in Supplemental Table S1.
Bacterial overexpression, purification and enzyme activity of recombinant OsPIMT1 and OsPIMT2
The coding regions for OsPIMT1, OsPIMT2, OsPIMT2pt and OsPIMT2nt were amplified and subcloned into the pET-28a vector (Novagen, Germany). The recombination proteins encoded by these genes, which contained a His-tag in the C terminal, were transformed into the E. coli host strain: Rosetta (DE3). The transformed strains were grown to reach A600-0.5 at 225 rpm in Luria–Bertani medium at 37 °C, and then were induced by 0.2 mM IPTG overnight at 18 °C. The induced cells were collected by centrifugation and vortexed in distilled water. The cells were resuspended in phosphate-buffered saline (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH = 7.4) and disintegrated by ultrasonication. The particulate and soluble fractions were analyzed by SDS-PAGE. Recombinant proteins in the soluble fraction were purified using nickel-charged affinity columns (GE Healthcare, USA) following the manufacturer’s instructions. The primers used to construct the prokaryotic expression vectors are listed in Supplemental Table S1.
Enzyme activity was determined using the ISOQUANT Isoaspartyl Detection Kit (Promega, USA) as described previously (Schurter and Aswad 2000) with minor modification. The final solution volume was 50 μl. The total proteins (20 μg) were incubated for 30 min at 30 °C in reaction buffer containing 0.1 M sodium phosphate (pH 6.8), 1 mM EGTA, 0.004 % sodium azide, 0.16 % Triton X-100, 20 mM AdoMet and PIMT enzyme. A concentration gradient (3.75–120 μg/ml) of recombinant OsPIMT1 or OsPIMT2nt proteins were added to the reaction buffer to initiate the reaction, and a reference analysis, containing a PIMT enzyme gradient (0.125- to 2-fold), was also carried out. The reactions were stopped by adding 10 μl stop solution (0.3 M phosphoric acid) and then the products were precipitated by centrifugation at 22,000g at 4 °C for 10 min. Finally, 40 μl of the supernatants were analyzed using HPLC to determine their S-adenosyl-l-homocystein (AdoHcy) contents at 260 nm. In the HPLC detection process, 20 µl of reaction sample was separated by the C18 reverse-phase column (4.6 mm × 150 mm, 5 μM) with a constant mobile phase concentration (90 % 50 mM potassium phosphate, pH 6.2, and 10 % methanol) using a flow rate of 1 ml min−1.
Sequence alignment and phylogenetic analysis
PIMT protein sequences from various plants were downloaded from NCBI. Multiple sequence alignments and the construction of a phylogenetic tree for PIMT were generated using MEGA5 (Tamura et al. 2011). The maximum likelihood method was used to construct the phylogenetic tree with 500 replicates (bootstrap values).
Plasmid construction
The coding region and promoter fragment (containing a 1400 bp promoter region upstream of ATG, the first exon and intron) were amplified using corresponding primers. Then the OsPIMT1 coding region was inserted into pCAMBIA1301 between the NcoI and BstEII sites to construct a CaMV 35S driven OsPIMT1 overexpression vector. At the same time, a GUS report vector, driven by the OsPIMT1 promoter, was constructed by substituting the CaMV 35S promoter upstream of the GUS report gene with the promoter fragment.
The OsPIMT1 RNAi plasmid construction procedure was based on a protocol described by Wang et al. (2004). A 165 bp fragment that was specific to OsPIMT1 was amplified using a pair of primers, each of which contained two different restrictive endonuclease sites. After two digestion and ligation cycles, this fragment was inserted into the RNAi vector, PTCK303, at the two flanking ends of the rice intron in opposite directions. This construct was driven by a UBQ promoter and formed a hairpin in vivo when the intron was excised after transcription.
For the transient assays using rice protoplasts, the OsPIMT1, OsPIMT2 and OsPIMT2pt coding regions were inserted into pCAMBIA1302 and fused with gfp under the control of the CaMV 35S promoter. N-OsPIMT2, encoding the first twenty amino acids in OsPIMT2, was generated by annealing two synthetic complementary ssDNAs. The whole sequence was as follows: 5′-GAGCTCATGTGCCTCGCCGCCGCCATCGCGTCCGCGTCCGCCTCACCCGCCCGCTGCCTGTCGCCCAAGCTT-3′ (restriction enzyme sites are underlined).
This N-OsPIMT2 fragment, which contained sticky ends, was directly ligated into PBI221GFP (a GFP report vector derived from PBI221) to construct the N-OsPIMT2:gfp fusion gene driven by the CaMV 35S promoter. In the nuclear targeting vector, PBI221GFP was modified by replacing gfp with mCherry, which encodes a red fluorescent protein, and a nuclear targeting transcriptional factor, OsbZIP72 (Lu et al. 2009; Yoshida et al. 2010), was subcloned in-frame with mCherry.
The primers used in this section are listed in Supplemental Table S1.
TZ assay
The TZ assay was performed as described by Das and Sen-Mandi (1988) with some modifications. Hulled seeds were imbibed in distilled water for 3 h and then cut longitudinally into two identical halves. The cut seeds were incubated in freshly prepared 1 × PBS containing (w/v) 0.1 % 2,3,5-triphenylte trazolium chloride for 25 min at 25 °C in a darkened incubator. After incubation, the seeds were immediately washed with distilled water and washed twice with 85 % ethanol. The stained seeds were air-dried in filter paper and observed using a Nikon SMZ745T stereoscopic microscope (Nikon, Japan).
Antibody preparation and Western-blot analysis
The synthesized peptide, which contained a number of specific OsPIMT1 regions, was coupled with Keyhole Limpet Hemocyanin and used to immunize mice. A hybridoma cell strain that recognized the specific epitope, KNQDGKVIRS, was selected for large-scale anti-OsPIMT1 antibody production and purification. The specific OsPIMT1 was tested using recombinant OsPIMT1 and OsPIMT2 proteins. The verified rice reference protein, HSP82 (annotated as heat shock protein), was used as the loading control (Li et al. 2011) in the Western blot analysis. Protein extracts were separated by 10 or 12 % SDS–PAGE, and transferred to a PVDF membrane by semi-dry electrophoretic blotting. The blot was cut according to its protein marker (PageRuler Pre-stained Protein Ladder, Pierce, USA), and the corresponding part of the blot was challenged with the anti-OsPIMT1 antibody or the anti-HSP82 antibody (Beijing Protein Innovation China), according to the manufacturer’s instructions. GOAT-anti-mouse peroxidase-conjugated secondary antibody (Pierce, USA) and a Luminol-based kit (EasySee® Western Blot Kit, TransGen Biotech, China) were used to detect the bands, following the manufacturer’s instructions.
Quantification of isoAsp content
The protein samples were extracted according to Oge et al. (2008) with minor modification. Briefly, 40 hand-isolated embryos were ground with quartz sand in 4 ml of extraction buffer (100 mM HEPES, pH 7.5, 500 mM EDTA, 100 mM DTT, 300 mM sucrose and 1 % Complete EDTA-Free Protease Inhibitor (Roche, USA). The slurry was collected in 5 ml centrifuge tubes and centrifuged at 22,000g and 4 °C for 10 min. The supernatant was filtered to remove the upper oily layer and the resultant protein was precipitated using 65 % ammonium sulfate for 1 h at 4 °C. The precipitated protein was centrifuged at 20,000g and 4 °C for 15 min after it had been stored for 1 h at 4 °C. The pellets were dissolved in 500 µl of 100 mM HEPES, pH 7.5.
The isoAsp content was quantified using the ISOQUANT Isoaspartate Detection Kit (Promega, USA) according to the manufacture’s protocol, but with minor modifications to the HPLC detection process. In the HPLC detection process, 20 µl of reaction sample was separated by the C18 reverse-phase column (4.6 mm × 150 mm, 5 μM) with a constant mobile phase concentration (90 % 50 mM potassium phosphate, pH 6.2, and 10 % methanol) using a flow rate of 1 ml min−1.
Subcellular localization analysis using rice protoplasts
Rice protoplasts were isolated as described previously (Zhang et al. 2011) with minor modification. The transfected plasmid was extracted using endotoxin-free plasmid DNA purification kits (NucleoBond® Xtra EF, Macherey–Nagel, Gemany) according to the manufacturer’s instructions. Typically, 110 μl of rice protoplasts in freshly prepared PEG solution [40 % (w/v) PEG 4000, 200 mM mannitol and 100 mM CaCl2] were transfected with 15 μg of plasmid DNA or plasmid mixture (plasmids harboring a target gene and a marker gene). The transfected protoplasts were captured by a FluoView FV1000 confocal laser scanning microscope (Olympus, Japan).
Artificial treatment and estimation of seed longevity
Newly harvested rice seeds were dried in an oven at 42 °C for 2 days to balance the water content and break seed dormancy. The artificial aging treatment used in this study was as described by Zeng et al. (2002) with some modifications. Seed from the mutant lines and ZH11 were stored at 42 °C and 88 % relative humidity for 21 days in a closed desiccator (BINDER GmbH, Germany) with a thermostatic moisture regulator. The treated seeds were sown on two layers of filter paper and germinated in an incubator at 30 °C/75 % RH with 14 h of light per day for 12 days. The germination percentage was the number of germinated seeds after 12 days. Seeds that had not been artificially aged were used as controls. One hundred seeds from each sample were treated, and there were three replicates for each sample.
The Nipponbare seeds were first treated for 0–24 days under the above conditions in order to estimate the reference aging curve. The treated seeds were then germinated in a petri dish with two layers of moist filter paper at 30 °C for 12 days. The germination energy was estimated 4 days after germination by counting the germinated seeds with a protruding radicle, and the germination percentage was recorded on day 12. Subsequently, the seeds from the transgenic plants and WT were treated for the same length of time and under the same conditions described above. Embryos were isolated by hand-cutting them from seeds that had undergone artificial aging. The isolated embryos were sterilized in 70 % ethanol for 30 s and then in 2 % sodium hypochlorite with stirring for 10 min. Immediately after sterilization, the isolated embryos were washed several times in sterile distilled water until the water turned clear. Then they were grown on half-strength Murashige and Skoog (MS) basal medium containing 200 mM sucrose. One hundred seeds were treated, and there were three replicates for each sample. Two independent biological replicate experiments were carried out.
Results
Isolation of OsPIMT genes in rice
The PIMT coding sequences in Arabidopsis and wheat were selected as reference sequences and blasted against the rice non-redundant nucleotide database of NCBI (National Center for Biotechnology Information). Two genes, Os08g0557000 and Os04g0481400, based on annotations from the Rice Annotation Project Database, were identified with up 70 % sequence identity to PIMT from Arabidopsis and wheat. The full length cDNA sequences were amplified using a combination of 5′ rapid amplification of cDNA ends (RACE) and 3′ RACE. The results indicated that the Os08g0557000 coding sequence (CDS) is 696 bp long, and is flanked by a 221 bp 5-untranslated region (UTR) and a 188 bp 3-UTR, whereas Os04g0481400 consisted of an 861 bp CDS, a 238 bp 5′-UTR and a 260 bp 3′-UTR. The Os08g055700 coding region shared 69.6, 69.5, and 72 % identities with AtPIMT1, AtPIMT2 and TaPIMT, respectively. Os04g0481400 has approximately 69–71 % similarity with Os08g0557000, AtPIMT1 and AtPIMT2, but has a higher identity (84 %) with TaPIMT. The alignments of the two candidate PIMT genes’ amino acid sequences with Arabidopsis and wheat PIMTs are shown in Fig. 1a. The proteins encoded by Os04g0481400 and Os08g055700 contained three conserved domains for AdoMet-dependent methyltransferase and two specific domains for the potential PIMT substrate recognition site (Kagan et al. 1997a). A phylogenetic tree was constructed to elucidate the evolutionary relationship between rice PIMT and PIMT proteins in other plants (Fig. 1b). Based on the phylogenetic tree, the grass family could be divided into two groups: one was PIMT1 and the other was PIMT2. The proteins encoded by Os08g055700 and Os04g0481400 were clustered in the PIMT1 and PIMT2 groups in the grass family, respectively. In addition, plant PIMT2 often contains an N-terminal extension (Dinkins et al. 2008; Verma et al. 2013; Xu et al. 2004), which is also found in Os04g0481400 protein sequence (Fig. 1a). Therefore, we designated Os08g055700 and Os04g0481400 as OsPIMT1 and OsPIMT2, respectively.
Sequence alignments and phylogenetic analysis of two PIMTs in rice. a Alignments of PIMT protein sequences from rice with those of Arabidopsis and wheat using the ClustalX 2.1 program. The three conserved regions shared by the different methyltransferase families, identified by AdoMet, are highlighted in gray and labeled as regions I, II and III. The two regions unique to PIMT are also highlighted in gray and labeled as Pre-I and Post-III. AtPIMT1 and AtPIMT2 are Arabidopsis PIMT1 and PIMT2, respectively; TaPIMT is wheat PIMT and Os08g0557000 and Os04g0481400 are the two putative rice PIMTs. b Phylogenetic analysis of PIMT proteins in plants. The phylogenetic tree was constructed using the maximum likelihood method. The numbers at the nodes indicate bootstrap values from 500 replicates. The two grass family PIMT groups are highlighted in gray. The scale bar represents the number of amino acid substitutions per site. The accession numbers for the PIMT sequences in sequence alignment and phylogenetic analysis can be found in the “Materials and methods” section
Purification and enzymatic characterization of OsPIMT1 and OsPIMT2
The OsPIMT1 and OsPIMT2 proteins were expressed with a C-terminal histidine tag in E. coli BL-21 (DE3) cells. OsPIMT2 contains a highly hydrophobic N-terminal extension (Supplemental Fig. S1A), so the truncated isoform OsPIMT2pt was also constructed by removing the partial transit peptide (the first 20 amino acids) of the OsPIMT2 N-terminus based on cleavage site prediction (Supplemental Fig. S1B). The recombinant OsPIMT1 was soluble (Supplemental Fig. S2A), but OsPIMT2 and OsPIMT2pt were both found to be expressed in the particulate fraction (Supplemental Fig. S2B). Amino acid composition analysis indicated that the OsPIMT2 insolubility was probably due to the highly hydrophobic N-terminal signal peptide (Supplemental Fig. S1B). Therefore, a fully truncated OsPIMT2, OsPIMT2nt, that lacked the total 56 amino acid N-terminal extension, was expressed and the results indicated that OsPIMT2nt was mainly expressed in the soluble fraction (Supplemental Fig. S2C). The recombinant OsPIMT1 and OsPIMT2nt were then purified by Ni+ affinity chromatography (Fig. 2a). The enzyme activities of the recombinant enzymes were determined using an HPLC-based method, and the results indicated that 3 μg OsPITM1 had equivalent enzymatic activity to 1 × commercial rrPIMT (commercial recombinant rat PIMT), but OsPIMT2nt had a lower specific activity than OsPIMT1, that is, 1 μg of OsPIMT1 was approximately equivalent to 4.5 μg of OsPIMT2 (Fig. 2b).
Purification and enzymatic comparison of OsPIMT1 and OsPIMT2. a Purification of OsPIMT1 and recombinant OsPIMT2nt (OsPIMT2nt lacked an N-terminal extension). Recombinant proteins in the soluble fraction were bound using nickel-charged affinity, and eluted by an imidazole solution concentration gradient. M protein marker, FT flow-through fractions; 25, 50 and 100 represent the imidazole concentrations in the elution buffer. b Determination of recombinant protein enzymatic activity using an ISOQUANT isoaspartyl Detection Kit (Promega), according to the manufacturer’s instructions and in a total volume of 50 µl. rrPIMT, rat recombinant PIMT enzyme provided with the kit. Different dilutions (from 1/8 × to 1 × represent the recommended concentrations used in the kit) were used during the analysis. Different amounts of OsPIMT1 and OsPIMT2nt were substituted for rrPIMT in the analysis. The error bars represent standard deviation (SD) from three parallel samples
OsPIMT response to stress treatment and abscisic acid induction
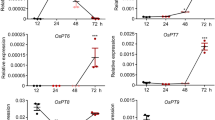
Previously, plant PIMTs were characterized by their inducible expression in contrast to human and bacteria, and they were found to be induced by multiple abiotic stresses and abscisic acid (ABA) in chickpea (Verma et al. 2010, 2013). We monitored OsPIMT1 and OsPIMT2 expression in seedlings under different environmental stresses and hormone treatments. OsPIMT1 was most sensitive to oxidative stress, which caused a threefold increase compared to the control (Fig. 3a). OsPIMT1 accumulation increased approximately twofold under cold and salt stress (Fig. 3a). OsPIMT2 was also induced by oxidative, cold and salt stress, but OsPIMT2 expression did not increase as much as OsPIMT1 when the plant was subjected to oxidative stress (Fig. 3a). In addition, OsPIMT2 was significantly, although not strongly, up-regulated by exposure to heat stress (Fig. 3a). Under ABA treatment, both the OsPIMT1 and OsPIMT2 transcript accumulations exhibited an elevated trend with increasing in treatment time, although expression OsPIMT1 or OsPIMT2 did not change at 1 or 12 h when compared with the previous time point, respectively (Fig. 3b). However, OsPIMT1 and OsPIMT2 transcript expressions did not change in the presence of exogenous salicylic acid (SA) or jasmonic acid (JA) (Supplemental Fig. S3A, B).
OsPIMT1 and OsPIMT2 expression pattern analyses. a–d qRT-PCR analysis of OsPIMT1 and OsPIMT2 transcripts at different abiotic stresses (a), in the present of 200 μM abscisic acid (ABA) (b), in different tissues (c), and in the embryos of mature seeds during germination (d). Cold, 4 °C; Heat, 45 °C; Oxidative, 20 μM methyl viologen; Salt, 200 mM NaCl; Drought, air-dried. The relative expression value was normalized to the UBQ5 reference gene and was calculated from at least three biological replicates. Error bars indicate the standard deviation, and an asterisk denotes a statistical significant difference (Student’s t test, P < 0.05) between the control and treatment. e Histochemical analysis of GUS expression driven by OsPIMT1 promoters. lf leaf, rt root, st stem, fl floral organ, ds dry seed, sd seedling geminated from mature seed. f Verification of the OsPIMT1 antibody. The recombinant OsPIMT1 and OsPIMT2nt, and the leaf total protein extracts were challenged with OsPIMT1 specific antibody. Around 1 µg of recombinant OsPIMT1 and OsPIMT2 ns and ~30 µg leaf total protein were separated in 10 % SDS PAGE, and probed with the OsPIMT1 antibody. g Confirmation of OsPIMT1 expression in embryos from mature seeds using Western blot analysis. Around 30 µg protein from the embryo and endosperm were separated by 10 % SDS PAGE. After transferred to the PVDF membrane, the proteins in the corresponding parts of the blot were challenged with the OsPIMT1 or HSP82 antibodies (loading control), respectively
OsPIMT1 and OsPIMT2 exhibited differential tissue-specific expressions
Plant genomes often contain two PIMT genes that encode PIMT1 and PIMT2, respectively (Mudgett and Clarke 1996; Xu et al. 2004), and these two genes show different tissue-specific expressions. It is therefore important to explore expression of both genes in rice seed. First, tissue specific expressions for both genes were characterized by qRT-PCR, and the results showed that OsPIMT2 was highly expressed in green tissues but barely detected in immature seeds (7 days after pollination), whereas, except for the relatively low expression in roots, OsPIMT1 expression was more evenly distributed among the tissues (Fig. 3c). OsPIMT1 was more abundant than OsPIMT2 in seeds, compared with leaves and stems (Fig. 3c). However, these results were not in agreement with those previously reported for chickpea and Arabidopsis (Verma et al. 2013; Xu et al. 2004), where the PIMTs were mainly found in the seeds in chickpea and PIMT2 transcripts are detected at low levels and only during seed maturation in Arabidopsis. We tested OsPIMT1 and OsPIMT2 mRNA levels in the embryos isolated from dry mature seeds and imbibed seeds separately to monitor their dynamic distribution during seed germination. The results showed that OsPIMT1 was more abundant in the embryos from mature seeds than in plant stems and the levels decreased sharply after imbibition (Fig. 3d), suggesting that OsPIMT1 was an essential requirement in the embryos from mature seeds. To fully understand OsPIMT1 expression, transgenic rice plants harboring the GUS gene construct, driven by the OsPIMT1 promoter, were generated by Agrobacterium-mediated transformation. The GUS-histochemical staining showed that the OsPIMT1 expression results were consistent with the qPCR analysis (Fig. 3c, e). In seeds, OsPIMT1 was present at low levels in the endosperm (only detected in the aleurone layer), but mainly detected in the embryo, and was also present after seedling formation (Fig. 3e).
OsPIMT1 monoclonal antibodies were generated using specific synthetic polypeptide and the specificity of the monoclonal antibody was verified using purified recombinant proteins. The results showed that the monoclonal antibody failed to detect recombinant OsPIMT2, but did detect OsPIMT1 from the whole protein extract and recombinant OsPIMT1 (Fig. 3f). Therefore, this monoclonal antibody provided enough specificity and sensitivity for OsPIMT1 detection. Additionally, OsPIMT1 expression in mature seeds was confirmed by Western blot analysis, and the result indicated that OsPIMT1 protein was mainly expressed in the embryo, but only at a minor level in the endosperm (Fig. 3g).
Cis-acting regulatory elements support similar, but distinctive, OsPIMT1 and OsPIMT2 expression patterns
The OsPIMT1 and OsPIMT2 promoter regions were screened for putative cis-acting regulatory elements using the PLACE online cis-acting regulatory element database (http://www.dna.affrc.go.jp/PLACE/) (Supplementary Table S1). The repeated MYC recognition site (CANNTG) (Abe et al. 2003) was found in both OsPIMT1 and OsPIMT2, and MYB recognition sites (GGATA and WAACCA) (Abe et al. 2003; Baranowskij et al. 1994; Bartels and Souer 2004) were present in the OsPIMT1 and OsPIMT2 promoter regions, both of which are known to function as cis-acting elements that promote dehydration and cold-induced expression. Surprisingly, we failed to find the ABA responsive elements (ABREs) in the promoter regions, which is commonly documented in the Arabidopsis and chickpea PIMT promoter regions (Verma et al. 2013; Xu et al. 2004). However, we did find two ABRE coupling elements (ABRE-CEs) (Kaplan et al. 2006; Nakabayashi et al. 2005; Niu 2002) in the OsPIMT1 and OsPIMT2 5′ UTRs. The stress responsive Ca2+/calmodulin binding sites (CGCGBOXAT; Yang and Poovaiah 2002) were present in the OsPIMT1 and OsPIMT2 promoters, some of which were adjacent to or overlapped with ABRE-CEs. In summary, both promoters contained many similar putative cis-regulatory elements in different positions, and a few cis-regulatory elements specific for the OsPIMT1 or OsPIMT2 promoter were also identified (Supplemental Table S3). The CAAT box in the pea (Pisum sativum L.) legA promoter (the major seed storage protein gene; Shirsat et al. 1989), was frequently present in the OsPIMT1 promoter region, but was not found in OsPIMT2. In contrast, the mesophyll-specific box, CACTFTPPCA1 (Gowik et al. 2004), was present, mostly in clusters, in the OsPIMT2 promoter. The difference of cis-element in promoter of OsPIMT1 and OsPIMT2 is consistent with the predominant expression in specific tissues of two genes. The occurrence of such similar, but distinctive, cis-regulatory elements in the promoters might explain the differential expressions of these two genes.
OsPIMT1 and OsPIMT2 exhibit differential subcellular localizations
In Arabidopsis and chickpea, PIMT1 and PIMT2 have different subcellular localizations (Dinkins et al. 2008; Verma et al. 2013). Typically, PIMT1 is mainly present in the cytosol and the nucleus, whereas PIMT2 is found in many subcellular compartments. In addition, PIMT2 often carries an N-terminal extension whereas PIMT1 does not. OsPIMT2 N-terminal targeting signal analysis by TargetP (Emanuelsson et al. 2007) and WoLF PSORT (Horton et al. 2007), indicated that OsPIMT2 contained a transit peptide in the N-terminal region, which was predicted to target chloroplasts (Supplemental Fig. S1B). In order to determine the subcellular localization of OsPIMT1 and OsPIMT2, we constructed a series of vectors harboring gfp fused with OsPIMT1 or OsPIMT2, and these vectors were transiently expressed in rice protoplasts isolated from leaves and shoots. GFP protein alone was distributed throughout the cytosol and nucleus (Fig. 4a), while OsPIMT1 also exhibited a similar distribution to GFP, and there was no distinct localization in subcellular compartments other than the nucleus (Fig. 4a), which was consistent with previous studies on PIMT1 in plants (Verma et al. 2013; Xu et al. 2004). The OsPIMT2:GFP fusion was present in the cytosol, the nucleus and chloroplasts (Fig. 4a). Due to multiple localization of PIMT2 in Arabidopsis and Chickpea, it is required to make a further investigation in the localization of OsPIMT2 in subcellular compartments. Therefore, OsPIMT2pt, which lacked a partial OsPIMT2 peptide based on the cleavage site prediction by TargetP (Supplemental Fig. S1B), was transformed into rice protoplast to assess the differential localization of OsPIMT2. Intriguingly, although OsPIMT2pt was also observed in cytosol, it was predominantly present in the chloroplasts (Fig. 4a).
Subcellular localizations of OsPIMT1 and OsPIMT2 in rice green tissue protoplasts. a Subcellular localizations of OsPIMT1, OsPIMT2 and OsPIMT2pt (OsPIMT2 lacked a 20 amino acid partial transit peptide at the N terminus). Each PIMT was fused to the N terminus of GFP and driven by a CaMV 35S promoter to form the CaMV 35S:PIMT:GFP construct. GFP alone is the CaMV 35S:GFP construct, which was used as a control. Merged images are shown as the overlapped views of GFP, chlorophyll (chlorophyll autofluorescence) and DIC (reflection differential interference contrast microscopy). All bars represent 5 µm. b Subcellular localization of N-OsPIMT2 (the first 20 amino acid N terminal segment of OsPIMT2). GFP alone or in the C-terminal fusion with N-OsPIMT2 was co-transformed with the nuclear targeting vector (bZIP72:mCherry). All fluorescence proteins were driven by a CaMV 35S promoter. Merged images are shown as the overlapped views of GFP, mCherry and chlorophyll autofluorescence. All bars represent 5 µm
Meanwhile, differential expression of OsPIMT2 and OsPIMT2pt raised our interest in the role of N-terminus of OsPIMT2 upstream the cleavage site. The vector carrying N-OsPIMT2 (the first 20 amino acids at the N-terminus of OsPIMT2) fused with GFP was co-transformed, along with the nuclear-localized marker vector expressing mCherry-fused OsbZIP72, into rice protoplast. The results shown that the N-OsPIMT2:GFP fusion was highly specific for nuclear localization (Fig. 4b), indicating that different parts of N-terminus endue OsPIMT2 with multiple localizations in different compartments. Taken together, these data support the differential, but complementary role, of OsPIMT1 and OsPIMT2 in cells.
Isolation of an OsPIMT1 T-DNA insertion line and preliminary assessment of seed longevity under artificial aging
We screened for T-DNA insertion lines in the Rice Mutation Database (http://rmd.ncpgr.cn) to explore the physiological role of OsPIMT1 in rice seed. A mutant with an insertion at the OsPIMT1 locus was detected. This line was characterized by a T-DNA insertion in the third intron (based on the genome structure of OsPIMT1; Fig. 5a). The insertion was confirmed by a pair of specific primers consisting of a primer localized at the T-DNA borders and a primer at OsPIMT1 locus flanking the insertion region (Supplemental Fig. S4A). The homozygous plants were identified by PCR and selected for further research (Supplemental Fig. S4B). Given the insertion was localized in the intron, we performed an RT-PCR to test whether the OsPIMT1 transcript was interrupted by this insertion and the results indicated that the insertion had no impact on the initial of transcription, but did lead to interruption of transcription downstream of the insertion site (Fig. 5b).
Molecular analysis and preliminary assessment of OsPIMT1 T-DNA Mutant pimt1-1 seed longevity. a Schematic diagram of the pimt1-1 insertion in the OsPIMT1 locus. Black rectangles represent exons; Open triangle, position of the T-DNA insertion in the third intron; R, right border of the T-DNA; L, left border of the T-DNA; T, specific primer for T-DNA (MT1-tDNA-T); S, sense primer upstream from T-DNA insertion (MT1-tDNA-S); A, antisense primer downstream from T-DNA insertion (MT1-tDNA-A). Arrows denote primer extension directions. b Verification of pimt1-1 loss-of-function insertion. RT-PCR was carried out to verify the insertion effects. 5′ Primer indicates the PCR product of the coding region upstream insertion site; S + A indicates the PCR product of the coding region spanning the insertion site; M, DNA marker; UBQ5, reference gene. c Histogram presentation of WT and pimt1-1 germination percentage before and after 21 days of aging treatment. The germination percentage was calculated from three parallel experiments with three replicates of each sample, and the values are the means of three parallel experiments. Error bars indicate SD; asterisk denotes a statistical significance (Student’s t test, P < 0.05). d Germination comparison of WT and pimt1-1 seeds 12 days after germination. The germinating seeds were from one of the three replicates that had produced similar results. e Shoot lengths of seedlings geminated from aging seeds 12 days after germination. Values represent mean ± SD from three parallel experiments. GS denotes germinating seeds
The seed viability of the homozygous population and its wild type japonica rice, ZH11 were tested using freshly harvested seeds. The results indicated that there were no significant differences between the homozygous population and ZH11 (Fig. 5c). Then we assessed seed vigor and longevity under artificially accelerated aging. In the accelerated aging treatment, the seeds were exposed to 42 °C and 88 % relative humidity (RH). After 21 days of treatment, the pimt1-1 seed germination percentage fell to 12.8 %, which was significantly lower than the WT (23.1 %; Fig. 5c, d). In additional, the average pimt1-1 shoot length was less than half the length of the WT after the artificial aging (Fig. 5e). These data indicated that the pimt1-1 seeds were more prone to seed viability losses than the WT when subjected to accelerated aging.
Generation of transgenic rice that overexpressed or suppressed OsPIMT1
We generated transgenic lines that overexpressed OsPIMT1 driven by the CaMV 35S promoter, and OsPIMT1 RNAi lines (Fig. 6a). The copy number of the T0 transgenic lines was first identified by DNA blot so that we could obtain homozygous overexpression (OE) lines with stable inheritance (Fig. 6b). The progeny of the OE lines with a single copy number were assessed for homozygosity using the segregation ratio test combined with PCR analysis. The progeny of each RNAi line generation were identified by PCR to screen positive plants for breeding. OsPIMT1 expression was estimated using RT-PCR analysis of the transgenic lines (Fig. 6c). The results indicated that the overexpression lines had higher OsPIMT1 expressions compared to the wild type Nipponbare, but OsPIMT1 expression in RNAi lines was barely detected. Seed embryos taken from the stable transgenic lines were also analyzed for OsPIMT1 expression and the results showed that expression of OsPIMT1 but not of OsPIMT2 had significantly changed in the OE lines and RNAi lines (Fig. 6d), which indicated that these transgenic lines could be used to investigate the physiological role of OsPIMT1 in seeds.
Construct of OsPIMT1 transformed vector and molecular analysis of transgenic plants. a Structure of vector of overexpression and RNAi. Overexpression vector harbors the construct consisting of OsPIMT1 CDS flanked by CaMV 35S promoter and the nopaline synthase (Nos) terminator. RNAi vector carrying the construct of two reverse complementary copies of specific region of OsPIMT1 spaced with a rice intron, and this construct was driven by a maize ubiquitin promoter. 35S promoter, CaMV 35S promoter; Nos, Nos terminator; Ubi-1, maize Ubiquitin promoter. b DNA gel blot of OsPIMT1 overexpression plants of T0 generation. ~100 μg total DNA from leaves of T0 transgenic and WT plants was digested with EcoRI and hybridized with hygromycin-resistant gene hptII probe (labeled with alkaline phosphatase). CK, OsPIMT1 overexpression plasmid; WT, wild type; 1–4, OsPIMT1 overexpression plants. c–d Relative abundance of OsPIMT genes in OsPIMT1 transgenic lines. OsPIMT1 and OsPIMT2 relative transcript abundance in three independent OsPIMT1 overexpression lines (OE1, OE2, and OE3) and two RNAi lines (Ri1 and Ri2) was first evaluated in leaves using RT-PCR (c). OsPIMT1 and OsPIMT2 relative transcript abundance in embryos of mature seeds was evaluated using qPCR in three OE lines and two RNAi lines (d). M, DNA Marker; WT, untransformed Nipponbare plants. For RT-PCR, one representative of three biological replicates was shown, and UBQ5 were used as a reference gene. For qPCR analysis, the relative expression value was normalized to the β-actin reference gene and was calculated from at least three biological replicates, and error bars indicate the standard deviation
Germination pattern and seed longevity differences between the WT and transgenic lines when they are subjected to artificial aging
Seeds harvested from the T3 transgenic lines were used for seed physiological analysis. Freshly harvested WT seeds were tested for final germination percentage and germination energy (germination percentage recorded at the early stage, typically the 3rd or 4th days, of germination period). The results indicated that germination energy began to decline 14 days after the aging treatment had begun, that the germination percentage exhibited a decrease 3 days later, and that the seeds lost all germination viability after 21 days of aging treatment (Fig. 7a). We divided the accelerated aging process into three stages, based on the germination energy and germination percentage of the WT seeds (Fig. 7a).
Characterization of OsPIMT1 transformed seeds under artificial aging treatment. a Seed aging curves [germination percentage (GR) and germination energy (GE)] of WT Nipponbare. Germination energy or germination percentage was defined as germination percentage after 4- or 12-day germination, respectively. Newly harvested seeds were stored at 42 °C/88 % RH for 24 days. There were 100 seeds and three replicates for each time point, and the values are the means of two parallel experiments. Error bars indicate SD. Three time points, 14, 17 and 21 days are boxed to highlight the decline in germination energy, the decline in germination percentage, and the complete loss of germinability. b–d Germination performance of newly harvested OsPIMT1 transformed seeds under artificial aging treatment. The treatment time points were 14, 17 and 21 days, which were based on the seed aging curves for WT. There were three samples for each sample point, in which 100 seeds were used to analyze the germination energy (b) and germination percentage (c). The embryos were hand-cut from the artificially aged seeds and germinated in 1/2 MS media in order to evaluate the germination percentage of isolated embryos (d). One representative of two biological experiments is shown here. The values are the mean of three replicates, and error bars indicate SD. OE1–3 and Ri1–2 denotes three overexpressing line and two RNAi lines, respectively. e Viability of the wild type and transformed seeds after 17 days of aging treatment. Red staining indicates viable seeds using TZ assay (All bars represent 1 mm)
After 14 days of the accelerated aging treatment (Supplemental Fig. S5), there was a variation in high germination energy values between the different seed lots (Fig. 7b). The seeds from the RNAi lines showed a sharp decline in both germination percentage and germination energy (Fig. 7b, c; Supplemental Fig. S5). In the overexpression lines, the OE1, OE2 and OE3 germination energies were higher than the WT germination energy (Fig. 7b), whereas the germination percentage of both the overexpression lines and the WT were not significantly different from the untreated seeds (Fig. 7c). In addition, we also examined the germination percentage of the isolated embryos incubated in 1/2 MS media to exclude variation between non-embryo tissues. Interestingly, isolated embryos of overexpression lines and WT maintain a high germination percentage similar to germination percentage of whole seeds after 14 days of aging treatment, while isolated embryos had germination percentage as low as the whole seeds in RNAi lines (Fig. 7c, d), which indicated that the difference in embryo vigor was closely related to seed longevity in the OE lines and RNAi lines in this stage. Meanwhile, isolated WT embryos showed a reduced germination pattern from 14 to 21 days (Fig. 7d), which was similarly observed in the whole seeds germination assay (Fig. 7c). This result indicated that the loss in germination percentage in the whole seeds might be mainly due to the loss of embryo vigor under aging treatment.
By the 17-day of treatment, the germination percentage of the RNAi lines had declined to 15 % (Fig. 7c), the WT seed germination percentage had sharply declined to 59 % and the seed germination percentage of the OE lines had decreased to 62–68 %, respectively (Fig. 7c), but the difference between the WT and OE lines was not significant. In contrast, the percentage germination for the isolated embryos was still approximately 80 % in the overexpression lines, but only 53 % for the WT (Fig. 7d). Embryo vigor after 17 days aging was also examined by tetrazolium (TZ) stain (Debeaujon et al. 2000). The results showed that the embryo staining had become faint in the RNAi line, which showed a loss of viability, and that the OE line had more viable embryos than the WT (Fig. 7e). The germination assay and TZ stain of the seed lots after 17 days aging indicated that OE embryo viability was more resistant to artificial aging than the WT. To further investigate the germination pattern differences between the whole seed and the isolated embryos, the integrity of aleurone layers was also tested by determining the α-amylase activity of embryo-free seeds with additional of exogenous gibberellin (GA). The results indicated that the change in α-amylase activity gradually decreased as the treatment time increased, but there were no significant differences between the transgenic lines and the WT (Fig. 8). Therefore, the comparison of the embryo and whole seed germination performances of the OE and WT lines suggested that the variation in the OE line embryos and whole seeds mainly resulted from physiological differences between the non-embryo parts of the seeds in this stage.
α-Amylase activity of endosperms of transformed and WT seeds after aging treatment. Determination of α-amylase activity of endosperms of transformed and WT seeds after aging treatment. Embryo-free endosperm was induced by 1 μM Exogenous GA for 24 h. One unit of activity was defined as the amount of enzyme that degrade 1 μg soluble starch per minute at 40 °C. Error bars indicate SD from three parallel samples
After 21 days of treatment, the viable seed percentage for WT was less than 5 %, the OE lines had germination percentages of 18, 20 and 14 %, respectively (Fig. 7c), and the RNAi lines were unable to complete germination at all. Similarly, the isolated embryos had a generally high germination percentage compared with that of whole seeds (Fig. 7d). As for germination percentage of isolated embryos, the OE lines had germination percentages of 25, 24, and 21 %, and the germination percentage of WT seeds remained at 9.8 %, which indicated that the reduction in embryo viability was not the only reason for the drop in the germination percentage of whole seeds after 21 days of treatment.
isoAsp levels fell in overexpression lines, but overaccumulated in RNAi lines under aging treatment
OsPIMT1 accumulation in the embryo was verified by Western blot (Fig. 9a), which confirmed that embryos from the OE lines had a higher OsPIMT1 expression than WT embryos, whereas OsPIMT1 was substantially suppressed in the RNAi lines (Fig. 9a). The isoAsp contents were determined by the PIMT-based HPLC method. The accumulation of isoAsp-containing proteins in the samples was measured as the aging treatment time length increased (Fig. 9b). The accumulation rate for isoAsp-containing proteins in OE1 was significantly lower than that in the WT at all three time points, whereas the RNAi lines tended to overaccumulate abnormal isoAsp during the whole treatment process (Fig. 9b), which indicated that the isoAsp accumulation differences in the OE line and RNAi line under artificial aging treatment resulted from the overexpression or suppression of OsPIMT1 in the transgenic lines.
Accumulation of OsPIMT1 protein in the embryos of mature seeds and isoAsp quantification after aging treatment. a Western blot analysis of OsPIMT1 protein accumulation in the embryos of newly harvested seeds from T3 transgenic plants. An OsPIMT1 specific antibody was used for immunoreactive detection. HSP82 is the loading control. OE1–3 and Ri1–2 denotes three overexpressing line and two RNAi lines, respectively. b isoAsp quantification over time. Error bars indicate SD from three parallel samples
Taken together, these data strongly suggested that there was a correlation between PIMT1 expression in seeds, and their longevity and germination vigor, which was also the case in the mutant line, and that the PIMT1-related longevity improvement is probably due to the increased repair of isoAsp-containing proteins that mainly accumulate in embryos after artificial aging treatment.
Discussion
PIMT is a protein-repairing enzyme that is found in a broad range of organisms. Plants have two PIMT isoenzymes (PIMT1 and PIMT2; Dinkins et al. 2008; Mudgett and Clarke 1993; Oge et al. 2008; Xu et al. 2004), which are mainly found in seeds, and have an unregulated response to abiotic and phytohormone treatments (Mudgett and Clarke 1996; Verma et al. 2010). Although PIMT was thought to be involved in seed vigor and longevity, there was no direct evidence to confirm this hypothesis until recent findings revealed that PIMT was shown to be involved in maintaining and enhancing germination vigor and longevity in Arabidopsis (Oge et al. 2008; Verma et al. 2013). However, the physiological relevance of PIMT in monocot seeds was uncertain and needed to be clarified. Therefore, we isolated PIMT genes, described their expression patterns and clarified the physiological relevance in rice, in order to elucidate the role of PIMT in higher plants and explore its potential importance in agriculture.
In Arabidopsis, AtPIMT2 was found in various cell compartments, and this was determined by its multiple transcriptional initiation sites and alternative spicing of the 5′ part of the coding region. However, AtPIMT1 was predominantly distributed in the cytosol and nucleus (Dinkins et al. 2008). Similarly, CaPIMT2 was mainly detected in the nucleus, whereas CaPIMT1 was localized in the cytosol (Verma et al. 2013). We found that OsPIMT1 was localized in the cytosol, whereas OsPIMT2 was found in many cell compartments, including the nucleus and chloroplasts. These results were consistent with previous studies that had concluded that PIMT2 and PIMT1 cooperatively repaired protein damage across the whole cell (Dinkins et al. 2008; Thapar et al. 2001; Verma et al. 2013).
The expression patterns of the two OsPIMT genes were similar under abiotic stress. The two genes were more sensitive to salinity, cold and oxidative stress. They exhibited different expression patterns from chickpea where PIMT was upregulated by dehydration and heat (Verma et al. 2013, 2010), which suggested that OsPIMTs were regulated in a different way in rice under abiotic stress. The promoter regions of the two PIMTs contained several important stress-response cis-elements, e.g. MYC and MYB, which respond to cold and dehydration stress (Abe et al. 2003; Agarwal et al. 2006; Chinnusamy et al. 2003, 2004; Lee et al. 2005; Urao et al. 1993). This may explain their similar expression patterns to some extent. Furthermore, OsPIMT1 and OsPIMT2 were both induced by ABA, which was consistent with previous reports (Mudgett and Clarke 1994; Oge et al. 2008; Verma et al. 2013). ABA biosynthesis was commonly enhanced under dehydration (Danquah et al. 2014; Lafitte et al. 2007; Tang et al. 2008). The two OsPIMT genes were not significantly affected by drought treatment, which suggested that other regulating factors in addition to ABA might be involved in the PIMT stress response to drought in rice. Interestingly, the ABRE cis-element, which is often found in the Arabidopsis and chickpea PIMT promoter regions (Mudgett and Clarke 1996; Verma et al. 2013; Xu et al. 2004), was not present in these two OsPIMT promoter regions. However, PIMT1 and PIMT2 contained two ABRE coupling elements in the 5′ UTR. ABA-inducible transcription typically requires more than two ABREs or the combination of an ABRE with a coupling element at appropriate positions in the promoter regions (Bartels and Souer 2004; Zhang et al. 2005), which means that ABRE-CE itself cannot function as an ABA-responsive element and the PIMT1 and PIMT2 were not regulated in the same way as they are in Arabidopsis and chickpea. Bioinformatics and high-throughput gene expression analysis showed that Ca2+/calmodulin binding protein was bound to the ABRE and ABRE-CE cis-elements during stress responses (Doherty et al. 2009; Kaplan et al. 2006; Reddy et al. 2011), which implied that they had a number of functions during stress responses. We identified several cis-elements that responded to stress by binding calmodulin-binding TF in the region adjacent to ABRE-CEs, which indicated that Ca2+/calmodulin-mediated pathways might be involved PIMT1 and PIMT2 stress responses.
Earlier studies have shown that PIMT was mainly found in seeds. The CaPIMT2 and CaPIMT1 expression patterns were fairly similar, but they were also detected in flowers, shoots, leaves and roots (Verma et al. 2013). OsPIMT2 did not show a seed-dominant expression pattern, which was different from a previous study on chickpea (Verma et al. 2013). Unusually, a leaf-related box (Gowik et al. 2004) was spread extensively, mostly in clusters, across the OsPIMT2 promoter, but, this structure was not observed in Arabidopsis and chickpea PIMT promoter regions, which suggested that OsPIMT2 might function in the green tissue, but not in seeds. Preliminary analysis of tissue specific OsPIMT1 expression showed that it was moderately distributed in various tissues, but mRNA levels were high in mature embryos, and a CAAT box, reported as being present in the legA promoter (Shirsat et al. 1989), was frequently found in the OsPIMT1 promoter region, but not in OsPIMT2. Taken together, the differences in OsPIMT1 and OsPIMT2 tissue specificity are probably due to different repair protein requirements across the whole plant.
PIMT activity is highest in the seeds, where non-enzymatic protein damage is thought to be prevalent during dehydration at maturity and during dry storage (Rajjou and Debeaujon 2008). The accumulation of isoAsp in seeds, due to aging or stress treatment, has been shown to adversely affect their vigor and viability, and such damage needs to be repaired if normal germination is to occur (Mudgett et al. 1997; Oge et al. 2008; Verma et al. 2013). Recently, Oge et al. (2008) showed that PIMT1 over accumulation could reduce the number of detrimental isoAsp residues in seed proteins, resulting in increased seed longevity and germination vigor. In RNAi line, suppression of OsPIMT1 caused isoAsp to overaccumulate, and accelerated the loss of seed vigor, which suggested that OsPIMT1 was involved in maintaining seed vigor and longevity. Similarly, overexpression of OsPIMT1 could increase seed germination energy because seed vigor was preserved in the transgenic lines, although the seed germination percentage only improved by a relatively small amount. Rice seed is an albuminous seed, in which the endosperm provides nutrients for germination and seedling growth. Therefore, attention has been paid to the changes that occur outside the embryo, since such changes would be expected to affect germination energy, seedling growth and vigor by affecting storage reserve mobilization. OsPIMT1 was highly expressed in the embryo, but not in the endosperm. There were no significant differences in aleurone layer α-amylase activity between the transgenic lines and the WT at a given time point, which partially suggested that OsPIMT1 overexpression and RNAi had no influence on the endosperm. It was noticed that the endosperm could be a limiting factor on germination depending on the treatment time, which was supported by the germination differences between the whole seeds and isolated embryos of the OE1 lines after 17 days treatment (Fig. 7c, d). The isolated embryos had higher germination percentages and this was also observed in wheat seed after artificial aging (Das and Sen-Mandi 1988; Petruzzelli and Taranto 1989). The greater germination percentage of isolated seed from the OE lines supported the conclusion that OsPIMT1 has a role in maintaining embryo vigor, and that OsPIMT1 is specifically involved in repairing isoAsp damage in the embryo proteome, which, in turn, enhances embryo and seed survival rates under artificial aging treatment.
Other repair pathways that stabilize the seed genome and proteome have been shown to play roles in enhancing seed vigor and longevity. Two DNA repair enzymes, AtLIG6 and AtOGG1, repair DNA damage in seeds under stress, thus extending seed longevity (Chen et al. 2012; Waterworth et al. 2010). Another important protein repair system, the methionine sulfoxide reductase repair system, has recently been shown to play a key role in the establishment and preservation of seed longevity (Chatelain et al. 2013). These findings draw attention to the role of the plant’s own repair systems and their effects on seed longevity and germination vigor. Although monocot seeds have a different structure to Arabidopsis and chickpea seeds, they undergo a similar process during germination, including hormone induction, protein synthesis and repair, and detoxification (Finkelstein 2010; Nonogaki 2006; Rajjou et al. 2012; Rock and Quatrano 1995). When taking into account the distinctive aspect for vigor and longevity of cereal seed, these plant repair systems may have promising applications in improving cereal crop seed storability and longevity.
References
Abe H, Urao T, Ito T, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. Plant Cell 15:63–78
Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281:37636–37645. doi:10.1074/jbc.M605895200
Baranowskij N, Frohberg C, Prat S, Willmitzer L (1994) A novel DNA binding protein with homology to Myb oncoproteins containing only one repeat can function as a transcriptional activator. EMBO J 13:5383–5392
Bartels D, Souer E (2004) Molecular responses of higher plants to dehydration. In: Hirt H, Shinozaki K (eds) Plant responses to abiotic stress, vol 4., Topics in current geneticsSpringer, Berlin Heidelberg, pp 9–38
Bernal-Lugo I, Rodriguez M, Gavilanes-Ruiz M, Hamabata A (1999) Reduced aleurone α-amylase production in aged wheat seeds is accompanied by lower levels of high-pI α-amylase transcripts and reduced response to gibberellic acid. J Exp Bot 50:311–317. doi:10.1093/jxb/50.332.311
Chatelain E, Satour P, Laugier E, Ly VuB, Payet N, Rey P, Montrichard F (2013) Evidence for participation of the methionine sulfoxide reductase repair system in plant seed longevity. Proc Natl Acad Sci 110(9):3633–3638
Chen T, Nayak N, Majee SM, Lowenson J, Schafermeyer KR, Eliopoulos AC, Lloyd TD, Dinkins R, Perry SE, Forsthoefel NR (2010) Substrates of the Arabidopsis thaliana protein isoaspartyl methyltransferase 1 identified using phage display and biopanning. J Biol Chem 285:37281–37292
Chen H, Chu P, Zhou Y, Li Y, Liu J, Ding Y, Tsang EWT, Jiang L, Wu K, Huang S (2012) Overexpression of AtOGG1, a DNA glycosylase/AP lyase, enhances seed longevity and abiotic stress tolerance in Arabidopsis. J Exp Bot 63:4107–4121
Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17:1043–1054. doi:10.1101/gad.1077503
Chinnusamy V, Schumaker K, Zhu JK (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55:225–236. doi:10.1093/jxb/erh005
Clarke S (2003) Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev 2:263–285
Danquah A, de Zelicourt A, Colcombet J, Hirt H (2014) The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32:40–52
Das G, Sen-Mandi S (1988) Root formation in deteriorated (aged) wheat embryos. Plant Physiol 88:983–986. doi:10.1104/pp.88.4.983
Debeaujon I, Leon-Kloosterziel KM, Koornneef M (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122(2):403–414
Dinkins RD, Majee SM, Nayak NR, Martin D, Xu Q, Belcastro MP, Houtz RL, Beach CM, Downie AB (2008) Changing transcriptional initiation sites and alternative 5′-and 3′-splice site selection of the first intron deploys Arabidopsis PROTEIN ISOASPARTYL METHYLTRANSFERASE2 variants to different subcellular compartments. Plant J 55:1–13
Doherty CJ, Van Buskirk HA, Myers SJ, Thomashow MF (2009) Roles for Arabidopsis CAMTA transcription factors in cold-regulated gene expression and freezing tolerance. Plant Cell Online 21:972–984
Emanuelsson O, Brunak S, von Heijne G, Nielsen H (2007) Locating proteins in the cell using TargetP, SignalP and related tools. Nat Protoc 2:953–971
Finkelstein R (2010) The role of hormones during seed development and germination. In: Davies P (ed) Plant hormones. Springer, Netherlands, pp 549–573. doi:10.1007/978-1-4020-2686-7_24
Gowik U, Burscheidt J, Akyildiz M, Schlue U, Koczor M, Streubel M, Westhoff P (2004) cis-Regulatory elements for mesophyll-specific gene expression in the C4 plant Flaveria trinervia, the promoter of the C4 phosphoenolpyruvate carboxylase gene. Plant Cell Online 16:1077–1090. doi:10.1105/tpc.019729
Horton P, Park K-J, Obayashi T, Fujita N, Harada H, Adams-Collier C, Nakai K (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35:W585–W587
Kagan RM, McFadden HJ, McFadden PN, O’Connor C, Clarke S (1997a) Molecular phylogenetics of a protein repair methyltransferase. Comp Biochem Physiol B: Biochem Mol Biol 117:379–385
Kagan RM, Niewmierzycka A, Clarke S (1997b) Targeted gene disruption of the Caenorhabditis elegans l-isoaspartyl protein repair methyltransferase impairs survival of dauer stage nematodes. Arch Biochem Biophys 348:320–328. doi:10.1006/abbi.1997.0362
Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H (2006) Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell Online 18:2733–2748. doi:10.1105/tpc.106.042713
Kim E, Lowenson JD, MacLaren DC, Clarke S, Young SG (1997) Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc Natl Acad Sci 94:6132
Lafitte H, Yongsheng G, Yan S, Li Z-K (2007) Whole plant responses, key processes, and adaptation to drought stress: the case of rice. J Exp Bot 58:169–175. doi:10.1093/jxb/erl101
Lee BH, Henderson DA, Zhu JK (2005) The Arabidopsis cold-responsive transcriptome and its regulation by ICE1. Plant Cell 17:3155–3175. doi:10.1105/tpc.105.035568
Li C, Clarke S (1992) A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance. Proc Natl Acad Sci 89:9885–9889
Li X, Bai H, Wang X, Li L, Cao Y, Wei J, Liu Y, Liu L, Gong X, Wu L (2011) Identification and validation of rice reference proteins for western blotting. J Exp Bot 62:4763–4772
Lu G, Gao C, Zheng X, Han B (2009) Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta 229:605–615
Mudgett MB, Clarke S (1993) Characterization of plant l-isoaspartyl methyltransferases that may be involved in seed survival: purification, cloning, and sequence analysis of the wheat germ enzyme. Biochemistry 32:11100–11111
Mudgett MB, Clarke S (1994) Hormonal and environmental responsiveness of a developmentally regulated protein repair l-isoaspartyl methyltransferase in wheat. J Biol Chem 269:25605–25612
Mudgett M, Clarke S (1996) A distinctly regulated protein repair l-isoaspartylmethyltransferase from Arabidopsis thaliana. Plant Mol Biol 30:723–737. doi:10.1007/BF00019007
Mudgett MB, Lowenson JD, Clarke S (1997) Protein repair l-isoaspartyl methyltransferase in plants (phylogenetic distribution and the accumulation of substrate proteins in aged barley seeds). Plant Physiol 115:1481–1489. doi:10.1104/pp.115.4.1481
Nakabayashi K, Okamoto M, Koshiba T, Kamiya Y, Nambara E (2005) Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: epigenetic and genetic regulation of transcription in seed. Plant J 41:697–709. doi:10.1111/j.1365-313X.2005.02337.x
Niu X (2002) Maize ABI4 binds coupling element1 in abscisic acid and sugar response genes. Plant Cell Online 14:2565–2575. doi:10.1105/tpc.003400
Nonogaki H (2006) Seed germination—The biochemical and molecular mechanisms. Breed Sci 56:93–105. doi:10.1270/jsbbs.56.93
Oge L, Bourdais G, Bove J, Collet B, Godin B, Granier F, Boutin JP, Job D, Jullien M, Grappin P (2008) Protein repair l-isoaspartyl methyltransferase 1 is involved in both seed longevity and germination vigor in Arabidopsis. Plant Cell 20:3022–3037. doi:10.1105/tpc.108.058479
Petruzzelli L, Taranto G (1989) Wheat aging: the contribution of embryonic and non-embryonic lesions to loss of seed viability. Physiol Plant 76:289–294. doi:10.1111/j.1399-3054.1989.tb06193.x
Rajjou L, Debeaujon I (2008) Seed longevity: survival and maintenance of high germination ability of dry seeds. CR Biol 331:796–805
Rajjou L, Duval M, Gallardo K, Catusse J, Bally J, Job C, Job D (2012) Seed germination and vigor. Annu Rev Plant Biol 63:507–533
Reddy ASN, Ali GS, Celesnik H, Day IS (2011) Coping with stresses: roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell Online 23:2010–2032. doi:10.1105/tpc.111.084988
Rock C, Quatrano R (1995) The role of hormones during seed development. In: Davies P (ed) Plant hormones. Springer, Netherlands, pp 671–697. doi:10.1007/978-94-011-0473-9_31
Schmittgen TD, Livak KJ (2008) Analyzing real-time PCR data by the comparative CT method. Nat Protoc 3:1101–1108. doi:10.1038/nprot.2008.73
Schurter BT, Aswad DW (2000) Analysis of isoaspartate in peptides and proteins without the use of radioisotopes. Anal Biochem 282:227–231. doi:10.1006/abio.2000.4601
Shen-Miller J (2002) Sacred lotus, the long-living fruits of China Antique. Seed Sci Res 12:131–143. doi:10.1079/SSR2002112
Shirsat A, Wilford N, Croy R, Boulter D (1989) Sequences responsible for the tissue specific promoter activity of a pea legumin gene in tobacco. Mol Gen Genet: MGG 215:326–331
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28:2731–2739
Tang R-S, Zheng J-C, Jin Z-Q, Zhang D-D, Huang Y-H, Chen L-G (2008) Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54:37–43. doi:10.1007/s10725-007-9225-8
Thapar N, Kim A-K, Clarke S (2001) Distinct patterns of expression but similar biochemical properties of protein l-isoaspartyl methyltransferase in higher plants. Plant Physiol 125:1023–1035
Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K (1993) An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence. Plant Cell 5:1529–1539. doi:10.1105/tpc.5.11.1529
Verma P, Singh A, Kaur H, Majee M (2010) PROTEIN l--ISOASPARTYL METHYLTRANSFERASE1 (CaPIMT1) from chickpea mitigates oxidative stress-induced growth inhibition of Escherichia coli. Planta 231:329–336
Verma P, Kaur H, Petla BP, Rao V, Saxena SC, Majee M (2013) PROTEIN l-ISOASPARTYL METHYLTRANSFERASE2 is differentially expressed in chickpea and enhances seed vigor and longevity by reducing abnormal isoaspartyl accumulation predominantly in seed nuclear proteins. Plant Physiol 161:1141–1157
Villa ST, Xu Q, Downie AB, Clarke SG (2006) Arabidopsis protein repair l-isoaspartyl methyltransferases: predominant activities at lethal temperatures. Physiol Plant 128:581–592
Visick JE, Cai H, Clarke S (1998) The l-isoaspartyl protein repair methyltransferase enhances survival of aging Escherichia coli subjected to secondary environmental stresses. J Bacteriol 180:2623–2629
Wang Z, Chen C, Xu Y, Jiang R, Han Y, Xu Z, Chong K (2004) A practical vector for efficient knockdown of gene expression in rice (Oryza sativa L.). Plant Mol Biol Rep 22:409–417. doi:10.1007/BF02772683
Waterworth WM, Masnavi G, Bhardwaj RM, Jiang Q, Bray CM, West CE (2010) A plant DNA ligase is an important determinant of seed longevity. Plant J 63:848–860. doi:10.1111/j.1365-313X.2010.04285.x
Xu Q, Belcastro MP, Villa ST, Dinkins RD, Clarke SG, Downie AB (2004) A second protein l-isoaspartyl methyltransferase gene in Arabidopsis produces two transcripts whose products are sequestered in the nucleus. Plant Physiol 136:2652
Yang T, Poovaiah BW (2002) A calmodulin-binding/CGCG box DNA-binding protein family involved in multiple signaling pathways in plants. J Biol Chem 277:45049–45058. doi:10.1074/jbc.M207941200
Yoshida S, Forno D, Cock J, Gomez K (eds) (1976) Routine procedure for growing rice plants in culture solution. In: Laboratory manual for physiological studies of rice. The International Rice Research Institute, 3rd edn. Los Baños, Laguna, Philippines, pp 61–66
Yoshida T, Fujita Y, Sayama H, Kidokoro S, Maruyama K, Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2010) AREB1, AREB2, and ABF3 are master transcription factors that cooperatively regulate ABRE-dependent ABA signaling involved in drought stress tolerance and require ABA for full activation. Plant J 61:672–685. doi:10.1111/j.1365-313X.2009.04092.x
Zeng D-L, Qian Q, Yasukumi K (2002) Study on storability and morphological index in rice (Oryza sativa L.) under artificial ageing. Acta Agron Sin 28:551–554
Zhang W, Ruan J, T-hD Ho, You Y, Yu T, Quatrano RS (2005) Cis-regulatory element based targeted gene finding: genome-wide identification of abscisic acid- and abiotic stress-responsive genes in Arabidopsis thaliana. Bioinformatics 21:3074–3081
Zhang Y, Su J, Duan S, Ao Y, Dai J, Liu J, Wang P, Li Y, Liu B, Feng D, Wang J, Wang H (2011) A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 7:30. doi:10.1186/1746-4811-7-30
Zhou Z, Robards K, Helliwell S, Blanchard C (2002) Ageing of stored rice: changes in chemical and physical attributes. J Cereal Sci 35:65–78
Acknowledgments
We thank Prof. Jiayang Li at Chinese Academy of Agricultural Sciences, Dr. Zhen Li at Institute of Genetics and Developmental Biology, Chinese Academy of Sciences for technical support of subcellular localization. We thank National key laboratory of Crop genetics improvement, Huazhong Agricultural University for the mutant of OsPIMT1 derived from variety ZH11. We also thank Prof. Kang Chong of Institute of Botany, Chinese Academy of Sciences for the RNAi vector. The research is supported by grants from The Hi-Tech Research and Development (863) Program of China (2014AA10A603, 2014AA10A604), The National Major Projects of Cultivated Transgenic New Crop Varieties Foundation of China (2008ZX001-006, 2011ZX001-006), The National Natural Science Foundation of China (31401471), The Special Foundation of Non⁃Profit Research Institutes of Fujian Province (2014R1021-8), and The Science Fund for Distinguished Young Scholars of Fujian Provincial Academy of Agricultural Sciences (2014JQ-3).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Accession numbers Sequence data from this article can be found in GenBank/EMBL data bases under the accession numbers listed below. The newly obtained cDNA sequences of Os08g0557000/OsPIMT1 and Os04g0481400/OsPIMT2 were deposited to GenBank under accession number KM527502 and KM675736, respectively. Accession Numbers used in sequence blast and phylogenetic analysis are in Supplemental Table S2.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Figure S1
Hydrophobicity analysis and targeting prediction of OsPIMT2 (TIFF 2288 kb)
Supplemental Figure S2
Prokaryotic expression analysis of OsPIMT1 and OsPIMT2 (TIFF 974 kb)
Supplemental Figure S3
Expression analysis of OsPIMT1 and OsPIMT2 under salicylic acid and jasmonic acid treatment (TIFF 883 kb)
Supplemental Figure S4
Verification of pimt1-1 T-DNA insertion and screening of homozygous pimt1-1 mutant plants (TIFF 231 kb)
Supplemental Figure S5
Overall view of germination performance of OsPIMT1 transgenic lines and WT after aging treatment (TIFF 5961 kb)
Supplemental Table S1
List of Primers used in this study (DOC 55 kb)
Supplemental Table S2
Accession numbers used in sequence blast and phylogenetic analysis (DOC 38 kb)
Supplemental Table S3
List of cis-regulatory elements in the 750-bp promoter regions of OsPIMT1 and OsPIMT2 (DOC 78 kb)
Rights and permissions
About this article
Cite this article
Wei, Y., Xu, H., Diao, L. et al. Protein repair l -isoaspartyl methyltransferase 1 (PIMT1) in rice improves seed longevity by preserving embryo vigor and viability. Plant Mol Biol 89, 475–492 (2015). https://doi.org/10.1007/s11103-015-0383-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0383-1