Abstract
Worldwide, plant viral infections decrease seriously the crop production yield, boosting the demand to develop new strategies to control viral diseases. One of these strategies to prevent viral infections, based on the immunomodulation faces many problems related to the ectopic expression of specific antibodies in planta. Camelid nanobodies, expressed in plants, may offer a solution as they are an attractive tool to bind efficiently to viral epitopes, cryptic or not accessible to conventional antibodies. Here, we report a novel, generic approach that might lead to virus resistance based on the expression of camelid specific nanobodies against Broad bean mottle virus (BBMV). Eight nanobodies, recognizing BBMV with high specificity and affinity, were retrieved after phage display from a large ‘immune’ library constructed from an immunized Arabic camel. By an in vitro assay we demonstrate how three nanobodies attenuate the BBMV spreading in inoculated Vicia faba plants. Furthermore, the in planta transient expression of these three selected nanobodies confirms their virus neutralizing capacity. In conclusion, this report supports that plant resistance against viral infections can be achieved by the in vivo expression of camelid nanobodies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant viruses cause devastating losses in agriculture, particularly, in economic crops traded across the world. This trading in turn, participates in spreading the viruses and expands the problems with plant infection (Prins et al. 2008). The worldwide virus propagation boosts the demand to develop new strategies to control viral diseases (Boonrod et al. 2004). Natural sources of plant resistance against viruses are limited and cannot be renewed once the virus managed to by-pass the innate plant resistance. Therefore, renewable forms of engineered plant resistance against viruses receive a lot of attention (Soosaar et al. 2005). Plant pathology, particularly plant virology, has long relied on the use of conventional monoclonal and polyclonal antibodies to diagnose the infection (Ziegler and Torrance 2002). Unfortunately, the large size and the complex architecture of these antibodies impose practical limitations for their application as in vivo therapeutics (Holliger and Hudson 2005). For applications where antibodies do not function satisfactorily, it might be an option to consider easier to manufacture, smaller and simpler antibody fragments (Ahmad et al. 2012). Antibody engineering yielded such small, but stable, fragments that perform the main function of an antibody in antigen recognition and binding (Holliger and Hudson 2005). Thus, over the last two decades, recombinant antibodies, such as single chain variable fragments (scFv), have been used as an interesting alternative to conventional antibodies (Ahmad et al. 2012). Many of these antibody formats were produced for different types of viruses infecting grassy (Harper et al. 1997; Ziegler et al. 1997; Toth et al. 1999; Zakri et al. 2010), shrub (Saldarelli et al. 2005; Orecchia et al. 2008; Nolke et al. 2009) and woody plants (Terrada et al. 2000). The efficacy of these smaller antibody variants was proven in a broad range of detection techniques, including viral diagnosis in pure preparations as well as in crude extracts from different infected plant tissues (Susi et al. 1998; Terrada et al. 2000).
Immunomodulation by ectopic expression of antigen-specific antibody fragments has a great potential in plant biology (Jobling et al. 2003), as well as in preventing viral infections (Villani et al. 2005). The concept of ectopic antibody expression was first established in mammalian cells and when expressed intracellularly they were called “intrabodies” (Marasco 1995). Likewise, antibodies produced by engineered plants were called “plantibodies” (De Jaeger et al. 2000; Ziegler and Torrance 2002). The proof of principle that plantibodies protect plants from viral attack was given in 1993 (Tavladoraki et al. 1993). Later, different recombinant antibodies were successfully expressed as immunomodulators in planta and were capable to inhibit virus accumulation in plant tissues (Boonrod et al. 2004; Villani et al. 2005; Nickel et al. 2008). Generally, functional antibodies expressed in plants can be used to either inhibit plant physiological processes or to establish pathogen resistance.
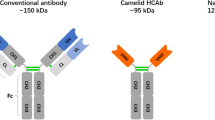
In Camelidae, about half of the antibodies circulating in blood are devoid of light chains. Nevertheless, these unique antibodies, referred to as heavy-chain antibodies (HCAbs), are able to bind their targets just as potently as conventional antibodies (Nguyen et al. 2001). The antigen-binding fragment of such HCAbs is reduced to one single domain of only 15 kDa, known as “VHH or nanobody” (Muyldermans 2013). Its potential as an attractive alternative for antigen-binding fragments such as scFvs derived from conventional antibodies has been demonstrated repeatedly in biotechnological, diagnostic and therapeutic applications (Holliger and Hudson 2005; Hassanzadeh-Ghassabeh et al. 2013; Gibbs 2005). This potency of nanobodies is based on their advantageous features including their small size, high solubility in aqueous solution, thermal stability, refolding capacity, long shelf life even at room temperature and good tissue penetration in vivo (Wesolowski et al. 2009; Muyldermans 2013). Furthermore, the minimal-sized monomeric nanobodies and their ability to bind epitopes that are cryptic or not accessible to conventional antibodies offer special advantages (Hassanzadeh-Ghassabeh et al. 2013). So far, multiple nanobodies have been successfully generated against numerous human and animal pathogens including protozoan parasites, bacteria and viruses (Wesolowski et al. 2009; Tokuhara et al. 2013; Desmyter et al. 2013; Vanlandschoot et al. 2011). Several recent reports have indicated the great potential of plants as efficient bioreactors for nanobody production (Jobling et al. 2003; Ismaili et al. 2007; Winichayakul et al. 2009; Teh and Kavanagh 2010; Tokuhara et al. 2013; De Buck et al. 2013; De Meyer et al. 2014a, b).
Broad bean mottle virus (BBMV), of the genus Bromovirus (Makkouk et al. 2012), is a seed-borne plant virus spreading in plants in Africa, Asia, Europe and Middle East (Makkouk et al. 1988). The BBMV viral particles are isometric with a diameter of 26 nm. The icosahedral (20 triangular faces) protein shell consists of 180 identical polypeptide subunits clustered as pentagonal and hexagonal structures. The shell packages the tripartite positive-sense single stranded genomic RNA, which includes three RNA molecules encoding four open reading frames (ORFs) (Bujarski 1998). Since many years, BBMV gained a large academic interest because high concentrations can be reached from infected cells, it is easy to purify and for its structural characteristics (Makkouk et al. 2012). The virus infects Cicer arietinum (chickpea), Pisum sativum (field pea) and Vicia faba (faba bean or broad bean) (Fortass and Bos 1992; Bawden et al. 2008). In addition, it is considered as one of the most important viruses in the Mediterranean region with a high economic impact. A possible loss in grain yield of 35–55 %, depending on the time of infection has been reported (Makkouk et al. 2012). It is well established that V. faba, one of the major crops cultivated intensively in West Asia and North Africa, is regularly infected with viruses, resembling BBMV (Makkouk et al. 1988). Possible seed transmission, incidence and effect on V. faba and some other crop species made the virus an important target in the crop improvement program of ICARDA (International Center for Agricultural Research in the Dry Areas) and worldwide.
Considering the advantages and potential of nanobodies, this work aimed to produce specific nanobodies against BBMV. The in planta transient expression of these nanobodies provides a proof of concept for their future exploitation to generate virus resistant transgenic plants.
Materials and methods
Ethics statement
This research was approved by the High Commission of Scientific Research and the National Sciences Research Ethics Board in Syria that operates in compliance with the International Convention for the Protection of Animals (ARTICLE 9: concerning animals used in scientific research). The experiments, maintenance and care of camelids complied with the guidelines of the National Committee of Scientific Ethics and Novel Technologies (chart of 2009). The experiments for this study were approved by the Local Scientific and Ethical Committee of the Atomic Energy Commission of Syria (AECS), Damascus, Syria, and the regulations of the General Commission for Scientific Agricultural Research (GCSAR), Deir El-Hagar Station for Camel Research, Damascus, Syria (Research Permit Number: 53-2007 and 155-2013).
Antigens and antibodies
Syrian isolates of plant viruses, BBMV, Bean yellow mosaic virus (BYMV), Alfalfa mosaic virus (AMV), Broad bean stain virus (BBSV) and Broad bean true mosaic virus (BBTMV) were collected, purified, concentrated to 0.4 mg/ml and characterized by the Plant Virology Laboratory at ICARDA. Briefly, BBMV was purified after a clarification step by adjusting the pH of the tissue homogenate with citric acid to pH 5.0 and low-speed centrifugation. Virus was concentrated by adding 8 % polyethylene glycol, and further purified by sucrose density-gradient centrifugation (Makkouk et al. 1988). Purified preparations of BBMV were treated with 0.2 % glutaraldehyde before camel immunization.
For ELISA tests, rabbit polyclonal antisera (provided by ICARDA) for BBMV (1:5,000) and AMV (1:2,000) were used to detect the respective virus by ELISA. The nanobodies bound on their antigen were revealed with either, rabbit anti-His antibody (Bethyl Laboratories Inc.) for His6-tagged nanobodies expressed in E. coli or with rabbit anti–nanobody (made in house) for untagged nanobodies expressed in plants. Detection of IgGs bound on antigen was performed with rabbit anti-camel antibody (Bethyl Laboratories Inc.). Subsequent detection of rabbit antibodies was done with anti-rabbit IgG conjugated to horseradish peroxidase (HRP) (Bethyl Laboratories Inc.). Nanobodies cloned in pMES4 phagemid vectors were expressed in E. coli strains (TG1 and WK6).
Camel immunizations and the fractionation of IgG subclasses
An adult (~2.5 years old) female camel (C. dromedarius) was immunized by seven subcutaneous injections, at weekly intervals, of 0.4 mg of glutaraldehyde-treated BBMV mixed with an equal volume of adjuvant. Blood samples were weekly collected before each injection and at days 42, 56 and 100 from the first injection. Serum was prepared and stored (as 50 % glycerol stocks) to monitor (at 1:2,000 dilution or as indicated) the camel immune response by ELISA.
An automated protocol of differential adsorption of IgGs on Hitrap-Protein A and Protein G columns (GE Healthcare) has been developed using FPLC (Abbady et al. 2011) to separate different IgG subclasses from camel plasma of day 56. Briefly, 5 ml camel serum (or plasma) was diluted with an equal volume of PBS and loaded at a flow rate of 5 ml/min on the Protein G column (5 ml) equilibrated with PBS. The flow through was collected as this fraction contains the IgG2 subclasses of antibodies that do not adsorb on Protein G. After washing with one column-volume PBS, IgG3 was eluted from the column with buffer A (150 mM NaCl, 0.58 % acetic acid, pH 3.5), IgG1 was then eluted with buffer B (100 mM glycine, pH 2.7). The column was washed intensively with PBS and the flow through was reloaded on the column to remove residual IgG1 and IgG3 from the first flow through (as all IgG isotypes from camel bind to Protein A). The flow through was loaded and captured on a Protein A column equilibrated in PBS. After washing the column with PBS, IgG2 was eluted using buffer A (brought at pH 4.5). The pH of each purified IgG fraction was immediately neutralized with 1.0 M Tris (pH 9.0), dialyzed against PBS and the IgG content quantified by UV absorption. The fractions were diluted to an IgG concentration of 1 mg/ml and aliquots were stored until further use at −20 °C.
Enzyme-linked immunosorbant assay
For direct ELISA, Maxisorb 96-well plates (Nunc) were coated with the different viruses at 0.1 μg/well in carbonate buffer by overnight incubation at 4 °C. Residual protein binding sites in the wells were blocked for 1 h at 37 °C with 5× blocking buffer (3 % skimmed milk or 1 % BSA) in TBS-T (20 mM Tris-base, 150 mM NaCl, 0.05 % Tween-20, pH 7.5). For sandwich ELISA, wells of microtiter plates were first coated with camel-anti-BBMV total IgG purified from serum of the immunized camel, at a dilution of 1:1,000 in carbonate buffer and residual protein binding sites were blocked as described before. Serial dilutions (from 0 to 1,000 ng/ml) of BBMV or of plant extracts 10 % (w/v) (1:10, 1:100 and 1:1,000) were prepared in 1× blocking buffer and then added to the wells and incubated for another 1 h at room temperature. Serial dilutions of primary antibodies were prepared and added to the wells according to what was indicated in each experiment. After several washes with TBS-T, detection of rabbit antisera was achieved with anti-rabbit HRP conjugate. The absorption at 450 nm was measured after adding peroxidase substrate 3,3′, 5,5′-tetramethylbenzidine (TMB; Sigma) followed by stopping buffer (1.0 M of H2SO4) to terminate the enzymatic reaction of peroxidase.
Construction of the nanobody library
A 50 ml blood sample was collected from the immunized camel at day 56 from the first day of immunization and the peripheral blood lymphocytes were prepared with the Lymphoprep kit (Nycomed). Later, RNA extraction was performed on purified lymphocytes with QIAamp RNA Blood Mini Kit (Qiagen). Twenty μg of total RNA was reverse transcribed into cDNA using oligo-dT12–18 (Invitrogen) as primer. Two subsequent PCR reactions were performed on this cDNA template to amplify the nanobody gene repertoire using specific primers (Abbady et al. 2011). Purified nanobody genes as well as the pMES4 phagemid were digested with PstI and BstEII restriction enzymes and then ligated using T4 DNA ligase (Invitrogen). Freshly prepared electro-competent E. coli TG1 cells were transformed with the ligated products and then plated on 24 × 24 cm plates containing LB agar supplemented with ampicillin and glucose. Colonies were scraped from the plates that were incubated overnight, washed and stored at −80 °C in LB medium supplemented with glycerol (final concentration of 20 %).
Panning of BBMV-specific nanobodies by phage display
A representative aliquot (250 µl) of the constructed nanobody library was grown to mid-logarithmic phase before infection with M13 helper phage (~20 times excess phages versus cells). After an overnight growth (in 250 ml), virions were purified from the culture supernatant by polyethylene glycol/NaCl precipitation and re-suspended in a total volume of 1 ml PBS. Phage particles (5 × 1011) were used for panning by incubating in MaxiSorb immunotubes (Nunc) pre-coated with native BBMV particles (10 μg for each round of panning). Phage particles carrying antigen-specific nanobodies were enriched by four consecutive rounds of in vitro selection. After each round of incubation and after washing off phage particles without BBMV-specific nanobodies, virus-bound phage particles were eluted for 10 min in 100 mM triethylamine (pH 11.0). Eluted phage particles were transferred to a fresh tube and the solution was immediately neutralized with 1.0 M Tris–HCl (pH 7.0), used to infect exponentially growing E. coli TG1 cells and plated on LB Agar with ampicillin and glucose. Individual colonies were picked, inoculated in LB medium with ampicillin and expression of soluble nanobody in the periplasm was induced by adding IPTG up to 1 mM. Soluble nanobodies extracted from the periplasm were tested in ELISA for their capacity to recognize BBMV.
Nanobody purification
The recombinant pMES4-nanobody plasmids were purified from E. coli TG1 cells that gave periplasmic extracts with positive signals in ELISA. These plasmids were sequenced and used to transform E. coli WK6 cells. Nanobodies expressed in these cells are tagged with six Histidines at their C-terminal, which allows their purification using nickel charged columns. A large scale production of recombinant nanobodies was performed by growing the bacteria in 250 ml shake flasks containing Terrific Broth medium (1.2 % tryptone, 2.4 % yeast extracts, 0.8 % glycerol, 17 mM KH2PO4, 72 mM K2HPO4) supplemented with 0.1 % glucose and 100 µg/ml ampicillin until reaching an optical density (at 600 nm) of 0.6–0.9. Expression of nanobody was induced by adding IPTG to 1 mM and incubating the culture for an additional 16 h at 28 °C (Arbabi Ghahroudi et al. 1997). After pelleting the cells, the periplasmic proteins were extracted by osmotic shock. Using FPLC, this periplasmic extract was loaded on 5 ml nickel charged columns. After washing, the bound proteins were eluted with a solution containing 500 mM imidazole. The eluted fraction was buffer-exchanged and concentrated on Vivaspin concentrators with a molecular mass cutoff of 5–10 kDa. The absorption at 280 nm and the extinction coefficient, as calculated from the amino acid sequence of each nanobody, were used to determine protein concentrations. The concentration of the nanobody solution was adjusted to 1 mg/ml and stored at −20 °C.
Biosensor analysis
The capacity of nanobodies to bind specifically their antigen was assessed by Surface Plasmon Resonance (SPR) using a SR7000DC instrument (Xantec). NbBBMV01 (250 μl) in 10 mM acetate buffer pH 5.0 at a concentration of 25 μg/ml was immobilized onto a research grade CMD200 M sensor chip according to the manufacturer’s instructions. All binding experiments were performed at 25 °C with 100 µl injections in HBS buffer (10 mM Hepes pH 7.5, 150 mM NaCl, 3.5 mM EDTA and 0.05 % Tween 20) at a flow rate of 50 μl/min and with a dissociation step of 240 s. A blank uncoated channel was used as an online reference during all injections. Epitope competition between anti-BBMV nanobodies was revealed by consecutive injections of BBMV (10 µg/ml) followed by each of the nanobodies (700 nM). Finally, rabbit anti-BBMV antibody (1:200) was injected over the two flow cells.
For affinity determination, native BBMV (10 µg/ml) was injected and run over immobilized NbBBMV01 and control channels. Measurement of the association kinetics was performed using injections of progressively increasing concentrations (0, 7, 35, 70, 350 and 700 nM) of each nanobody. The sensorgrams were fitted by subtracting the signal from the reference flow cell and were globally treated using Scrubber 2 software (www.cores.utah.edu). Short pulses (15 μl) of regeneration buffer (6 M guanidine HCl) were used to regenerate the nanobody-immobilized chip between the injections without any detectable effect on binding capacity of the nanobody afterwards.
Immunoblotting and coomassie blue staining of SDS-PAGE
The mini-Protean II system (BioRad) was used for SDS-PAGE following the manufacturer’s instructions. Gels were prepared with stacking and running acrylamide gels of 5 and 12 %, respectively. After electrophoresis, the proteins in the gel were stained by soaking the gel in coomassie blue for 2 h followed by destaining in 5 % acetic acid, 10 % methanol. For immunoblotting, 25 µg of plant proteins were separated on gels and blotted onto 0.45 µm nitrocellulose membranes (BioRad) using 1× blotting buffer (25 mM Tris-base, 200 mM glycine, 0.1 % SDS and 20 % methanol). After incubation in 5× blocking buffer, membranes were incubated with primary antibodies or nanobodies at the indicated dilutions for 1 h at room temperature. After several washes with TBS-T, the membranes were incubated with goat anti-rabbit HRP conjugated antibodies (1:2,000) for 1 h at room temperature. Bands were revealed by adding AEC (3-amino-9-ethylcarbazole) chromogen substrate in acetate buffer in presence of hydrogen peroxide.
Nanobody-mediated in vitro inhibition of BBMV
Five microliters of purified BBMV solution at 1 μg/ml were separately incubated with the three nanobodies (NbBBMV01, NbBBMV12 and NbBBMV22) in 50 ml of phosphate buffer at a final concentration 5 ng/μl was incubated for 2 h at room temperature. Later, the nanobody and virus mixture was inoculated into V. faba plants (15 plants per nanobody) by rubbing the upper surface of the leaf in the presence of abrasive Celite. Local and systemic symptoms (plant upper leaves) were monitored 5, 15, 25 days post inoculation (d.p.i.).
Co-immunoprecipitation and dot blot assays
Plant leaves agroinfiltrated (see below) for expressing nanobody NbBBMV01, NbBBMV12 or NbBBMV22 were inoculated with BBMV virus particles. Eighteen h after inoculation, plant tissues were harvested and total extracts were prepared in PBS buffer. BBMV virus particles in plant extracts were immunocaptured with rabbit polyclonal IgG and Protein A-coated agarose beads (Roche Diagnostic GmbH), following the manufacturer’s protocol. Briefly, 100 µl of plant extract was incubated for 30 min at room temperature with affinity-purified rabbit polyclonal IgG1, diluted 1/1,000. Protein-A-coated agarose beads were added to the mixture and washed three times with TBS-T buffer (20 mM Tris-base, 150 mM NaCl, 0.05 % Tween 20, pH 7.5). After washing the beads, IgG1-BBMV was eluted in 50 μl of buffer A (150 mM NaCl, 0.58 % acetic acid, pH 3.5) and used in a dot blot. Equal volumes of immunocaptured solutions were blotted onto 0.45 µm nitrocellulose membranes (BioRad) using 1× blotting buffer (25 mM Tris-base, 200 mM glycine, 0.1 % SDS and 20 % methanol) and using the dot blot instrument (BioRad). After incubation in the 5× blocking buffer, membranes were incubated with the primary rabbit-anti-BBMV antibodies at the indicated dilution for 1 h at room temperature. After several washes with TBS-T, the membranes were incubated with a goat anti-rabbit HRP conjugated antibody (1:2,000) for 1 h at room temperature. Bands were revealed by adding AEC chromogen substrate in acetate buffer in presence of hydrogen peroxide.
Tissue blotting immunoassay (TBIA)
BBMV virus particles were detected by TBIA following the protocol described by Lin et al. (1990). Briefly, the dried membranes were blocked for 20 min with 5 % non-fat dry milk and 0.5 % bovine serum albumin (BSA) (Sigm-Aldrich) in TBS-T. Without rinsing, the membranes were then placed for 90 min into a combination of primary antibody specific for each virus and a secondary enzyme-labeled anti-animal antibody. Membranes were then rinsed for 10 min in TBS-Tween, each for 5 min. Finally, membranes were immersed in a solution containing AEC chromogen substrate in acetate buffer and hydrogen peroxide.
In vivo nanobody expression after agroinfiltration
Three different fragments of ORFs (NbBBMV01, NbBBMV12 or NbBBMV22) were amplified by PCR with 5′-specific primers introducing a XbaI site, and 3′-specific primers introducing a SacI site, for sub-cloning into the cytoplasmic plant expression vector pBI121 (Clontech) containing the 35S cauliflower mosaic virus (CaMV) promoter and the CaMV terminator. Agrobacterium tumefaciens, strain LBA4404 was transformed with each of the expression plasmids by electroporation. A. tumefaciens suspensions were co-infiltrated into the leaves of thirteen wild-type V. faba plants in presence of A. tumefaciens carrying pBI 61-P19 (P19, suppressor of silencing of Tomato bushy stunt virus) to enhance the acquired transient expression in vivo (Voinnet et al. 2003).
Virus inoculation and monitoring of infection
BBMV virus particles were inoculated into plants using a purified virus solution at 1 µg/ml. Four days after infiltration, the agroinfiltrated V. faba leaves were mechanically inoculated with 1 µg/ml of BBMV by rubbing the upper surface of the leaf in the presence of abrasive Celite. Local and systemic symptoms (plant upper leaves) were monitored at 5, 15 and 21 d.p.i. Inoculated tissues were harvested on a daily basis. Harvested tissues were kept at −80 °C until further use.
Viral RNA extraction and diagnosis of BBMV content
BBMV RNA and plant RNA were extracted from plant tissues using Trizol® (Invitrogen) according to the manufacturer’s instructions. For cDNA synthesis, 2 µg of total RNA was used for reverse transcription (Superscript III, Invitrogen) as described in the manufacturer’s protocol. Quantitative Real-Time PCR (qPCR) was performed using SYBR Green I Dye (BioRad) in a StepOne® PCR Real-time (Applied Biosystems). The transcripts of EF1-alpha, used as an internal reference gene, were also amplified under the same conditions. The amount of each transcript, normalized to the internal reference gene, was analyzed with the StepOne™ Software (version 2.1). The standard curve method (diagnostic option) was used to inspect the BBMV content in planta. Three replicates of the PCR amplifications were performed for each cDNA sample in each run. The qPCR runs were repeated three to five times on different treated plants. Data were expressed as the mean value ± SEM of individual experiments.
Results
Evaluation of camel immune response against BBMV
The BBMV proteinaceous coat represents an interesting antigenic structure because of its symmetric shape, composed of almost 200 copies of a single protein. To generate nanobodies against this intact structure, a dromedary was injected seven times with an emulsified inactivated virus, and serum was collected at several time points during the immunization to monitor the elicited immune response by ELISA (Fig. 1a). Obviously, a significant and specific immune response against BBMV was raised from day 35 onwards. Furthermore, the camel serum collected at day 56 (1 week after the last boost) was serially diluted to determine the titer of the virus-specific antibodies (Fig. 1a, inset). The IgGs in the camel serum, diluted up to 3 × 10−5 (v/v), can still be detected to recognize the BBMV particles.
BBMV-specific immune response in the immunized camel. a The BBMV reactivity of IgG from serum samples (diluted to 1:1,500), taken from the immunized camel at several time points after the first injection of BBMV, was tested in ELISA on wells with (Circle BBMV) or without (Triangle No Ag, for no antigen) immobilized BBMV a inset, ELISA titration of the camel serum (from day 56) using serial dilutions (v:v). b Left panel represent the reactivity of camel IgG (at dilution 1:1,000) against BBMV as measured in an ELISA. The purified total antibodies (IgGs) and the different IgG sub-classes (IgG1, 2 and 3) purified from camel serum at day 56 were tested separately (black bars denoted BMV). Diluted rabbit anti-BBMV (R-a-BBMV) (1:5,000) was used as positive control, and the negative control was in the absence of antibodies (No Ab, for no antibody). IgGs were also tested in wells without antigen immobilized (open bars, denoted by ‘No Ag’, for no antigen). Image at the right end is an SDS-PAGE (10 % acrylamide) of IgG protein samples purified by differential Protein A and Protein G affinity chromatography; IgG3 (lane 1), IgG1 (lane 2) and IgG2 (lane 3)
Sera of camelids contain conventional (IgG1) and heavy chain-only antibodies (HCAbs), which can be separated into two distinct fractions (IgG2 and IgG3) by differential adsorption on Protein A and Protein G chromatography (Muyldermans et al. 2009). These IgG subclasses were purified as a mixture (total IgG) or separated fractions (IgG1, 2 and 3) from the immunized camel serum (day 56), and were tested in ELISA to evaluate the presence of BBMV-specific antibodies in each of the isotype fractions (Fig. 1b). Interestingly, all IgG subclasses contain virus-specific antibodies, and the signal in ELISA is comparable to that of total camel IgG or for polyclonal rabbit anti-BBMV antibody. The integrity and the purity of the camel IgG subclasses was verified by separating the proteins under reducing conditions by 12 % SDS-PAGE, followed by coomassie blue staining (Fig. 1b). This gel clearly distinguishes the conventional IgG1, with its heavy and light chains, and the HCAbs (IgG2 and IgG3) comprising smaller heavy chains (Fig. 1b).
Construction of an ‘immune’ VHH library and ‘biopanning’ for specific anti-BBMV nanobodies
The encouraging results of the camel immunization stimulated us to construct a nanobody library from the blood sample taken at day 56. The PCR amplified VHH genes from this sample were digested by PstI/BstEII and ligated into the pMES4 phagemid at a 3:1 ratio. The ligated material was used to transform the E. coli strain TG1, which resulted in a large nanobody library of 6.5 × 107 transformants, where more than 98 % of the colonies contained cells with a pMES4 vector having a gene insert of the size of a nanobody. Nanobodies in this library can be expressed as fusions with the PIII coat protein and displayed on M13 virions, enabling the retrieval of specific nanobodies for BBMV by panning. The isolation of BBMV specific binders was performed by four consecutive rounds of solid-phase in vitro selections in immunotubes coated with native BBMV. Using this approach, a clear enrichment of phage particles, carrying antigen-specific nanobodies, was observed from the third round of panning onwards (data not shown).
Examining the sequence of individual nanobody-expressing colonies from the enriched antigen-specific pool, confirmed the presence of eight distinct nanobodies, which were referred to as NbBBMV01, −03, −10, −11, −12, −13, −20 and −22 (Fig. 2a). The alignment of the deduced encoded amino acid sequences of these nanobodies shows a different amino acid composition in the three complementary determining regions (CDR), and mainly in the CDR3 (Fig. 2a). The NbBBMV12 is the only one derived from a VHH germline gene, as it contains most of the hallmark amino acid substitutions in framework 2 (amino acids at position 42, 49, 50 and 52) (Nguyen et al. 2000). Apart from the conserved disulfide bridge between Cys23 and Cys104 that is present in all clones, an extra interloop disulfide bridge, frequently occurring between CDR1 and CDR3 in camel nanobodies, was only present in this nanobody (Fig. 2a) (Saerens et al. 2008). In contrast, all other clones lack an interloop disulfide bond (clone NbBBMV13 has an intraloop disulfide bond in its CDR3) and the VHH-hallmark amino acids in framework region 2 indicate that these variable domains were derived from a VH germline gene. However, these nanobodies definitely originated from a HCAb as the presence of a Arg at position 118 instead of Trp would prevent interaction with the variable domain of a light chain (Muyldermans 2013).
Characteristics of anti-BBMV nanobodies. a Amino acid sequence alignment of different anti-BBMV nanobodies. Frameworks (FR) and hyper variable regions (CDR) are indicated and numbered according to IMGT (Lefranc et al. 2003). b Phylogeny tree of protein sequences demonstrating the homology between the different anti-BBMV nanobodies. c Protein characteristics of these nanobodies including sequence length (aa), theoretical size (kDa), extinction coefficient (OD280), iso-electric point (pI), the number of expected disulfide bonds (S–S), the recovery (mg/L of culture) and the EC50 (nM). d The reactivity and specificity of anti-BBMV nanobodies (dilution 1:500) and antibodies (R-a-BBMV “rabbit anti-BBMV” and R-a-AMV “rabbit anti-AMV”; dilution 1:3,000) were tested in ELISA against different immobilized (0.1 µg/well) viruses (BBMV, AMV, BYMV, BBTMV and BBSV)
A phylogenetic analysis of these sequences confirms the different origin of NbBBMV12 from the other nanobodies (Fig. 2b). The analysis of the amino acid sequence of these nanobodies predict minor differences in the protein characteristics such as size or molecular weight, extinction coefficient and iso-electric point (Fig. 2c). The expression yields of these nanobodies ranged between 1.5 and 18 mg/L of bacterial culture and most of them were highly reactive with the BBMV in ELISA, showing a significant median effective concentration (EC50) in the nanomolar (NbBBMV11, −12, −13, −20 and −22) and sub-nanomolar (NbBBMV01, −03 and −10) range towards the immobilized antigen (Fig. 2c).
We further tested the specificity by ELISA of the soluble nanobodies from all eight clones against four different fabaceae viruses: BYMV, AMV, BBSV and BBTMV (Fig. 2d). Rabbit anti-BBMV and anti AMV-specific polyclonal antibodies were used as reference, and wells without a coated virus were our negative controls. The data confirmed the high specificity of all selected nanobodies towards BBMV and no cross reaction was noticed for any of the other viruses.
Anti-BBMV nanobodies target a unique epitope
The recognition of the most appropriate epitope is crucial to exert a maximal therapeutic effect (Ladner 2007). Hence, we designed an epitope binning experiment on native BBMV particles for our 8 nanobodies by sandwich SPR (surface plasmon resonance) (Fig. 3a). We first covalently immobilized NbBBMV01 on the activated matrix of one sensor channel by amine coupling, while the second channel was left empty to obtain a constant reference value. Signals from both channels after flowing the virus were recorded in a sensorgram of refractive index units (RIU) as a function of time (Fig. 3a). The injected BBMV is captured on the sensor surface containing the immobilized nanobody, resulting in a signal shifting from the base line. Moreover, since the SPR signal is affected by the mass of molecules captured on the sensor chip, the sensorgram revealed an additional signal upon injecting NbBBMV01 in the mobile phase. This is explained by the multiple repetition of the protein coat epitope, exposed on the surface of the virus captured by the immobilized NbBBMV01. However, the subsequent injections of any other nanobody failed to increase the signal. In contrast, the injection of polyclonal antibody of BBMV did raise the signal. Therefore, it is suggested that all nanobodies target one single, overlapping epitope. The captured BBMV was cleared and the sensor surface was regenerated using high salt (6 M guanidine HCl) washes. After several regeneration cycles, the binding capacity of the immobilized NbBBMV01 was not affected and it was still able to bind the antigen without a significant decrease in SPR signal.
Epitope mapping and binding affinity of anti-BBMV nanobodies. a Epitope mapping analysis by SPR sensorgram. (I) NbBBMV01 was first immobilized; (II) BBMV (10 µg/ml) in the mobile phase was captured by the first nanobody (dashed arrow ‘BBMV); (III) an excess of NbBBMV01 was injected over the captured virus (dashed arrow NbBBMV01) followed by sequential injections of other nanobodies (700 nM) (each represented by dashed arrow and labeled with the name of the Nb); (IV) a final injection of rabbit polyclonal anti-BBMV (dashed arrow R-a-BBMV, 1:200); regeneration with guanidinium HCl (last dashed arrow). SPR values were corrected for signal from control channel lacking the capturing nanobody. b Rate plane with iso-affinity diagonals (RaPID) plot of anti-BBMV nanobodies. The kinetic rate values kon and koff for all nanobodies as determined by biosensor measurements were plotted on a two-dimensional diagram so that nanobodies located on the same diagonal line have identical KD values
The SPR can also be used for the quantitative and kinetic analyses of nanobody/BBMV interaction without the need to label the reactants, which might interfere with binding. From a plot of the resonance signal versus time (sensorgram) and using increasing concentrations of the injected nanobodies (from 0 to 700 nM) (Fig. 3b), the association (k on) and dissociation (k off) rate constants for the interaction were determined and the affinity (KD) of the binding was then calculated for each of the anti-BBMV nanobodies. The binding kinetics yielded kon values between 105 and 106 M−1s−1 and koff values are in the range of 4 × 10−3–4 × 10−4 s−1. From these kinetic rate constants corresponding K D values ranging from 0.6nM to 30 nM were calculated.
NbBBMVs inhibit plant virus infectivity in vitro
The inhibition of viral infectivity by anti-BBMV nanobodies was investigated in vitro using wild type V. faba plants. BBMV was firstly pre-incubated with three different nanobodies, NbBBMV01, NbBBMV12 or NbBBMV22, separately and then used for plant inoculation. Plants challenged with the virus, pre-blocked with any of the three nanobodies, completely lacked symptoms of viral infections at 15 d.p.i. In contrast, plants inoculated with BBMV alone developed clear infection symptoms after 15 d.p.i.
Apart from (lack of) visible infection symptoms, infection status was also detected by a real-time PCR diagnostic assay of BBMV-cp RNA accumulation in systemic leaves (Fig. 4a). Inoculation with BBMV showed an increase in percentage of viral RNA content to 400 and 800 % at 5 and 15 d.p.i. respectively (Fig. 4a). Interestingly, untreated plants or leaves treated with the nanobody-neutralized virus, correlated also with the lack of cp-viral RNA accumulation.
In vitro inhibition of BBMV infection by nanobodies. a Leaves of V. faba plants were inoculated with BBMV virus only, BBMV virus mixed with NbBBMV01, −12 or −22, or mock inoculated. Systemic tissues were harvested at three different time points (white, dashed and black bars) and absolute quantification of BBMV-cp transcripts were analyzed by monitoring the Ct values. Time zero was considered 100 % of BBMV content and was used to normalize the presence of virus in plant tissue. TBIA to detect BBMV using rabbit anti-BBMV in healthy (−) and infected (+) plants (1), tissues infected with mixture of virus and NbBBMV01 (2), tissues detected with rabbit anti-AMV (3) and in absence of detecting rabbit antibody (4). Magnified image (×100) from infected tissue blot showed the stained vascular bundles (arrows), indicating the presence of virus (inset figure). b Sandwich ELISA measuring the viral content (mg/g) in the plant tissue extracts (10 %, w:v), taken (after 15 days) from the systemic leaves from the different treatments. In this technique, purified camel anti-BBMV IgGs (C-a-BBMV at 1:200) was used as a capturing antibody for the viruses from the plant extracts. Bound viruses were detected using rabbit (R-a-BBMV) antibody (1:5,000) followed with a HRP-conjugated goat anti-rabbit IgG antibody (G-a-R-HRP) (inset figure)
In addition, a tissue blotting immunoassay (TBIA) was performed to confirm the presence of BBMV particles in the stem imprints from infected plants using specific polyclonal antibody or the nanobody NbBBMV01. As expected, a magnified image from infected tissue blots showed the stained vascular bundles, indicating the presence of virus in the transmitter parts of the plant (Fig. 4a, inset).
Furthermore, BBMV was detected in the extracts from systemic leaves of treated plants at 15 d.p.i. by ELISA (Fig. 4b). The viral content was quantified in 1 g of the plant tissue by a sandwich ELISA method using two different species of anti-BBMV specific antibodies (Fig. 4b, inset drawing). As determined by comparisons to a standard curve of known concentrations of pure BBMV, infected plants accumulated about 20 mg of virus per g of wet plant tissue (Fig. 4). In contrast to those plants, plants challenged with nanobody-neutralized BBMV exhibited an immune phenotype and showed undetectable traces of virus, similarly to the untreated plant (Mock). Furthermore, following the same procedure, these nanobodies were unable to protect plants from an AMV virus infection (data not shown).
Transient expression of functional anti-BBMV nanobodies in planta
The NbBBMV01,−12 and −22 were transiently expressed in planta to assess whether these nanobodies have a relevant in vivo biological activity on the infectivity of BBMV. Wild type V. faba plant leaves were infiltrated with agrobacteria containing a vector expressing nanobody genes under the control of CaMV 35S promoter. A vector carrying P19 gene of Tomato bushy stunt virus was used to enhance the transient expression of the nanobodies by suppression of any putative gene silencing effect (Voinnet et al. 2003). Five days after infiltration, RT-PCR quantitative analysis indicated that transcripts of the three nanobodies appeared in almost the same amounts, and decreased 10 days after agroinfiltration (Fig. 5a). Furthermore, western blot analysis indicated that all three nanobody proteins were expressed and could be detected in the extracts from plant leaves, 5 days after agroinfiltration (Fig. 5b).
In planta nanobody transient expression inhibits BBMV propagation a V. faba plant tissues agroinfiltrated with P19, NbBBMV01, −12 or −22, or mock were harvested at 5 and 10 d.p.i. The relative nanobody gene expression was analyzed using the comparative Ct method. b Immunoblotting of protein extracts (25 µg/lane) from treated leaves and detection of nanobodies with rabbit anti-nanobody polyclonal antibody (1:3,000). c Reactivity of the nanobodies expressed in plants was tested via an indirect ELISA against immobilized BBMV (0.1 µg/well). Tested protein extracts (10 % w:v) included those from inoculated (IL) and systemic leaves (SL). Values at the top of columns refer to the estimated quantity (ng) of each nanobody (NbBBMV01, −12 and −22) in 1 g of plant tissue. d Dot blot of the immunoprecipitated BBMV from the protein extract (200 µl of 10 % w:v) of the inoculated leaves from the plants expressing nanobodies NbBBMV01, −12 and −22. Leaf extracts from untreated mock plant (negative control) and from P19-expressing plant inoculated with the virus (positive control) were used as well. The immunoprecipitation was performed using camel anti-BBMV antibody (subclass IgG1, 10 µg), and blots were revealed using either polyclonal anti-nanobody (R-a-Nb; 1:3,000) or anti-BBMV (R-a-BBMV; 1:5,000), followed with a HRP-conjugated goat anti-rabbit antibody
To determine whether the in planta-expressed nanobodies are functional and reactive to their antigen, protein extracts from agroinfiltrated leaves (IL) were tested in ELISA against the immobilized BBMV (Fig. 5c). In contrast to mock and P19-expressing plants, leave extracts from plants expressing NbBBMV01, −12 or −22 contained proteins that were reactive to the antigen and resulted in detectable signals. Obviously, all expressed nanobodies in these extracts were able to bind to different extents to their immobilized antigen. Interestingly, protein extracts of the systemic leaves (SL) from all these plants failed in such interaction, suggesting that nanobodies expression was a local phenomenon. Thus, in contrast to the virus, in planta expressed nanobodies are unable to migrate from the infiltrated leaves (Fig. 5c). Using standard curves for known concentrations of each of the three bacterial expressed nanobodies, it was possible to estimate the amount of the plant-expressed nanobodies at 72 ± 2, 15.5 ± 8, 99 ± 15 ng from 1 g of plant tissue for NbBBMV01, −12 and −22, respectively (Fig. 5c).
Moreover, to investigate whether in planta-expressed nanobodies complex the BBMV in vivo, we inoculated nanobody-agroinfiltrated leaves with BBMV, and 18 h later, possible virus/nanobody complexes were immunoprecipitated using polyclonal anti-BBMV antibodies. Subsequently, blots of the precipitated proteins were detected either by a rabbit anti-nanobody or anti-BBMV antibodies. Obviously, untreated plants (mock) did not show any presence of BBMV particles, and BBMV-inoculated plants revealed presence of significant amounts of BBMV (Fig. 5d). As expected, only blots of precipitated material from the nanobody-expressing plants, and not from mock or P19 plants, were detected by anti-nanobody polyclonal antibody, suggesting a direct in vivo interaction between the expressed nanobodies and the precipitated virus (Fig. 5d).
Agroinfiltrated leaves expressing NbBBMVs prevent viral infection
Additional experiments were performed to determine whether in planta transient expression of nanobodies immuno-protects plants against BBMV infection. For these BBMV resistance tests in the greenhouse, thirteen individual plants were selected to express each of the anti-BBMV nanobodies, separately. At 15 d.p.i., wild-type plants or P19-expressing plants inoculated with BBMV developed viral symptoms on the upper parts of the plant. In contrast, agroinfiltrated leaves expressing NbBBMV01, −12 or −22 completely lacked symptoms of viral infection at 15 d.p.i. (Fig. 6a). Furthermore, TBIA on the stem imprints from all treated plants proved the blocking of virus movement in nanobody-expressing plants (Fig. 6b). This virus blocking may explain the absence of virus-infection symptoms in other parts of the treated plants (i.e. the systemic leaves) (Fig. 6a).
Phenotype of local resistance to viral infection conferred by nanobodies transient expression. V. faba plants were lifted without treatment (mock), or inoculated with BBMV directly or five days after being infiltrated with agrobacteria with P19 or nanobody genes (NbBBMV01, −12 and −22). a Apical leaves of wild-type plants or those expressing P19 showed typical symptoms of Bromovirus infection. b TBIA for the detection of BBMV in control and treated plants using rabbit anti-BBMV (1), NbBBMV01 (2) and no antibody (3). c Systemic tissues from the different conditions were harvested and absolute quantification of BBMV-cp gene in the mRNA was evaluated from their Ct values at two different time points (7 and 21 days). Time zero was considered to possess 100 % of BBMV content, used to normalize the virus presence in plant tissue. Inset panel is from sandwich ELISA measuring the viral content (mg/g) in the systemic leaves (at day 21), of the plants under the different experimental conditions
Detection of viral infection, based on an absolute quantification of BBMV-cp transcripts in systemic parts by RT-PCR, was performed on the treated plants 7 and 21 d.p.i (Fig. 6c). The percentage of viral content was estimated in comparison with the standard amount of viral RNA. Control and P19-expressing plants showed a normal multiplication of BBMV virus to reach 600 and 500 % at 7 d.p.i. and 1,200 and 1,000 % at 21 d.p.i. respectively (Fig. 6c). Sandwich ELISA on viral particles accumulating in plant tissue of systemic parts confirmed the real-time quantification (Fig. 6c, inset diagram). Clearly, the lack of visible symptoms on both inoculated and systemic leaves correlated with the lack of BBMV particles in ELISA at 7 d.p.i. Furthermore, additional challenging experiments using different, but closely related plant viruses such as AMV, BBTMV or BYMV revealed that the resistance was restricted to BBMV only. Hence, these nanobodies create a target-specific virus protection and fail to create a broad virus resistance (data not shown).
Discussion
Integrated pest management strategies to control viral infections of plants put generally the focus on either a reduction of virus-transmitter vectors (e.g. insects, nematodes) or on breeding virus-resistant plants. In the last two decades, alternative promising strategies were proposed to overcome plant infection by viruses, such as expression of antibodies, or its antigen binding fragments (e.g. scFv), directed against viral proteins (Tavladoraki et al. 1993; Fecker et al. 1997; Boonrod et al. 2004; Villani et al. 2005; Gargouri-Bouzid et al. 2006; Prins et al. 2005). These reports showed that the expression of recombinant antibody targeting non-structural viral proteins such as protease (Gargouri-Bouzid et al. 2006; Bouaziz et al. 2009) or RdRp (Boonrod et al. 2004) might provide plant resistance to the cognate virus to a similar level as plants expressing an ectopic antibody targeting the viral structural coat protein (Tavladoraki et al. 1993).
After the initial successes of these proof-of-concepts whereby a transgenic plant expressing a scFv showed virus resistance, surprisingly the number of similar reports increased only slightly (Prins et al. 2008). Possibly, the impact of virus-specific recombinant antibodies is too much depended on: (1) the quantity of functional antibodies (yield and stability), (2) the localization (intracellular targeting) and (3) the binding efficiency (affinity, avidity and targeted epitope) of these antibodies (Safarnejad et al. 2011). We hypothesized that scFv expression in plants might be a limiting factor and that single domain antibodies or nanobodies might be a better option. Although the production of significant amounts of nanobodies in seeds has recently been indicated (Tokuhara et al. 2013; De Buck et al. 2012), the ectopic expression of nanobodies in planta for virus attenuation is lacking (De Meyer et al. 2014b). Note that nanobodies (from alpaca) against Tulip virus X were only developed for diagnostic purposes (Beekwilder et al. 2008). To demonstrate that virus-specific nanobodies, derived from HCAbs elicited in dromedaries after immunization, may provide a virus-resistant phenotype when expressed in planta, we chose the BBMV as a model because of its important impact on legume crops.
The simple, robust and homogenous structure of most plant and animal viruses are ideal to raise antibodies (Ziegler and Torrance 2002). The BBMV proteinaceous coat represents an interesting antigenic structure because of its icosahedral symmetric shape composed of 180 of similar copies of a single coat protein (Speir et al. 1995; Dzianott and Bujarski 1991; White and Fischbach 1973). Evidently, the immunized camel elicited an important immune response against BBMV, in which HCAbs sub-isotypes targeting the antigen by virtue of one single domain were certainly implicated. A nanobody ‘immune’ library constructed from these HCAbs allows the retrieval after phage display pannings of antigen-specific, monoclonal nanobodies, affinity-matured in vivo in the dromedary. This strategy generates routinely nanobodies of (sub) nanomolar affinity (Lauwereys et al. 1998; Conrath et al. 2001; Saerens et al. 2004). In contrast, with scFvs this is much more difficult to achieve since after the immunization step, the VH and VL gene pools are amplified separately and subsequently reassembled in scFv constructs so that the original VH–VL pair as it was affinity-matured is lost. This VH–VL shuffling might lead to suboptimal affinity and stability of the scFv (Harmsen and De Haard 2007). Nevertheless, several reports are available on the identification of scFv against Cucumber mosaic virus (CMV), a virus of the Bromoviridea family, like BBMV (Fukuzawa et al. 2011; Di Carli et al. 2010; Villani et al. 2005; Chae et al. 2001; Ziegler et al. 1995). These scFv were retrieved from a synthetic library (Ziegler et al. 1995) or from amurine ‘immune’ scFv library (Chae et al. 2001).
The phage display and biopanning of our ‘immune’ nanobody library lead to the identification of multiple BBMV-specific nanobodies. Remarkably, (1) all these nanobodies recognize an overlapping epitope on the virus; and (2) only one nanobody (NbBBMV12) originated from a recombination involving a VHH germline gene, whereas all other nanobodies resulted from a recombination of a VH germline gene with the D-Jh genes. At first sight this mono-specific epitope recognition might be surprising, however, it is well established that single-domain antibodies (VHH and shark V-NARs as well) prefer to interact with concave surfaces such as the catalytic cleft of enzymes (Lauwereys et al., 1998; De Genst et al. 2006). So, here as well, it might be that the BBMV coat contains one single (repeated) immunodominant region on its surface. The second surprising observation, the predominance of the VH hallmark imprint in the BBVM-specific nanobodies, has also been observed for a few other antigens (Monegal et al. 2012; Kastelic et al. 2009). However, although the VHH framework-2 hallmarks are absent in most of our nanobodies, it is clear that these nanobodies are derived from HCAbs, since the conserved Tryptophan at position 118, critical for VL association has been substituted by Arginine. The emergence of such ‘VH-like’ nanobodies in the B cell receptor repertoire of camelids was explained previously (Muyldermans et al. 2009; Muyldermans 2013).
It was shown before that targeting a structural component of the virus-like coat protein with scFv might dramatically affect the infectivity of some plant viruses such as Tomato spotted wilt virus (TSWV) (Prins et al. 2005). In our work, it was demonstrated as well that the in vitro pre-incubation of nanobodies and their viral target inhibited the BBMV infectivity and arrested its spreading in plants. Similar virus attenuation by nanobodies has been observed previously, for example with phage TP901-1 infecting Lactococcus lactis (Desmyter et al. 2013). However to the best of our knowledge, this is the first report describing the nanobody-induced modulation of viral infection in plants.
To support the in vitro virus attenuation by nanobodies in plants we assessed a transient expression in plant leaves after agroinfiltration of nanobody expression clones and its capacity to reduce in vivo virus infectivity. With this accelerated approach we demonstrated the production of functional nanobodies, assisted by suppression of the transgene silencing effect (Voinnet et al. 2003), within a few days. The in vivo transient expression of NbBBMV01, NbBBMV12 or NbBBMV22 in V. faba seems to protect the modified leaves very effectively against virus infection probably by a direct contact between BBMV and the cognate nanobody as suggested by the immunoprecipitation experiment (Fig. 5d). This trans-nanobody binding capacity could, create a barrier against the virus proliferation in plants, similar to some trans-scFv mode-of-action (Prins et al. 2008). The monoclonal nanobodies expressed in plants retain their antigen-specificity as the plant immunity was restricted to BBMV and could not be broadened to AMV, BBTMV and BYMV. Evidently, stable transgenic plants should be generated to determine the stability of the recombinant nanobodies and the quantity of the (functional) nanobody, required to protect the plant against BBMV.
References
Abbady AQ, Al-Mariri A, Zarkawi M, Al-Assad A, Muyldermans S (2011) Evaluation of a nanobody phage display library constructed from a Brucella-immunised camel. Vet Immunol Immunopathol 142(1–2):49–56
Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen NB, Hamid M (2012) scFv Antibody: principles and Clinical Application. Clin Dev Immunol 2012:980250
Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S (1997) Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett 414(3):521–526
Bawden FC, Chaudhuri RP, Kassanis B (2008) Some properties of broad-bean mottle virus. Ann Appl Biol 38(4):774–784
Beekwilder J, Houwelingen A, Beckhoven J, Speksnijder A (2008) Stable recombinant alpaca antibodies for detection of Tulip virus X. Eur J Plant Pathol 121:477–485
Boonrod K, Galetzka D, Nagy PD, Conrad U, Krczal G (2004) Single-chain antibodies against a plant viral RNA-dependent RNA polymerase confer virus resistance. Nat Biotechnol 22(7):856–862
Bouaziz D, Ayadi M, Bidani A, Rouis S, Nouri-Ellouz O, Jellouli R, Drira N, Gargouri-Bouzid R (2009) A stable cytosolic expression of VH antibody fragment directed against PVY NIa protein in transgenic potato plant confers partial protection against the virus. Plant Sci 176(4):489–496
Bujarski JJ (1998) Bromovirus isolation and RNA extraction. Methods Mol Biol 81:183–188
Chae JS, Choi JK, Lim HT, Cha SH (2001) Generation of a murine single chain Fv (scFv) antibody specific for cucumber mosaic virus (CMV) using a phage display library. Mol Cells 11(1):7–12
Conrath KE, Lauwereys M, Galleni M, Matagne A, Frere JM, Kinne J, Wyns L, Muyldermans S (2001) Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in the camelidae. Antimicrob Agents Chemother 45(10):2807–2812
De Buck S, Virdi V, De Meyer T, De Wilde K, Piron R, Nolf J, Van Lerberge E, De Paepe A, Depicker A (2012) Production of camel-like antibodies in plants. Methods Mol Biol 911:305–324
De Buck S, Nolf J, De Meyer T, Virdi V, De Wilde K, Van Lerberge E, Van Droogenbroeck B, Depicker A (2013) Fusion of an Fc chain to a VHH boosts the accumulation levels in Arabidopsis seeds. Plant Biotechnol J 11(8):1006–1016
De Genst E, Silence K, Decanniere K, Conrath K, Loris R, Kinne J, Muyldermans S, Wyns L (2006) Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc Natl Acad Sci U S A 103(12):4586–4591
De Jaeger G, De Wilde C, Eeckhout D, Fiers E, Depicker A (2000) The plantibody approach: expression of antibody genes in plants to modulate plant metabolism or to obtain pathogen resistance. Plant Mol Biol 43(4):419–428
De Meyer T, Eeckhout D, De Rycke R, De Buck S, Muyldermans S, Depicker A (2014a) Generation of VHH antibodies against the Arabidopsis thaliana seed storage proteins. Plant Mol Biol 84(1–2):83–93
De Meyer T, Muyldermans S, Depicker A (2014b) Nanobody-based products as research and diagnostic tools. Trends Biotechnol 32(5):263–270
Desmyter A, Farenc C, Mahony J, Spinelli S, Bebeacua C, Blangy S, Veesler D, van Sinderen D, Cambillau C (2013) Viral infection modulation and neutralization by camelid nanobodies. Proc Natl Acad Sci U S A 110(15):E1371–E1379
Di Carli M, Villani ME, Bianco L, Lombardi R, Perrotta G, Benvenuto E, Donini M (2010) Proteomic analysis of the plant-virus interaction in cucumber mosaic virus (CMV) resistant transgenic tomato. J Proteome Res 9(11):5684–5697
Dzianott AM, Bujarski JJ (1991) The nucleotide sequence and genome organization of the RNA-1 segment in two bromoviruses: broad bean mottle virus and cowpea chlorotic mottle virus. Virology 185(2):553–562
Fecker LF, Koenig R, Obermeier C (1997) Nicotiana benthamiana plants expressing beet necrotic yellow vein virus (BNYVV) coat protein-specific scFv are partially protected against the establishment of the virus in the early stages of infection and its pathogenic effects in the late stages of infection. Arch Virol 142(9):1857–1863
Fortass M, Bos L (1992) Broad bean mottle virus in Morocco; variability in interaction with food legume species and seed transmission in faba bean, pea and chickpea. Neth J Plant Pathol 98:329–342
Fukuzawa N, Ishihara T, Itchoda N, Tabayashi N, Kataoka C, Masuta C, Matsumura T (2011) Risk-managed production of bioactive recombinant proteins using a novel plant virus vector with a helper plant to complement viral systemic movement. Plant Biotechnol J 9(1):38–49
Gargouri-Bouzid R, Jaoua L, Rouis S, Saidi MN, Bouaziz D, Ellouz R (2006) PVY-resistant transgenic potato plants expressing an anti-NIa protein scFv antibody. Mol Biotechnol 33(2):133–140
Gibbs WW (2005) Nanobodies. Sci Am 293(2):78–83
Harmsen MM, De Haard HJ (2007) Properties, production, and applications of camelid single-domain antibody fragments. Appl Microbiol Biotechnol 77(1):13–22
Harper K, Kerschbaumer RJ, Ziegler A, Macintosh SM, Cowan GH, Himmler G, Mayo MA, Torrance L (1997) A scFv-alkaline phosphatase fusion protein which detects potato leafroll luteovirus in plant extracts by ELISA. J Virol Methods 63(1–2):237–242
Hassanzadeh-Ghassabeh G, Devoogdt N, De Pauw P, Vincke C, Muyldermans S (2013) Nanobodies and their potential applications. Nanomedicine (Lond) 8(6):1013–1026
Holliger P, Hudson PJ (2005) Engineered antibody fragments and the rise of single domains. Nat Biotechnol 23(9):1126–1136
Ismaili A, Jalali-Javaran M, Rasaee MJ, Rahbarizadeh F, Forouzandeh-Moghadam M, Memari HR (2007) Production and characterization of anti-(mucin MUC1) single-domain antibody in tobacco (Nicotiana tabacum cultivar Xanthi). Biotechnol Appl Biochem 47(Pt 1):11–19
Jobling SA, Jarman C, Teh MM, Holmberg N, Blake C, Verhoeyen ME (2003) Immunomodulation of enzyme function in plants by single-domain antibody fragments. Nat Biotechnol 21(1):77–80
Kastelic D, Frkovic-Grazio S, Baty D, Truan G, Komel R, Pompon D (2009) A single-step procedure of recombinant library construction for the selection of efficiently produced llama VH binders directed against cancer markers. J Immunol Methods 350(1–2):54–62
Ladner RC (2007) Mapping the epitopes of antibodies. Biotechnol Genet Eng Rev 24:1–30
Lauwereys M, Arbabi Ghahroudi M, Desmyter A, Kinne J, Holzer W, De Genst E, Wyns L, Muyldermans S (1998) Potent enzyme inhibitors derived from dromedary heavy-chain antibodies. EMBO J 17(13):3512–3520
Lefranc MP, Pommie C, Ruiz M, Giudicelli V, Foulquier E, Truong L, Thouvenin-Contet V, Lefranc G (2003) IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev Comp Immunol 27(1):55–77
Lin NS, Hsu YH, Hsu HT (1990) Immunological detection of plant viruses and a mycoplasma-like organism by direct tissue blotting on nitrocellulose membranes. Phytopathology 80:824–828
Makkouk KM, Bos L, Rizkallah A, Azzam OI, Katul L (1988) Broad bean mottle virus: identification, host range, serology, and occurrence on faba bean (Vicia faba) in West Asia and North Africa. Neth J Plant Pathol 94(4):195–212
Makkouk K, Pappu H, Kumari SG (2012) Virus diseases of peas, beans, and faba bean in the Mediterranean region. Adv Virus Res 84:367–402
Marasco WA (1995) Intracellular antibodies (intrabodies) as research reagents and therapeutic molecules for gene therapy. Immunotechnology 1(1):1–19
Monegal A, Olichon A, Bery N, Filleron T, Favre G, de Marco A (2012) Single domain antibodies with VH hallmarks are positively selected during panning of llama (Lama glama) naive libraries. Dev Comp Immunol 36(1):150–156
Muyldermans S (2013) Nanobodies: natural single-domain antibodies. Annu Rev Biochem 82:775–797
Muyldermans S, Baral TN, Retamozzo VC, De Baetselier P, De Genst E, Kinne J, Leonhardt H, Magez S, Nguyen VK, Revets H, Rothbauer U, Stijlemans B, Tillib S, Wernery U, Wyns L, Hassanzadeh-Ghassabeh G, Saerens D (2009) Camelid immunoglobulins and nanobody technology. Vet Immunol Immunopathol 128(1–3):178–183
Nguyen VK, Hamers R, Wyns L, Muyldermans S (2000) Camel heavy-chain antibodies: diverse germline V(H)H and specific mechanisms enlarge the antigen-binding repertoire. EMBO J 19(5):921–930
Nguyen VK, Desmyter A, Muyldermans S (2001) Functional heavy-chain antibodies in Camelidae. Adv Immunol 79:261–296
Nickel H, Kawchuk L, Twyman RM, Zimmermann S, Junghans H, Winter S, Fischer R, Prufer D (2008) Plantibody-mediated inhibition of the potato leafroll virus P1 protein reduces virus accumulation. Virus Res 136(1–2):140–145
Nolke G, Cobanov P, Uhde-Holzem K, Reustle G, Fischer R, Schillberg S (2009) Grapevine fanleaf virus (GFLV)-specific antibodies confer GFLV and Arabis mosaic virus (ArMV) resistance in Nicotiana benthamiana. Mol Plant Pathol 10(1):41–49
Orecchia M, Nolke G, Saldarelli P, Dell’Orco M, Uhde-Holzem K, Sack M, Martelli G, Fischer R, Schillberg S (2008) Generation and characterization of a recombinant antibody fragment that binds to the coat protein of grapevine leafroll-associated virus 3. Arch Virol 153(6):1075–1084
Prins M, Lohuis D, Schots A, Goldbach R (2005) Phage display-selected single-chain antibodies confer high levels of resistance against tomato spotted wilt virus. J Gen Virol 86(Pt 7):2107–2113
Prins M, Laimer M, Noris E, Schubert J, Wassenegger M, Tepfer M (2008) Strategies for antiviral resistance in transgenic plants. Mol Plant Pathol 9(1):73–83
Saerens D, Kinne J, Bosmans E, Wernery U, Muyldermans S, Conrath K (2004) Single domain antibodies derived from dromedary lymph node and peripheral blood lymphocytes sensing conformational variants of prostate-specific antigen. J Biol Chem 279(50):51965–51972
Saerens D, Conrath K, Govaert J, Muyldermans S (2008) Disulfide bond introduction for general stabilization of immunoglobulin heavy-chain variable domains. J Mol Biol 377(2):478–488
Safarnejad MR, Jouzani GS, Tabatabaei M, Twyman RM, Schillberg S (2011) Antibody-mediated resistance against plant pathogens. Biotechnol Adv 29(6):961–971
Saldarelli P, Keller H, Dell’Orco M, Schots A, Elicio V, Minafra A (2005) Isolation of recombinant antibodies (scFvs) to grapevine virus B. J Virol Methods 124(1–2):191–195
Soosaar JL, Burch-Smith TM, Dinesh-Kumar SP (2005) Mechanisms of plant resistance to viruses. Nat Rev Microbiol 3(10):789–798
Speir JA, Munshi S, Wang G, Baker TS, Johnson JE (1995) Structures of the native and swollen forms of cowpea chlorotic mottle virus determined by X-ray crystallography and cryo-electron microscopy. Structure 3(1):63–78
Susi P, Ziegler A, Torrance L (1998) Selection of single-chain variable fragment antibodies to black currant reversion associated virus from a synthetic phage display library. Phytopathology 88(3):230–233
Tavladoraki P, Benvenuto E, Trinca S, De Martinis D, Cattaneo A, Galeffi P (1993) Transgenic plants expressing a functional single-chain Fv antibody are specifically protected from virus attack. Nature 366(6454):469–472
Teh YH, Kavanagh TA (2010) High-level expression of Camelid nanobodies in Nicotiana benthamiana. Transgenic Res 19(4):575–586
Terrada E, Kerschbaumer RJ, Giunta G, Galeffi P, Himmler G, Cambra M (2000) Fully “recombinant enzyme-linked immunosorbent assays” Using genetically engineered single-chain antibody fusion proteins for detection of citrus tristeza virus. Phytopathology 90(12):1337–1344
Tokuhara D, Alvarez B, Mejima M, Hiroiwa T, Takahashi Y, Kurokawa S, Kuroda M, Oyama M, Kozuka-Hata H, Nochi T, Sagara H, Aladin F, Marcotte H, Frenken LG, Iturriza-Gomara M, Kiyono H, Hammarstrom L, Yuki Y (2013) Rice-based oral antibody fragment prophylaxis and therapy against rotavirus infection. J Clin Invest 123(9):3829–3838
Toth RL, Harper K, Mayo MA, Torrance L (1999) Fusion proteins of single-chain variable fragments derived from phage display libraries are effective reagents for routine diagnosis of potato leafroll virus infection in potato. Phytopathology 89(11):1015–1021
Vanlandschoot P, Stortelers C, Beirnaert E, Ibanez LI, Schepens B, Depla E, Saelens X (2011) Nanobodies(R): new ammunition to battle viruses. Antivir Res 92(3):389–407
Villani ME, Roggero P, Bitti O, Benvenuto E, Franconi R (2005) Immunomodulation of cucumber mosaic virus infection by intrabodies selected in vitro from a stable single-framework phage display library. Plant Mol Biol 58(3):305–316
Voinnet O, Rivas S, Mestre P, Baulcombe D (2003) An enhanced transient expression system in plants based on suppression of gene silencing by the p19 protein of tomato bushy stunt virus. Plant J 33(5):949–956
Wesolowski J, Alzogaray V, Reyelt J, Unger M, Juarez K, Urrutia M, Cauerhff A, Danquah W, Rissiek B, Scheuplein F, Schwarz N, Adriouch S, Boyer O, Seman M, Licea A, Serreze DV, Goldbaum FA, Haag F, Koch-Nolte F (2009) Single domain antibodies: promising experimental and therapeutic tools in infection and immunity. Med Microbiol Immunol 198(3):157–174
White RA, Fischbach FA (1973) An x-ray scattering investigation of broad bean mottle virus in solutions of various electron densities. J Mol Biol 75(3):549–558
Winichayakul S, Pernthaner A, Scott R, Vlaming R, Roberts N (2009) Head-to-tail fusions of camelid antibodies can be expressed in planta and bind in rumen fluid. Biotechnol Appl Biochem 53(Pt 2):111–122
Zakri AM, Ziegler A, Torrance L, Fischer R, Commandeur U (2010) Generation and characterization of a scFv against recombinant coat protein of the geminivirus tomato leaf curl New Delhi virus. Arch Virol 155(3):335–342
Ziegler A, Torrance L (2002) Applications of recombinant antibodies in plant pathology. Mol Plant Pathol 3(5):401–407
Ziegler A, Torrance L, Macintosh SM, Cowan GH, Mayo MA (1995) Cucumber mosaic cucumovirus antibodies from a synthetic phage display library. Virology 214(1):235–238
Ziegler A, Macintosh SM, Torrance L, Simon W, Slabas AR (1997) Recombinant antibody fragments that detect enoyl acyl carrier protein reductase in Brassica napus. Lipids 32(8):805–809
Acknowledgments
The authors would like to thank the Director General of the Atomic Energy Commission of Syria and the head of the Molecular Biology and Biotechnology department for their continuous support throughout this work. We thank Mr. Adnan Al-Assad at General Commission for Scientific Agricultural Research (GCSAR), Deir El-Hagar Station for Camelid Research (Damascus, Syria), for housing and immunizing camels. We also thank the lab. of Patrice Dunoyer, IBMP/CNRS institute, Strasbourg, FRANCE for kindly providing pBI61-P19 construct and the lab of Sunil K. Mukherjee, ICGEB, New-Delhi, INDIA for kindly providing empty pBI121 vector.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ghannam, A., Kumari, S., Muyldermans, S. et al. Camelid nanobodies with high affinity for broad bean mottle virus: a possible promising tool to immunomodulate plant resistance against viruses. Plant Mol Biol 87, 355–369 (2015). https://doi.org/10.1007/s11103-015-0282-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-015-0282-5