Abstract
For detection of the plant pathogenic Tulip virus X (TuVX), a panel of six recombinant antibodies was identified. To this end, a repertoire of variable domains from heavy-chain immunoglobulins (VHH) was cloned from an alpaca, which had been immunized with TuVX. Binding domains were selected by phage display and panning on immobilized TuVX particles. Recombinant VHH antibodies were tested for sensitivity in a sandwich ELISA, and were demonstrated to be readily able to distinguish TuVX-infected tulip leaf material from uninfected leafs. No cross-reactivity of the VHH antibodies to related flexiviridae was observed. Recombinant VHHs maintained their reactivity upon storage at −20°C for over a year. The effect of incubation at higher temperatures for prolonged time was studied. Two out of three VHH proteins retained activity after several weeks of storage at 37°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibodies have a long history of application in the detection of plant pathogens. They are key elements in routine immunological tests, due to their low price, high specificity and reliable and fast performance. For this reason, immunoassays are essential to test the phytosanitary status of large volumes of plant material. For example, for the trade of tulip material, 1.7 million extractions for virus analysis are performed in The Netherlands annually.
Immunological tests for detection of plant pathogenic viruses usually involve the use of antiserum, raised in rabbit, chicken or mouse. Since the characteristics of these antisera differ between individual animals, their application in certified tests requires exhaustive tests for every new batch produced. For this reason, monoclonal antibodies obtained by hybridoma technology, or recombinant antibodies expressed in micro-organisms have been considered as an alternative. Over the past 15 years, selection of recombinant monoclonal antibodies by phage display has been developed (Hoogenboom 2005). This involves construction of libraries of antibody fragments, expressed on the surface of bacteriophages, and selection of desired antibody fragments from the library. In most cases, the fragments consist of single-chain variable fragments (scFvs), which are fusions of the variable domains of antibody heavy and light chains through a flexible synthetic linker. Recombinant scFvs have mainly been applied in the medical research field, but have also been described for a number of plant viruses (reviewed in Ziegler and Torrance 2002) and a few other plant pathogens (Griep et al. 1998). However, a large scale transition of plant pathogen detection from classical antisera to recombinant antibodies (such as scFvs) has not been made.
Solubility and stability are an important issue for recombinant antibodies. Though a number of studies report on high accumulation of soluble recombinant antibody fragments like scFv, others describe poor solubility and a propensity to irreversibly aggregate (Moss et al. 2003; Hamilton et al. 2003; Holliger and Hudson 2005). In recent years, antibody sources have been explored that give rise to soluble recombinant antibodies with a higher frequency. Among those are heavy-chain IgG antibodies from camelids, such as camels, dromedaries and llamas (Ghahroudi et al. 1997). Remarkably, camelid heavy-chain IgGs do not involve a light-chain, and lack the first constant domain of the heavy chain, which is replaced by an exceptionally long hinge region (Hamers-Casterman et al. 1993). The variable part of these antibodies (referred to as the VHH domain) is a single immunoglobulin-folded protein domain of about 140 amino acids, which does not involve artificial linkers. Generally, VHH domains can be produced in large quantities in microbial recombinant systems as stable, water-soluble molecules (Ghahroudi et al. 1997; Muyldermans 2001).
In this study, Tulip virus X (TuVX) was applied as an example of a plant pathogen for development of novel immunological detection tools. TuVX is among the more abundant pathogens for tulip. It is a filamentous, positive-stranded RNA virus, belonging to the family of flexiviridae and the genus of potex viruses. Its natural hosts are Tulipa species. Symptoms of infection comprise chlorotic or necrotic grey–brown streaking of leaves and streaks of intensified pigment (or of necrosis) in petals (ICTVdB-Management 2006). The amount of TuVX tests (currently 7,000 per year in The Netherlands) is expected to increase, firstly because infection incidence seems to be increasing, and secondly, because an increasing number of countries impose a quarantine status on this virus.
TuVX is considered by several governments as a pathogen of potential quarantine concern on flower bulb imports. Therefore we set out to isolate recombinant antibodies for detection of TuVX from an immunized alpaca for standardized application.
Materials and methods
Library construction and panning
A VHH library was created from lymphocyte RNA from alpaca which had been immunized with TuVX particles. A 3-year old female alpaca (ID-Lelystad, The Netherlands) was immunized with 100 μg of purified virus material (supplied by PPO Lisse, The Netherlands, purified from tulip material), suspended in Stimune (Cedi diagnostics, Lelystad), according to institutional guidelines. After 4 and 8 weeks, the immunization was repeated. After 5 and 9 weeks, 150 ml blood was drawn from the neck vein and immediately mixed with heparin. Peripheral lymphocytes were purified using a Ficoll gradient, and total RNA was purified using the RNAeasy kit (Qiagen, The Netherlands). For each RNA sample, cDNA was generated using random hexamers. VHH fragments were amplified from first-strand cDNA with VHH specific primers (De Haard et al. 2002). Amplified fragments were pooled and ligated into the pCANTAB-5E vector (Pharmacia) in frame with the M13 gene 3 for expression of VHH-g3p fusion protein. Recombinant plasmids were introduced into competent Escherichia coli TG1 cells by electroporation, and about 107 individual recombinants were obtained and pooled.
Phage particles were obtained by inoculating 109 library cells into 25 ml 2xYT medium with 100 μg ml−1 ampicillin and 1% glucose growing at 37°C and 250 rpm. After the culture had reached a density of ±2.4 × 108 cells ml−1, it was infected with helper phage VCSM13 for 1 h and then centrifuged. The bacterial pellet was resuspended in YT medium with 100 μg ml−1 ampicillin and 50 μg ml−1 kanamycin for the production of phages, and further incubated overnight at 30°C and 250 rpm. Particles were harvested by PEG-precipitation.
The phage displayed VHH antibodies were panned against four different concentrations (0.4, 2, 10, and 25 μg ml−1) of TuVX particles coated in ELISA plates. Phages binding to the TuVX coating were eluted with trypsin and used to infect four batches of 4.8 × 108 E. coli TG1 cells. The four batches of cells were plated separately on solid LB medium with 1% glucose and 100 μg ml−1 ampicillin and the obtained colonies were pooled. To further enrich for TuVX-positive antibodies the panning procedure was repeated twice.
Recloning of selected VHH fragments for expression
After enrichment by three rounds of panning against TuVX, pooled phages were used to infect E. coli TG1. Phagemid DNA was isolated from 4 × 107 E. coli cells. The VHH inserts were amplified by PCR to add the appropriate restriction sites, digested with NdeI and SfiI at the primer ends and ligated into the CM194 expression vector (CatchMabs, The Netherlands), a derivative of pET12a (Novagen). Expression of the VHH recombinant antibody is under control of the T7 promoter. The C-terminus of the VHH fragment is fused to a VSV-G tag (YTDIEMNRLGK) for detection purposes, followed by a C-terminal 6× His tag for purification.
For expression, plasmids were introduced into E. coli BL21-AI. This strain carries a chromosomal insertion of the gene encoding T7 RNA polymerase in the araB locus of the araBAD operon, allowing expression of the recombinant VHH antibody in the presence of l-arabinose. Inserts of 28 randomly picked colonies were amplified and subjected to sequence analysis.
Expression and purification of recombinant VHH antibodies
For expression, 200 ml of 2xYT medium containing 100 μg ml−1 of ampicillin was inoculated with an overnight culture of BL21-AI carrying the VHH expression plasmids. Cultures were grown to an OD600 of 0.6–0.8 at 37°C at 250 rpm. Subsequently expression of the antibodies was induced by adding 0.02% l-arabinose, and continued by incubation of the culture for 4 h at 37°C at 250 rpm. Bacteria were harvested by centrifugation and the pellet was resuspended in 20 ml of 9M urea in PBS and incubated for 30 min at room temperature to allow cell lysis and solubilization of intracellular inclusion bodies. The cell lysate was centrifuged at 15,500 RCF for 2 h at 4°C. The clarified supernatant was diluted with 180 ml 50 mM Tris–HCl pH 9 with 150 mM NaCl (TN buffer) and incubated for 1 h to allow refolding of the antibodies. The material was subsequently centrifuged at 15,500 RCF for 2 h at 4°C and the supernatant was used to purify the recombinant 6× His tagged VHH antibodies with Ni-NTA metal-affinity chromatography.
For purification, the clarified cell lysate including the refolded VHH antibodies was brought to a final concentration of 10 mM imidazole and incubated for 1 h with 2.5 ml of Ni-NTA resin (Qiagen) pre-equilibrated with TN buffer. The resin mixture was loaded into a column, drained and subsequently washed with 50 ml of TN buffer containing 20 mM imidazole and finally proteins were eluted with 25 ml of TN buffer containing 250 mM imidazole.
The eluate was dialyzed against 5 l of TN buffer in three steps, to remove imidazole, and stored in 2 ml batches at −20°C.
Antigen-coated plate ELISA
Wells of a 96-well microtitre plate (Greiner, The Netherlands) were coated for 2 h at 27°C with 100 μl of TuVX, helper phage VCS-M13, Potato virus S (PVS; isolate IJsselster), Potato virus X (PVX; isolate PVX3), Pepino mosaic virus (PepMV; isolate P1), Kalanchoe mosaic virus (KMV; isolate Kal6), or Chrysanthemum virus B (CVB; isolate RR). TuVX was diluted to 2 μg ml−1 in sodium carbonate buffer (0.1 M, pH 9.6). All other virus stocks were of unknown concentration as they were isolated with a background of nucleic acids from plants. The extracts were known to be suitable for immunological detection proved by regular testing with conventional polyclonal antiserum (Prime Diagnostics). The virus stocks were diluted five-fold in 0.1 M sodium carbonate buffer pH 9.6. After virus coating, wells were washed once with 200 μl of TN-buffer and blocked with 150 μl of TNE buffer (2% non-fat dried milk powder in TN buffer) for 1 h at 27°C. Further steps were carried out at room temperature. Wells were emptied and incubated for 1 h with 100 μl of recombinant VHH antibody (5 μg ml−1 in TNE buffer). After washing three times with 200 μl of TNT buffer (TN buffer containing 0.05% Tween-20), wells were incubated for 60 min with 100 μl of monoclonal Anti-VSV-G peroxidase conjugate (Sigma) diluted 1:2,000 in TNE buffer. Wells were emptied and washed three times with 200 μl of TNT buffer followed by washing three times with 200 μl of TN buffer. 100 μl of TMB substrate solution (Pierce) was added per well and incubated for 30 min maximum in the dark. To stop the reaction, 50 μl of 2 N sulphuric acid was added to each well, after which the absorbance was measured at 415 nm in a microplate reader (EL808, Bio-Tek Instruments Inc., The Netherlands). The data were analyzed using the software linked to the microplate reader (KC4, KinetiCalc version 3.3 ref 10 for Windows XP, Bio-Tek Instruments Inc. Titertek, The Netherlands).
DAS-ELISA
Wells of a 96-well microtitre plate (Greiner) were coated overnight at 4°C with 100 μl of polyclonal rabbit anti TuVX gamma-immunoglobulin (serum obtained from PPO, Lisse) (2 μg ml−1 in sodium carbonate buffer, pH 9.6). Subsequent steps were carried out at room temperature. Wells were washed once with 200 μl TN buffer and blocked with 150 μl TNE buffer for 90 min. Wells were emptied, 100 μl either healthy, naturally infected or spiked leaf extract diluted 1:10 and 1:100 in 2% TNE buffer was added. The spiked leaf extract contained a dilution series of TuVX (16, 8, 4, 2, 1, 0.5, 0.25 or 0 μg ml−1). The plate was incubated for 60 min at room temperature, after which the wells were emptied, washed three times with 200 μl TNT buffer and incubated for 1 h with 100 μl diluted VHH 5 μg ml−1 in TNE buffer. Detection proceeded in the same way as described above for the direct ELISA procedure.
For storage experiments the VHH antibody stocks were divided into four portions, and stored at either −20, 4, 21 or 37°C. Sodium azide (0.5%) was added to prevent microbial contamination. The stocks were diluted to 0.05 μg ml−1 in TNE buffer; otherwise the assay was carried out as described above.
Results
Recombinant antibodies recognizing TuVX
Recombinant antibodies for detection of TuVX were obtained from alpaca. As a first step, a female alpaca was immunized and boosted twice with 100 μg purified TuVX particles. A VHH library was created from lymphocyte RNA from this animal, and the library was subjected to three rounds of panning on TuVX material. After the last panning, VHH inserts were mass-excised and transferred into an expression vector where the C-terminus of the encoded VHH domain is fused to a VSV-tag, followed by a C-terminal His-6 tag.
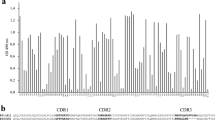
The DNA sequences of 28 selected VHHs were analyzed. The resulting sequences have been deposited to Genbank (accession numbers EF455532 to EF455558). The deduced protein sequences are shown in Fig. 1. Variation is mainly confined to the complementarity-determining regions (CDRs), which are involved in the binding of the antigen. Among the 28 analyzed sequences, five sequence groups could be distinguished by variations in the CDR regions. Group 1 (TVX72, 78, 94, 88, 71, 82, 91, 86 and 81) is distinguished by its CDR3 sequence (NANWGATN). Group 2 (TVX84 and 76), group 3 (TVX92) and group 4 (TVX73, 89, 90, 85, 93, 87, 74 and 79) have comparable CDR1 and CDR2 regions to group 1, but differ in their CDR3 region. The CDR3 region of group 4 is clearly extended, having five to eight residues more than the other groups. The feature of an extended CDR3 region is considered to be typical for camelid antibodies. Group 5 (TVX80, 83, 69, 75, 95, 96, 70 and 77) is clearly distinguished from the other groups by its CDR3 region, but also by its completely different CDR1 and CDR2 regions, where the CDR2 region is three residues larger in size than in the other groups. This indicated that, in contrast to unselected VHH clones (not shown) clusters of VHH domains can be distinguished in the TuVX-selected population.
The selected VHH molecules all belong to the same subfamily of camelid antibodies. Four distinct subfamilies in llama heavy-chain VHH domains have been distinguished (Harmsen et al. 2000). These subfamilies are defined by the incidence of a number of key-residues in the VHH amino-acid sequence. Typically the anti-TuVX VHH sequences have subfamily 2 features, such as the Y residue at position 38, the number of residues in CDR2 (seven instead of typically eight), the N at positions 62 and 100. None of the CDR1 and CDR3 regions contains any additional cystein residues, which only occur in subfamilies 3 and 4. Remarkably, the residue at position 48 in all anti-TuVX VHH sequences is a V, while this position has a strong preference for F (subfamily 1) or L (subfamily 2), and V is found only in one out of 152 VHH sequences described by Harmsen et al. (2000).
Most selected VHH molecules could easily be purified in significant quantities. For each sequence group, a representative clone was selected for further analysis. Clone TVX69 (group 5) displayed a continuous tendency to precipitate during purification, while all others tested remained soluble. Three VHH domains (TVX90, TVX92 and TVX76) were expressed in 200 ml cultures and purified from inclusion bodies by Ni-affinity chromatography. As exemplified in Fig. 2, the expressed proteins were the dominant protein in the E. coli cell pellet (Lane A), and Ni-affinity purification removed most of the other proteins (Lane E). The yields of purified recombinant proteins TVX76, TVX90 and TVX92 were 4.2, 6.4 and 5.4 mg per 200 ml culture, respectively.
Protein purification. Shown is a 15% SDS PAGE gel, stained by Coomassie BB. Indicated are the positions of relevant size markers in kDa. Lane A: Total culture of TVX72. Lane B: Soluble protein, after cell lysis and refolding. Lane C: Flow-through of the Ni-NTA column; Lane D: Eluted protein from the Ni-NTA column; Lane E: TVX72 after dialysis, as it is stored and applied in the tests
Antibody specificity
Antibodies were tested by ELISA using the VSV-tag for detection. Firstly, reactivity to TuVX particles which had been directly immobilized on ELISA wells was assessed, and compared to reactivity to bacteriophage VCSM13 as a control. VCSM13 particles are also filamentous, but otherwise unrelated to TuVX. All three tested clones reacted equally well with TuVX, and did not produce a signal with VCSM13 (Table 1). This indicated that the selected antibodies could recognize TuVX.
The specificity of TVX76, TVX90 and TVX92 was further investigated using a number of flexiviridae. For this purpose, the potex viruses Potato virus S (PVS), Potato virus X (PVX), Kalanchoe mosaic virus (KMV) and Pepino mosaic virus (PepMV), and the carlavirus Chrysanthemum virus B (CVB) were compared to TuVX in an ELISA experiment where the virus material was directly coated in the ELISA plate wells. In all experiments, signals did not exceed those of the buffer (carbonate buffer) or uninfected plant material (Table 1), in contrast to the TuVX material. Thus, it appeared that the antibodies are specific for TuVX, though it cannot be excluded that the direct coating interfered with the integrity of the other potex and carla viruses.
DAS-ELISAs
Application of plant pathogen antibodies routinely includes double antibody sandwich ELISA tests (DAS-ELISA). The activity of TVX76 in particular was further tested using a DAS-ELISA. As capture probe, polyclonal rabbit anti TuVX serum was used. Detection of virus material in different dilutions was tested. Virus particles were tested in dilutions between 16 μg ml−1 and 0.25 μg ml−1, either in carbonate buffer, 10-fold diluted tulip leaf extract or 100-fold diluted tulip leaf extract. For all tested virus dilutions, significant signals were observed. The background signal (in the absence of virus particles) was less than 2% of the maximal signal, while 0.25 μg ml−1 virus dilution gave a signal which was more than 10-fold higher than the background (Fig. 3) The DAS-ELISA system was further tested on leaf material from TuVX-infected plants. The 1:10 and 1:100 fold diluted infected plant material produced clearly positive ELISA signals (arrows in Fig. 3). The detection limit is comparable to that of other serological virus tests, leading us to conclude that the TVX76 VHH allows sensitive detection of TuVX in infected plant material. Similar results were obtained for TVX90 and TVX92 (not shown, see below).
DAS-ELISA result for TVX76 with different virus dilutions and infected plant material. Squares represent virus particles suspended in a 1:10 diluted tulip leaf extract. Circles represent virus particles suspended in 1:100 diluted tulip leaf extract. Triangles represent virus particles suspended in carbonate buffer. The values on the right (above the 0) are the background levels of uninfected leaf material or carbonate buffer. The solid arrow indicates the signal from a 1:10 diluted extract from naturally infected tulip leaf material, while the open arrow represents the signal of 1:100 diluted extract from naturally infected tulip leaf material
Storage
Camelid single chain antibody fragments are attractive for diagnostics because they are generally less prone to precipitation and aggregation, as compared to scFv antibody fragments (Holliger and Hudson 2005). To substantiate this further, purified antibodies TVX90, TVX92 and TVX76 were tested in DAS-ELISA for their resistance to prolonged storage at different temperatures. For this purpose, antibodies were diluted 100-fold more than in the above described DAS-ELISA, to facilitate monitoring of changes in detection efficiency. After initial testing, antibody stocks of 0.1 μg ml−1 were stored at −20, +4, 21 or 37°C for several weeks. No special precautions were taken to enhance protein stability, except that a low amount of azide was added to prevent microbial contamination. At regular intervals, antibodies were diluted to critical concentrations, and DAS-ELISAs on a range of concentrations of TuVX diluted in TNE buffer were performed.
The lowest concentration of virus at which a threshold signal of four times the background (signal in the absence of virus material) was observed (Table 2), was determined. The reactivity of antibodies TVX90 and TVX92 did not change upon storage for 8 weeks at −20 or 4°C. In general, TVX76 was more sensitive to the temperature treatments. Its reactivity was reduced after storage at 4°C, and it did not resist storage of more than 1 week at room temperature or 37°C. Antibody TVX92 on the other hand was still highly reactive after 5 weeks of storage at 37°C, and only after 8 weeks showed a clear decrease in activity. Antibody TVX90 behaved similarly to TVX92. Thus, two out of three antibodies showed excellent behaviour during storage.
Discussion
Camelid VHH domains form a special class of recombinant antibodies. VHH domains from camels, dromedaries and llamas have been described (Harmsen et al. 2000). In this paper, for the first time, the isolation of recombinant antibodies from Alpaca is described. Alpacas are part of the camelid family, and are often classified as a subspecies of the llama. However, recent DNA marker analyses have demonstrated that, while the llama (Lama glama) has been domesticated from guanacos, the alpaca is descended from the vicuña, and should be classified as Vicugna pacos (Kadwell et al. 2001). The selected alpaca VHH domains show good sequence homology to the known VHH domains from other camelids; for instance the protein sequence of TVX72 is 80% identical to that of a L. glama VHH domain (accession CAD22462). In this study, an alpaca was involved for acquiring VHH domains because of its smaller size and generally tame behaviour. The sequences shown in Fig. 1 thus represent the first immunoglobulin sequence information for the Vicugna family.
This also is the first report on recombinant antibodies from camelid origin for phytosanitary purposes. In this field, recombinant antibodies have mainly been derived from human or mouse naïve libraries (Ziegler and Torrance 2002), and concern scFv antibody fragments. Though some have reported successful application of these fragments, (Holliger and Hudson 2005) note that the application of scFvs has been severely hampered by their tendency to precipitate, referring likely to numerous unpublished disappointments in scFv proteins, including our own. Poor solubility has for instance been reported for a His-purified scFv for detection of cocaine, which precipitated during 2 weeks of storage, irrespective of the concentration, unless it was further purified and lyophilized (Moss et al. 2003). Another His-purified scFv for detection of melanoma-associated proteoglycan was described to be only soluble to 150 μg ml−1 during storage at 4°C (Hamilton et al. 2003). For comparison, all VHH stocks tested by us were stable at 4°C in concentrations exceeding 0.5 mg ml−1, and only one out of these six selected VHH domains suffered from any precipitation.
Some clear advantages have been noted for camelid VHH domains over scFv fragments, in particular relating to robustness. For instance, VHH domains have the ability to refold after melting (Ewert et al. 2002). A comparison has been made between VHH antibody fragments and mouse monoclonal antibodies (van der Linden et al. 1999). While most mouse monoclonal antibodies retain hardly any reactivity after 2 h incubation at 70°C, VHH fragments preserved full binding even after 2 h incubations at 90°C. Binding capabilities of VHH domains and soluble human VH3 domains have been compared after temperature treatments (Ewert et al. 2002). It was observed that the binding capacities of VHH domains were almost fully recovered after heating to 80°C, while soluble human VH3 fragments irreversibly precipitate at temperatures higher than 65°C. Similar observations have been made on a panel of VHHs for detection of diverse antigens (Goldman et al. 2006).
In our study, we tested the storage behaviour of TuVX-binding VHH domains, and observed that activity is best maintained after storage at −20 or +4°C, while two out of three tested VHH domains resisted incubation at 37°C for 5 weeks. Though this study does not include a comparison with monoclonal antibodies or scFv molecules, this behaviour at least parallels that of commonly used purified IgGs and alkaline phosphatase-conjugated antibodies originating from rabbit. In our opinion, this represents a clear benefit of the VHH over the scFv type of recombinant antibodies, which, in our hands, remained very poorly stable upon storage at −20°C, or transportation at ambient temperature.
Immunological detection of plant pathogens is predominantly performed with classical antisera raised by immunization of rabbits. However, the policy of governments is aimed to reduce animal suffering and to require constant quality of the performance of an assay. The use of recombinant antibodies allows implementation of diagnostic sera production in standard microbiological laboratories according to ISO17025 certification. Facilities to produce polyclonal sera are demanding to set-up or maintain and are less robust in production capacity.
Diagnostics using immunochemistry is still under constant innovation. Examples are the development of easy-to-handle, field-compatible tests such as lateral-flow (Delmulle et al. 2005), but also more complex test set-ups like flow-cytometry (Chitarra et al. 2002), and multiplex detection using Luminex (Rao et al. 2004; Peters et al. 2007) or protein chips (Taitt et al. 2004). Use of recombinant antibodies, fused to specific tags, may facilitate multiplex design (Wingren and Borrebaeck 2006). Such tags may comprise DNA (Jongsma and Litjens 2006), His-stretches (Larsson et al. 2005) or many other oligo-peptidic moieties. The availability of recombinant antibodies facilitates the development of such multiplex immunological assays.
Abbreviations
- CDR:
-
complementarity-determining region
- scFv:
-
single-chain variable fragment
- VHH:
-
variable domains from heavy-chain immunoglobulin
- TuVX:
-
Tulip virus X
- PVS:
-
Potato virus S
- PVX:
-
Potato virus X
- PepMV:
-
Pepino mosaic virus
- KMV:
-
Kalanchoe mosaic virus
- CVB:
-
Chrysanthemum virus B
References
Chitarra, L. G., Langerak, C. J., Bergervoet, J. H., & van den Bulk, R. W. (2002). Detection of the plant pathogenic bacterium Xanthomonas campestris pv. campestris in seed extracts of Brassica sp. applying fluorescent antibodies and flow cytometry. Cytometry, 47, 118–126.
De Haard, J. J. W., Hermans, P., Landa, I., & Verrips, C. T. (2002). Protein arrays. Patent application WO 02/48193.
Delmulle, B. S., De Saeger, S. M. D. G., Sibanda, L., Barna-Vetro, I., & Van Peteghem, C. H. (2005). Development of an immunoassay-based lateral flow dipstick for the rapid detection of Aflatoxin B1 in pig feed. Journal of Agricultural and Food Chemistry, 53, 3364–3368.
Ewert, S., Cambillau, C., Conrath, K., & Pluckthun, A. (2002). Biophysical properties of camelid V(HH) domains compared to those of human V(H)3 domains. Biochemistry, 19, 3628–3636.
Ghahroudi, M. A., Desmyter, A., Wyns, L., Hamers, R., & Muyldermans, S. (1997). Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Letters, 414, 521–526.
Goldman, E. R., Anderson, G. P., Liu, J. L., Delehanty, J. B., Sherwood, L. J., Osborn, L. E., et al. (2006). Facile generation of heat-stable antiviral and antitoxin single domain antibodies from a semisynthetic llama library. Analytical Chemistry, 78, 8245–8255.
Griep, R., van Twisk, C., van Beckhoven, J. R. C. M., van der Wolf, J. M., & Schots, A. (1998). Develpment of specific recombinant monoclonal antibodies against the lipopolysaccharide of Ralstonia solanacearum race 3. Phytopathology, 88, 795–803.
Hamers-Casterman, C. A. T., Muyldermans, S., Robinson, G., Hamers, C., Songa, E. B., Bendahman, N., et al. (1993). Naturally occurring antibodies devoid of light chains. Nature, 363, 446–448.
Hamilton, S., Odili, J., Pacifico, M. D., Wilson, G. D., & Kupsch, J. M. (2003). Effect of imidazole on the solubility of a His-tagged antibody fragment. Hybrid, 22, 347–355.
Harmsen, M. M., Ruuls, R. C., Nijman, I. J., Niewold, T. A., Frenken, L. G., & de Geus, B. (2000). Llama heavy-chain V regions consist of at least four distinct subfamilies revealing novel sequence features. Molecular Immunology, 37, 579–590.
Holliger, P., & Hudson, P. J. (2005). Engineered antibody fragments and the rise of single domains. Natural Biotechnology, 23, 1126–1136.
Hoogenboom, H. R. (2005). Selecting and screening recombinant antibody libraries. Natural Biotechnology, 23, 1105–1116.
ICTVdB-Management (2006). 00.056.0.01.019. Tulip virus X. In C. Büchen-Osmond (Ed.) ICTVdB – The Universal Virus Database, version 4. New York: Columbia University.
Jongsma, M. A., & Litjens, R. H. (2006). Self-assembling protein arrays on DNA chips by auto-labeling fusion proteins with a single DNA address. Proteomics, 6, 2650–2655.
Kadwell, M., Fernandez, M., Stanley, H. F., Baldi, R., Wheeler, J. C., Rosadio, R., et al. (2001). Genetic analysis reveals the wild ancestors of the llama and the alpaca. Proceedings of the Royal Society B Biological Sciences, 268, 2575–2584.
Larsson, C., Bramfeldt, H., Wingren, C., Borrebaeck, C., & Hook, F. (2005). Gravimetric antigen detection utilizing antibody-modified lipid bilayers. Analytical Biochemistry, 345, 72–80.
Moss, J. A., Coyle, A. R., Ahn, J. M., Meijler, M. M., Offer, J., & Janda, K. M. (2003). Tandem IMAC-HPLC purification of a cocaïne-binding scFv antibody. Journal of Immunological Methods, 281, 143–148.
Muyldermans, S. (2001). Single domain camel antibodies: Current status. Journal of Biotechnology, 74, 277–302.
Peters, J., Sledz, W., Bergervoet, J., & Van der Wolf, J. M. (2007). An enrichment microsphere immunoassay for the detection of Pectobacterium atrosepticum and Dickeya dianthicola in potato tuber extracts. European Journal of Plant Pathology, 117, 97–107.
Rao, R. S., Visuri, S. R., McBride, M. T., Albala, J. S., Matthews, D. L., & Coleman, M. A. (2004). Comparison of multiplexed techniques for detection of bacterial and viral proteins. Journal of Proteome Research, 3, 736–742.
Taitt, C. R., Golden, J. P., Shubin, Y. S., Shriver-Lake, L. C., Sapsford, K. E., Rasooly, A., et al. (2004). A portable array biosensor for detecting multiple analytes in complex samples. Microbial Ecology, 47, 175–185.
van der Linden, R. H., Frenken, L. G., de Geus, B., Harmsen, M. M., Ruuls, R. C., Stok, W., et al. (1999). Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochimica et Biophysica Acta, 1431, 37–46.
Wingren, C., & Borrebaeck, C. A. (2006). Antibody microarrays: Current status and key technological advances. OMICS, 10, 411–427.
Ziegler, A., & Torrance, L. (2002). Applications of recombinant antibodies in plant pathology. Molecular Plant Pathology, 3, 401–407.
Acknowledgements
The authors wish to acknowledge Irma Veijn, Erwin Houtzager (CatchMabs BV, The Netherlands) and Cees Waalwijk for technical advice, Joop van Doorn (PPO Lisse, The Netherlands) for TuVX material, polyclonal serum and tulip material, and the DWK programme 397III-31 from the Dutch Ministry of Agriculture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Beekwilder, J., van Houwelingen, A., van Beckhoven, J. et al. Stable recombinant alpaca antibodies for detection of Tulip virus X . Eur J Plant Pathol 121, 477–485 (2008). https://doi.org/10.1007/s10658-007-9265-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9265-y