Abstract
Tree pollen chimera 7 (TPC7), a hypoallergenic Bet v 1 tolerogen against birch pollen allergy, induces the formation of novel, huge protein bodies (referred to as TPC7 bodies) in rice endosperm, and is accumulated in high level. In the present study, we found that native Bet v 1 and TPC9, analog proteins of TPC7, were also deposited into novel protein bodies in rice endosperm. However, the novel protein bodies in Bet v 1 and TPC9 rice were much smaller and less abundant than those in TPC7 rice, reflected in lower amounts of accumulation of Bet v 1 and TPC9 than that of TPC7. A domain swapping experiment between TPC7 and Bet v 1 revealed that the latter half of TPC7 is important for the formation of the TPC7 body. We found that chaperons and folding enzymes such as BiP and protein disulfide isomerase were localized within the TPC7 body. TPC7 protein was extracted from TPC7 seeds as large aggregates with molecular masses greater than 669 kDa, or approximately 75 kDa under native or semi-native conditions. These TPC7 aggregates are thought to be responsible for the induction of TPC7 body formation. TPC7 accumulated to a maximum level of 550 μg/seed, which amounts to 23 % of total seed protein, while Bet v 1 and TPC9 accumulated much lower levels. The TPC7 body represents a promising reservoir, which may serve as a fusion partner for high-level production and sequestering storage of recombinant proteins.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Protein bodies (PBs), which can serve as deposition sites of seed storage proteins, are ideal production platforms for high-value recombinant proteins, such as pharmaceuticals, vaccines and antibodies. In rice seeds, more than 80 % of total seed proteins accumulate in PBs as storage proteins (Simmonds 1978). These proteins are thereby protected from proteases and desiccation during seed maturation by membrane boundaries, and they are subsequently hydrolyzed and utilized as a nitrogen source for the germinating seedling (Müntz 1998). Thus, PBs have long been considered to be suitable sites for deposition and storage of large amounts of recombinant products. Rice seeds harbor two types of protein bodies: the endoplasmic reticulum (ER)-derived protein body PB-I, which contains several types of prolamin, and the protein storage vacuole PB-II, which comprises glutelins and globulin (Takaiwa et al. 1999). Many other cereal grains contain only either ER-derived PBs or protein storage vacuoles (PSVs). Specific protein–protein interactions among storage proteins and chaperone binding proteins and the formation of disulfide bridges are important for the formation of ER-derived PBs (Kumamaru et al. 2007; Vitale and Ceriotti 2004).
Transgenic plants accumulating various types of therapeutic proteins or peptides in PBs of seeds or other tissues have been generated to produce high levels of recombinant proteins. In rice endosperm, some recombinant proteins have been deposited into PB-I or/and PB-II (Nicholson et al. 2005; Suzuki et al. 2011; Takaiwa et al. 2009; Torres et al. 2001; Wakasa et al. 2013). Notably, several recombinant proteins induce the formation of novel, unique storage protein bodies that differ from PB-I and PB-II, which accumulate high levels of these proteins. Tree pollen chimera 7 (TPC7), a hypoallergenic Bet v 1 tolerogen against birch pollen allergy, accumulates at high levels in novel, huge spherical PBs, referred to as TPC7 bodies, in transgenic rice endosperm (Wang et al. 2013). When human IL-10 is expressed in rice endosperm, this protein is deposited into numerous granules that are smaller than PB-I (Yang et al. 2012a). Hypoallergenic Der f 2 derivatives with altered intramolecular disulfide bonds also accumulate in novel ER-derived PBs, which exhibit unusual physicochemical properties such as atypical electron densities or digestibility by gastrointestinal enzymes (Yang et al. 2012b). In light of their extremely high levels of protein accumulation, TPC7 bodies represent a promising reservoir for high-level production and sequestration storage of recombinant proteins.
TPC7 is a versatile hypoallergenic derivative of Bet v 1 generated by DNA shuffling of the major allergens of trees belonging to the Fagales order Bet v 1 family (Wallner et al. 2007). Wallner et al. (2007) also generated another chimeric molecule, TPC9, which displays low allergenicity and high immunogenicity to Bet v 1. In the present study, we generated transgenic rice plants expressing Bet v 1 and TPC9 in their endosperm. To investigate the biogenesis mechanisms of TPC7 bodies, we compared Bet v 1 and TPC9 rice with TPC7 rice and analyzed the structural status of TPC7 deposited in the endosperm and the involvement of chaperons and folding enzymes that are implicated in protein quality control.
Materials and methods
Plant material and growth conditions
Rice plants (Oryza sativa cv. Kita-ake) were grown at 25 °C/20 °C (12/12 h) under natural light conditions in pots (12 cm diameter) containing a commercial soil mixture (Bonsol No. 1; Sumitomo Chemicals, Osaka, Japan) with 14-14-14 chemical fertilizer.
Generation of transgenic rice
DNAs encoding Bet v 1 and TPC9 were synthesized by GenScript Corporation (NJ, USA), which was optimized by employing codons that are preferentially used in genes encoding many major rice seed storage proteins and by avoiding AT rich sequences, including potential mRNA destabilizing sequences, polyadenylation signals and some rare codons (Takaiwa et al. 2008). DNAs encoding T-B and B-T were generated using the modified Bet v 1 and TPC9. The modified TPC9 and Bet v 1, T-B and B-T genes with a glutelin B1 (GluB-1) signal peptide (72 bp) sequence and an ER retention signal (KDEL) attached to the N- and C-termini, respectively, were ligated downstream of the endosperm-specific 2.3 kb GluB-1 promoter and upstream of a 0.65 kb GluB-1 terminator. The resulting constructs were introduced into the destination-binary vector pHGW using MultiSite Gateway LR Clonase II Plus Enzyme Mix (Invitrogen, CA, USA). The rice plants were transformed with these newly constructed plasmids by Agrobacterium-mediated transformation as previously described (Qu and Takaiwa 2004). We generated 20–30 independent lines of each type of transgenic rice.
Protein extraction and Western blot analysis
Protein extraction of mature seeds and Western blotting were performed as described previously (Wang et al. 2013) with minor modifications. Extraction buffer (50 mM Tris–HCl [pH 6.8], 4 M urea, 4 % [v/v] SDS, 1 % [v/v] 2-mercaptoethanol) was added to the tissue powder and vortexed at room temperature. The mixture was centrifuged at 13,000×g for 10 min and the supernatant was used for Western blotting. For Western blotting used to investigate disulfide bonds, proteins were extracted in buffer [50 mM Tris–HCl (pH 6.8), 0.5 % (v/v) SDS], and electrophoresed with 2× loading buffer [40 % (v/v) glycerol, 8 % SDS, 0.4 % BPB, 0.2 M Tris–HCl (pH 6.8)]. The band images were scanned and quantified using ImageQuant TL (GE Healthcare Uppsala, Sweden). Polyclonal antibodies against TPC7, OsBiP1 (Os02g0115900), OsBiP4/OsBiP5 (OsBiP4&5; Os05g0428600, Os08g0197700), OsPDIL1;1 (Os11g0199200), OsPDIL2;3 (Os09g0451500), calnexin (CNX; Os04g0402100) and OsTIP3 (Os10g0492600) were prepared previously (Kudo et al. 2013; Oono et al. 2010; Wakasa et al. 2011, 2012; Wang et al. 2013; Yasuda et al. 2009).
The total protein concentration was measured by the RC DC Protein Assay (Bio-Rad Laboratories, CA, USA) according to the manufacturer’s instructions. The recombinant protein yields were estimated by SDS-PAGE and Western blotting. For SDS-PAGE, the lower bands corresponding to non-glycosylated proteins of TPC7, Bet v 1 and TPC9 were separated from the other storage proteins, while the upper bands corresponding to glycosylated proteins were not. Therefore, the yields of unglycosylated proteins were first estimated by SDS-PAGE using BSA as a standard. Then, the ratios of upper bands to lowers bands were estimated by Western blotting, and total recombinant protein yields were calculated using these ratios and the yields of unglycosylated proteins.
Indirect immunohistochemical analysis by confocal laser scanning microscopy and transmission electron microscopy (TEM)
Immunohistochemical analysis by confocal laser scanning microscopy was performed as described previously (Wang et al. 2013) with minor modifications. The primary antibody was used at a 1:300 dilution. The specimens were observed with a confocal laser scanning microscope (Fv10i, Olympus, Tokyo, Japan) using Alexa 488 and rhodamine filter sets. Photographs taken with the confocal laser scanning microscope were used to count the numbers and measure the diameters of the huge PBs. TEM was performed as described previously (Wang et al. 2013).
RNA preparation and RT-PCR
Total RNA was prepared from mature seeds of non-transgenic (NT) and transgenic rice plants as previously described (Yasuda et al. 2005). Then, cDNA was synthesized from 500 ng of total RNA using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Osaka, Japan) according to the manufacturer’s instructions. PCR amplifications were performed using GoTaq Green Master Mix (Promega, WI, USA) as follows: 22, 25, 28 and 31 cycles of 95 °C for 30 s, 55 °C for 30 s and 72 °C for 10 s. To amplify TPC7, Bet v 1 and TPC9, the primer set (5′-CCCTTTCAAGTACGTGAAGG-3′ and 5′-CATCCTCCATCAGGGGTTGC-3′), which can amplify all of TPC7, Bet v 1 and TPC9, was used. The actin-encoding gene (Os03g0718100) was amplified as an internal reference using the primer set (5′-GCGTGGACAAAGTTTTCAACCG-3′ and 5′-TCTGGTACCCTCATCAGGCATC-3′).
Glycosylation analysis
Total seed proteins extracted for Western blotting used to investigate disulfide bonds were suspended in reaction buffer and incubated in the presence or absence of Endo H or PNGase F at 37 °C for 1 h, according to the manufacturer’s protocol (New England Biolabs) without denaturalization at 100 °C in the denaturing buffer. The reaction products were used for SDS–PAGE and Western blot analysis.
Sequential protein extraction in native and semi-native conditions
Native and semi-native proteins were extracted from maturing seeds (15–25 DAF) using a sequential extraction method. First, protein was extracted with 500 μl of native extraction buffer (50 mM Tris–HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 10 mM glucose, 5 U/ml hexokinase and 1× Complete mini EDTA-free Protease Inhibitor Cocktail [Roche, Basel, Switzerland]) by homogenization with Biomasher II (Nippi, Tokyo, Japan) followed by vortex for 5 min. After centrifugation at 13,000×g for 10 min and removal of supernatant, the residue was homogenized with semi-native buffer (0.2 % Triton X-100, 50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA and 1× Complete mini EDTA-free Protease Inhibitor Cocktail) for further extraction of protein. After extraction in the same way as native protein extraction, the residue was subjected to further protein extractions with semi-native extraction buffer containing 0.5, 1.0 and 2.0 in sequence.
Native PAGE and gel filtration analysis
Native PAGE was performed using the same gels and running buffer as standard PAGE except for the lack of SDS and urea. Electrophoresis was performed at 4 °C.
Gel filtration analysis was performed as described previously (Wakasa et al. 2009). Native protein extract without Triton X-100 and semi-native protein extract containing 0.2 % Triton X-100 were applied to a Sephacryl S-300 HR (GE Healthcare) column and immediately subjected to fraction collection with 50 μl per tube using ice-cold native extraction buffer without Triton X-100 and semi-native extraction buffer containing 0.2 % Triton X-100, respectively. A Gel Filtration Calibration Kit HMW (GE Healthcare) was used to determine the molecular weight of the TPC7 aggregates.
Results
Generation of transgenic rice accumulating Bet v 1 and TPC9 in the endosperm
The amino acid sequence homology between TPC7, Bet v 1 and TPC9 is greater than 90 % (Fig. 1a). All of these proteins possess one cysteine residue at position 112, one glycosylation site at position 83 and five phosphorylation sites between positions 40 and 90. At position 118, Bet v 1 and TPC9 possess a phosphorylation site but TPC7 does not (Fig. 1a).
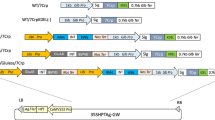
Generation of Bet v 1 and TPC9 transgenic rice. a Alignment of TPC7, Bet v 1 and TPC9 sequences. The red triangle, the red bar and red dashed squares represent cysteine residues, glycosylation sites and phosphorylation sites, respectively. The shaded areas represent either identical residues found in all three proteins (black) or residues with similar characters (gray). b Schematic representation of the gene construct used for expression of TPC7, Bet v 1 and TPC9 in transgenic rice seeds. TPC7, Bet v 1 and TPC9 genes were expressed under the control of the 2.3 kb glutelin B1 (GluB-1) promoter. GluB1 pro, GluB1 ter, SP and KDEL represent the 2.3 kb GluB-1 promoter, 0.65 kb 3′-untranslated region, the GluB-1 signal peptide sequence and the tetrapeptide endoplasmic reticulum (ER) retention signal, respectively. c Seed phenotype of non-transgenic (NT) TPC7, Bet v 1 and TPC9 rice. The photographs in the bottom row show cross sections. d Western blot analysis and SDS-PAGE of mature seeds of NT, TPC7, Bet v 1 and TPC9 rice. Total proteins were extracted from seeds of NT, TPC7, Bet v 1 and TPC9 rice and separated by 15 % SDS-PAGE. Immunodetection with anti-TPC7 antibody (upper) or Coomassie Brilliant Blue (CBB) staining (lower). The upper and lower bands revealed by immunodetection indicate glycosylated and unglycosylated polypeptide, respectively
In a previous study, we generated transgenic rice accumulating TPC7 in seeds as an oral vaccine against birch pollen allergy by expressing this protein as a secretory protein using the N-terminal signal peptide and the C-terminal KDEL tag (ER retention signal) under the control of an endosperm-specific glutelin promoter (Fig. 1b, Wang et al. 2013). In the present study, we generated transgenic rice expressing Bet v 1 and TPC9 in the endosperm in the same manner as for TPC7 rice. The GluB-1 signal peptide and the KDEL were linked to the N- and C- termini of Bet v 1 and TPC9, respectively, and these gene constructs were specifically expressed under the control of the rice major SSP glutelin 2.3 kb GluB-1 promoter (Fig. 1b). Aberrant seed phenotypes such as floury and shrunken features were observed in TPC7 rice seeds (Fig. 1c). Bet v 1 and TPC9 rice seeds also showed aberrant phenotypes, although these phenotypes were not so predominant as they were in TPC7 rice (Fig. 1c). Western blot analysis was performed to detect TPC7, Bet v 1 and TPC9 protein in seeds using an anti-TPC7 antibody. TPC7, Bet v 1 and TPC9 were detected as two bands with molecular masses of 17 and 20 kDa (Fig. 1d). Wang et al. (2013) showed that a portion of TPC7 protein expressed in endosperm is glycosylated, and that the upper band represents glycosylated TPC7 protein while the lower unglycosylated. As Bet v 1 and TPC9 also have a candidate glycosylation site at the same position as that of TPC7 (Fig. 1a), these two bands (from both Bet v 1 and TPC9 rice) are thought to be derived from glycosylated and unglycosylated protein, respectively.
Formation of few huge PBs in Bet v 1 and TPC9 rice
To investigate the intracellular localization of Bet v 1 and TPC9 in endosperm cells and whether Bet v 1 and TPC9 induce the formation of huge PBs, we performed indirect immunohistochemical analysis using confocal laser scanning microscopy. We stained sections of maturing seeds with rhodamine B to visualize ER-derived PB-Is (red), and we immunostained the sections with anti-TPC7 antibody to visualize TPC7, Bet v 1 and TPC9 localization (green). Huge, spherical PBs designated TPC7 bodies were often observed in TPC7 rice, and fluorescent signals from TPC7 were detected within the TPC7 body (Fig. 2a), as reported previously (Wang et al. 2013). Some cells in the endosperm were full of TPC7 bodies (Fig. 2a). Huge, spherical PBs were also observed in Bet v 1 and TPC9 rice, and fluorescent signals from Bet v 1 and TPC9 were detected within the huge PBs (Fig. 2b, c). However, the number of huge PBs in Bet v 1 and TPC9 rice was much lower than that in TPC7 rice. In particular, huge PBs were rarely observed in Bet v 1 rice. The number density of the huge PBs in subaleurone cells of TPC7, Bet v 1 and TPC9 rice was approximately 1,200, 100 and 250 mm−2, respectively (Fig. 2d). Most of the huge PBs were 5–20 μm in diameter (Fig. 2e). The average diameter of the huge PBs in TPC7, Bet v 1 and TPC9 was 12, 8 and 10 μm, respectively (Fig. 2e). In TPC7 rice, several extremely huge TPC7 bodies with diameters up to 30 μm were observed (Fig. 2a, e).
Indirect immunohistochemical analysis of TPC7, Bet v 1 and TPC9 in subaleurone layer cells in maturing seeds of TPC7 (a), Bet v 1 (b) and TPC9 (c) rice, respectively. Red signal indicates PB-Is labeled with rhodamine, and green signal indicates immunofluorescence of TPC7, Bet v 1 and TPC9 immunostained with anti-TPC7 antibody. Bars in upper and lower figures represent 20 and 5 μm, respectively. d The number densities of huge PBs in subaleurone cells of TPC7, Bet v 1 and TPC9 rice. Error bars represent standard deviation (n = 3). d The diameters of huge PBs in subaleurone cells of TPC7, Bet v 1 and TPC9 rice. Each dot represents the diameter of a huge PB (n = 169 for TPC7, n = 55 for Bet v 1 and n = 68 for TPC9). Horizontal bars represent means
The accumulation levels of TPC7, Bet v 1 and TPC9 protein
We measured the accumulation levels of TPC7, Bet v 1 and TPC9 protein in seeds by Western blot analysis following electrophoresis by SDS-PAGE. TPC7 protein accumulated at levels up to 550 μg/seed, while Bet v 1 and TPC9 accumulated at levels of approximately 170 and 80 μg/seed, respectively (Table 1). TPC7, Bet v 1 and TPC9 accounted for 23, 4 and 2 % of total seed proteins, respectively. The total seed protein content of TPC7 rice was much lower than that of NT rice because the TPC7 rice seeds were shrunken and smaller than NT rice seeds (Table 1; Fig. 1c). By contrast, the seed weights of Bet v 1 and TPC9 rice were not significantly lower than that of NT rice, and their total protein contents were almost identical to that of NT rice (Table 1).
To investigate the transcript levels of TPC7, Bet v 1 and TPC9 in seeds, we performed RT-PCR. The mRNA levels of seed were almost identical in TPC7, Bet v 1 and TPC9 (Fig. 3). This result suggests that the different protein accumulation levels observed among TPC7, Bet v 1 and TPC9 were not due to variations in mRNA levels.
The intermolecular disulfide bonds of Bet v 1 and TPC9
TPC7, Bet v 1 and TPC9 contain one free cysteine residue at position 113 (Fig. 1a). TPC7 is dimerized via intermolecular disulfide bonds between free cysteine residues, and the disulfide bond is important for TPC7 body formation (Wang et al. 2013). To investigate whether Bet v 1 and TPC9 are dimerized via disulfide bonds in the endosperm, we extracted proteins from mature seeds using buffer lacking any reducing agents. When the proteins were subjected to electrophoresis by 12.5 and 7.5 % PAGE and analyzed by Western blotting, the 17 and 20 kDa bands corresponding to the monomer proteins of TPC7, Bet v 1 and TPC9 were only weakly detected (Fig. 4a). Instead, three bands with molecular masses between 35 and 40 kDa were detected (Fig. 4a, b). These three bands are thought to represent dimers formed via intermolecular disulfide bonds. The upper, middle and lower bands are thought to represent dimers composed of two glycosylated proteins, one glycosylated–one unglycosylated protein and two unglycosylated proteins, respectively. The relative intensities of the bands between 35 and 40 kDa to the bands between 17 and 20 kDa of TPC7 were slightly higher than those of Bet v 1 and TPC9. To determine whether the glycosylation affect the banding patterns, the seed proteins extracted in buffer lacking reducing agents were digested by either endoglycosidase H (Endo H) or peptide-N-glycosidase F (PNGase F). The upper bands between 17 and 20 kDa were shifted to the lower bands in TPC7, Bet v 1 and TPC9 rice (Fig. 4c). The upper two bands between 35 and 40 kDa, corresponding to the dimer proteins, were also shifted to the lowest bands (Fig. 4c). The relative intensities of the dimer bands to the monomer bands of TPC7, Bet v 1 and TPC9 were not affected by deglycosylation.
Western blot analysis of mature seeds of TPC7, Bet v 1 and TPC9 rice in buffer lacking reducing agents. Total proteins were extracted from seeds in extraction buffer without reducing agents and separated by 12.5 % (a) and 7.5 % (b) SDS-PAGE. Dashed and solid lines indicate monomer and dimer proteins, respectively. c N-Glycosylation analysis of proteins extracted in buffer without reducing agents. Total proteins extracted in buffer without reducing agents were digested in the absence (−) or presence (+) of either Endo H or PNGase F. Reaction mixtures were separated by 10 % SDS-PAGE. TPC7, Bet v 1 and TPC9 proteins were detected with anti-TPC7 antibody
In addition to these three bands, some minor bands greater than 50 kDa were observed in TPC7, Bet v 1 and TPC9 rice (Fig. 4a, b). TPC7, Bet v 1 and TPC9 are thought to generate high molecular mass aggregates via hydrophobic interactions along with the formation of intermolecular disulfide bonds, even in buffer containing detergent.
Domain swapping of TPC7 and Bet v 1
To investigate which domains are important for the formation of numerous huge PBs, we performed domain swapping between TPC7 (which forms numerous huge PBs) and Bet v 1 (which rarely forms huge PBs). The homology of the amino acid sequences from position 1–31 between TPC7 and Bet v 1 is relatively low, but that from position 32–160 is quite high (Fig. 1a). Therefore, we swapped the amino acid sequences between positions 1 and 31 and between positions 31 and 160 in TPC7 and Bet v 1. The protein consisting of amino acids 1–31 from TPC7 and 32–160 from Bet v 1 was designated T-B, and the converse protein was designated B-T (Fig. 5a). The GluB-1 signal peptide and the KDEL ER retention signal were linked to the N- and C- termini of T-B and B-T, respectively, and these gene constructs were expressed under the control of the 2.3 kb GluB-1 promoter. Accumulated T-B and B-T in seeds were detected as two bands, which corresponded to glycosylated and unglycosylated protein, respectively (Fig. 5b). We observed sections of maturing seeds stained with toluidine blue by optical microscopy and carried out immunocytochemical studies using anti-TPC7 antibody. Huge protein bodies were rarely observed in T-B rice (Fig. 5c), and T-B protein was detected in PB-I and PB-II (Fig. 5d). On the other hand, huge PBs were often observed in B-T rice (Fig. 5c). B-T protein was mainly observed in huge PBs (Fig. 5d). The number density of huge PBs in B-T rice was approximately 300 mm−2 (Fig. 5e). The huge PBs were 5–25 μm in diameter, with an average diameter of 14 μm (Fig. 5f). These results indicate that the amino acid sequence of TPC7 between positions 32 and 160 is important for the formation of huge PBs.
Domain swapping of TPC7 and Bet v 1. a Schematic representation of the gene construct used for domain swapping. GluB1 pro, GluB1 ter, SP and KDEL represent the 2.3 kb GluB-1 promoter, 0.65 kb 3′-untranslated region, GluB-1 signal peptide sequence and tetrapeptide endoplasmic reticulum (ER) retention signal, respectively. b Western blot analysis and SDS-PAGE of mature seeds of T-B and B-T rice. Total proteins were extracted from seeds of non-transgenic (NT), T-B and B-T rice and separated by 15 % SDS-PAGE. Immunodetection with anti-TPC7 antibody (upper) or Coomassie Brilliant Blue (CBB) staining (lower). The upper and lower bands in immunodetection represent glycosylated and unglycosylated proteins, respectively. c Sections of subaleurone layers of developing seeds of NT, T-B and B-T rice were stained with toluidine blue and observed by optical microscopy. Arrows indicate huge PBs. Bars = 20 μm. d Immunoelectron micrographs of T-B and B-T observed by TEM. The section samples were reacted with anti-TPC7 antibody as the primary antibody. Arrow indicates huge PB. PB-I, protein body I; PB-II, protein body II. Bars = 500 nm. e The number densities of huge PBs in subaleurone cells of T-B and B-T rice. Error bars represent standard deviation (n = 3). f The diameters of huge PBs in subaleurone cells of T-B and B-T rice. Each dot represents the diameter of a huge PB (n = 117 for B-T). Horizontal bars represent means
Localization of proteins involved in protein folding
Wang et al. (2013) showed that the expression of molecular chaperone genes involved in ER quality control, such as several BiPs, are induced in maturing seeds expressing TPC7 and that OsBiP1 is localized within the TPC7 body. We therefore investigated the intracellular localization of other chaperone proteins and enzymes involved in protein folding in TPC7 rice seeds. Sections of maturing TPC7 rice seeds were immunostained with antibodies raised against OsBiP4&5, OsPDIL1;1, OsPDIL2;3, OsPDIL1;4 and CNX (Fig. 6). OsBiP4&5, OsPDIL1;1 and OsPDIL2;3 signals were detected within the TPC7 bodies, while OsPDIL1;4 signals were not. These results suggest that some BiPs and PDILs are involved in the formation of TPC7 bodies. The intermolecular disulfide bond of TPC7 may be catalyzed by OsPDIL1;1 and OsPDIL2;3. CNX signals were detected around the surfaces of TPC7 bodies, while OsTIP3 signals were not detected within or around the peripheries of TPC7 bodies (Fig. 6). These results indicate that the TPC7 body is enclosed by ER-derived membrane and not by vacuole-derived membrane.
Subcellular localization of proteins involved in protein folding. Sections of subaleurone layer in maturing seeds of TPC7 rice were reacted with antibodies raised against OsBiP4&5, OsPDIL1;1, OsPDIL2;3, OsPDIL1;4, calnexin (CNX) and OsTIP3, respectively. Red signal indicates PB-Is labeled with rhodamine, and green signal indicates immunofluorescence of OsBiP4&5, OsPDIL1;1, OsPDIL2;3, OsPDIL1;4, CNX and OsTIP3 immunostained with each antibody. Bars represent 5 μm
Detection of high molecular mass aggregates of TPC7 by native PAGE and gel filtration analysis
Western blot analysis in the absence of a reducing agent showed that TPC7 is not only dimerized via intermolecular disulfide bonds, but it is also oligomerized by other types of intermolecular interactions (Fig. 4). To investigate the oligomerization status of TPC7 deposited in the endosperm, we extracted proteins from maturing TPC7 rice seeds with native or semi-native buffer containing 0, 0.2, 0.5, 1.0 and 2.0 % Triton X-100 in a sequential extraction method. We then performed native PAGE using these native and semi-native extracts. In native extracts without Triton X-100, major bands of approximately 75 kDa were detected as well as smear signals larger than 75 kDa and relatively strong signals larger than 250 kDa (Fig. 7A). In semi-native extract containing 0.2 and 0.5 % Triton X-100, smear signals larger than 75 kDa and relatively strong signals larger than 250 kDa were observed, but 75 kDa band was not (Fig. 7a). These results suggest that 75 kDa aggregates are fully extracted by native buffer and residual TPC7 proteins extracted by semi-native buffer form aggregates larger than 75 kDa. With increasing concentrations of Triton X-100, the densities of smear bands larger than 250 kDa decreased, and these bands were not present in proteins extracted with buffer containing 2.0 % Triton X-100 (Fig. 7a). No bands smaller than 75 kDa were detected. These results indicate that TPC7 forms aggregates of approximately 75 kDa and aggregates larger than 75 kDa, in which the largest aggregates are more than 250 kDa, in the endosperm.
Native PAGE and gel filtration analysis of native extract of TPC7 rice seeds. a Native PAGE of native and semi-native extracts of maturing seeds of TPC7 rice. Proteins were sequentially extracted with native buffer and semi-native buffer with 0, 0.2, 0.5, 1.0 and 2.0 % Triton X-100. Proteins were separated by 12.5 and 7.5 % native PAGE. Immunodetection was performed with anti-TPC7 antibody. b Gel filtration analysis of native extracts without Triton X-100 and semi-native extracts containing 0.2 % Triton X-100 of maturing seeds of TPC7 rice. Each fraction was analyzed by immunoblot analysis using antibodies raised against TPC7, OsBiP1, OsBiP4&5, OsPDIL1;1, OsPDIL2;3 and calnexin (CNX). The bars on the panels show the molecular weight markers (669, 440, 150 kDa)
To investigate the state of TPC7 aggregates larger than 250 kDa in detail, we subjected native extracts without Triton X-100 and semi-native extracts containing 0.2 % Triton-X to gel filtration analysis. The fractions were analyzed by Western blotting using antibodies raised against TPC7, OsBiP1, OsBiP4&5, OsPDIL1;1, OsPDIL2;3 and CNX. In native extracts without Triton X-100, strong TPC7 signals were detected in fractions larger than 669 kDa (Fig. 7b). Weak TPC7 signals were also detected in fractions smaller than 669 kDa. The 75 kDa aggregate signal was stronger than that of the larger aggregates in native PAGE (Fig. 7a), indicating that aggregates larger than 669 kDa may be dissociated after electrophoresis. OsBiP1, OsBiP4&5, OsPDIL1;1, OsPDIL2;3 and CNX were also present in fractions larger than 669 kDa, although stronger OsBiP1, OsBiP4&5, OsPDIL1;1 and OsPDIL2;3 signals were detected in fractions smaller than 669 kDa (Fig. 7b). In semi-native extract containing 0.2 % Triton X-100, the strong TPC7 signals were detected in the fractions larger than 669 kDa. Weak signals of OsBiP1, OsBiP4&5, OsPDIL1;1, OsPDIL2;3 and CNX were also present in fractions larger than 669 kDa (Fig. 7b). These results demonstrate that TPC7 forms huge aggregates larger than 669 kDa in maturing seed endosperm. Some proteins involved in protein folding, such as OsBiP1, OsBiP4&5, OsPDIL1;1, OsPDIL2;3 and CNX, may be included in the huge TPC7 aggregates.
Discussion
In the endosperm of cereal grains, prolamins are generally deposited into ER-derived PBs. Prolamins form aggregates, which involves specific protein–protein interactions among the prolamins with the help of chaperone binding proteins and the formation of disulfide bridges (Kumamaru et al. 2007; Vitale and Ceriotti 2004). In maize ER-derived PBs, α-zeins fill most of the core and are surrounded by a thin layer of β- and γ-zeins (Lending and Larkins 1989). During rice PB-I formation, 10 kDa prolamin initially accumulates and forms a core inside PB-I, and other Cys-rich prolamins are subsequently synthesized to cover the core 10 kDa prolamin (Saito et al. 2012). Maize prolamin γ-zein and its N-terminal domain (Zera) are oligomerized and form PBs not only in the original host (maize seeds) but also in a broad spectrum of eukaryotic cells (Geli et al. 1994; Torrent et al. 2009a, b). Protein assembly is responsible for their retention in the ER and for the formation of ER-derived PBs (Kawagoe et al. 2005; Müntz 1998; Pompa and Vitale 2006).
Many allergens form homodimers or oligomers, e.g., Phl p 7 from Timothy grass pollen, Equ c 1 from horse and Ara h 1 from peanut (Lascombe et al. 2000; Maleki et al. 2000; Verdino et al. 2002). Bet v 1-dimers have been observed in pollen extracts and in recombinant allergen preparations (Ferreira et al. 1998; Friedl-Hajek et al. 1999; Kaul et al. 2003; Krebitz et al. 2000; Vieths et al. 1998). A Bet v 1 trimer forms high molecular weight aggregates (Campana et al. 2011). In the current study, we demonstrated that TPC7 protein formed huge aggregates larger than 669 kDa in maturing rice seeds (Fig. 7). OsBiP1, OsBiP4&5, OsPDIL1:1, OsPDIL2;3 and CNX were also detected in the same fractions as the huge TPC7 aggregates (Fig. 7b). In addition, OsBiP1, OsBiP4&5, OsPDIL1:1 and OsPDIL2;3 were observed within the TPC7 body, and CNX was observed around the periphery of TPC7 bodies (Fig. 6). This result suggests that TPC7 forms huge aggregates in maturing seeds via self-interaction of TPC7 and that some chaperone proteins and enzymes involved in protein folding co-localize with TPC7. The signals of huge aggregates larger than 669 kDa decreased after electrophoresis, and they were not detected in the extracts containing high concentrations of detergent (Fig. 7a). This result indicates that intermolecular associations of the huge aggregates are relatively loose. In addition to huge aggregates, 75 kDa aggregates that included TPC7 were observed in the native extracts lacking detergent (Fig. 7a). Smear bands larger than 75 kDa were also observed in native extracts and semi-native extracts with low concentrations of detergent (Fig. 7a). While the smear bands of high-molecular weight decreased with increasing concentrations of detergent, the bands around 75 kDa were detected even in extracts containing 2 % Triton X-100 (Fig. 7a). These results indicate that TPC7 forms approximately 75 kDa aggregates with strong intermolecular associations, while TPC7 also forms aggregates larger than 75 kDa with relatively weak intermolecular associations.
Around the surfaces of TPC7 bodies, CNX signals were detected, but OsTIP3 signals were not (Fig. 6), demonstrating that the membrane of the TPC7 body is derived from the ER. Wang et al. (2013) showed that most glycans linked to TPC7 are high mannose-type glycans, suggesting that most of the TPC7 protein is retained in the ER and further deposited into the ER. The retention of TPC7 in the ER is thought to be caused by KDEL and the formation of huge aggregates. Notably, even TPC7 without KDEL induced TPC7 body formation in rice endosperm, although its size was smaller than that of TPC7 containing KDEL (Wang et al. 2013), suggesting that KDEL is not necessary for the formation of TPC7 bodies, while it affects the formation of TPC7 bodies to some extent. In addition to KDEL, TPC7 aggregates are thought to be responsible for ER retention and the formation of TPC7 bodies. These results suggest the following model regarding the formation mechanism of TPC7 bodies in the endosperm (Fig. 8). After TPC7 proteins are translated on the ER as secretory proteins, a portion of these proteins are glycosylated in the ER lumen. Then, TPC7 is folded with the help of CNX and BiP, and a portion of TPC7 proteins are dimerized via intermolecular disulfide bonds catalyzed by PDIL. Monomers and dimers of TPC7 form 75 kDa aggregates via relatively strong intermolecular associations. The 75 kDa aggregates further form huge aggregates larger than 669 kDa with weak intermolecular associations, which causes ER retention of TPC7 protein. Accumulation of substantial levels of TPC7 protein in the ER induces the formation of huge ER-derived PBs. Some of the proteins involved in protein folding may be included in the huge aggregate and enclosed in the huge PBs.
In the present study, Bet v 1 and TPC9, the analog proteins of TPC7, were expressed in rice endosperm. Although huge PBs were observed in transgenic rice seeds expressing Bet v 1 and TPC9, they were fewer and of reduced size compared to those derived from TPC7 (Fig. 2). TPC7 protein accumulated at levels up to 550 μg/seed, while Bet v 1 and TPC9 accumulated much lower levels (Table 1). Despite having a greater than 90 % amino acid sequence homology to TPC7, Bet v 1 and TPC9 failed to induce the formation of numerous huge PBs, and these proteins accumulated at much lower levels than TPC7. On the other hand, the mRNA levels of TPC7, Bet v 1 and TPC9 in each seed were almost identical (Fig. 3). These results suggest that the behaviors of TPC7, Bet v 1 and TPC9 after transcription, such as their structural status, interactions with molecular chaperones, transport efficiency to huge PBs and degradation characteristics, are responsible for the different levels of protein accumulation and differences in the formation of huge PBs. Among the behaviors of proteins after transcription, we thought that disulfide bond formation might be an important factor for inducing huge PB formation. Disulfide bond formation among prolamins is responsible for prolamin accumulation and PB formation (Kawagoe et al. 2005; Müntz 1998; Pompa et al. 2006). While mutated TPC7, in which cysteine was changed to alanine in the TPC7 amino acid sequence, generates TPC7 bodies, the morphology of these PBs is distorted and their size is reduced, suggesting that the intermolecular disulfide bonds of TPC7 are important for the formation TPC7 bodies (Wang et al. 2013). TPC7, Bet v 1 and TPC9 each possess one cysteine residue and form intermolecular disulfide bonds (Fig. 4). The relative intensity of the dimer bands to the monomer bands of TPC7 were higher than those of Bet v 1 and TPC9 (Fig. 4), indicating that less dimerization is related to the reduced formation of huge PBs in Bet v 1 and TPC9. We also performed domain swapping analysis between TPC7 and Bet v 1 to investigate which domains are responsible for the formation of huge PBs (Fig. 5). No huge PBs were observed in T-B rice, while numerous huge PBs were observed in B-T rice. The number and size of the huge PBs were larger than those in Bet v 1 rice (Fig. 5e, f). These results indicate that the latter half of TPC7 between positions 32 and 160 is important for the formation of numerous huge PBs. The latter halves of TPC7 and Bet v 1 are highly homologous and include only one mismatched amino acid sequence with different chemical properties, threonine (TPC7)/lysine (Bet v 1 and TPC9) at position 81 (Fig. 1a). This single amino acid difference may significantly affect the conformational status of TPC7, Bet v 1 and TPC9. Position 118 of Bet v 1 and TPC9 was predicted to be a phosphorylation site, while that of TPC7 was not (Fig. 1b). Phosphorylation of proteins significantly affects protein conformational changes. For example, aggregation of some neuroproteins is promoted/disrupted by phosphorylation or dephosphorylation (Liu et al. 2007; Sato et al. 2013; Schneider et al. 1999). Dephosphorylation at position 118 in TPC7 may be important for the formation of TPC7 aggregates and for subsequent huge PB formation. Further study is needed to characterize the key properties of TPC7 bodies. Proteome analysis of TPC7 body isolated from TPC7 seeds might be helpful to investigate proteins involved in formation of TPC7 body, and construction of TPC7 deletion series may be effective to identify the important domains of TPC7.
In the endosperm of TPC7 rice, numerous huge TPC7 bodies were observed, and TPC7 bodies filled some of the subaleurone layer cells. The accumulation level of TPC7 was 550 μg/seed, which amounted to 23 % of total seed proteins (Table 1). We previously reported that the level of TPC7 is approximately 200 μg/seed (Wang et al. 2013). Since the rice was grown using nitrogen-containing fertilizer in the present experiment, both the total seed protein level and the TPC7 protein level were higher than those observed in the previous experiment. The total seed protein content of TPC7 rice was much lower than that of NT rice, and the TPC7 rice seeds were shrunken and smaller than NT rice seeds (Table 1; Fig. 1c). The high expression levels of TPC7 as secretory protein in rice endosperm elicited ER stress (Wang et al. 2013), leading to the distortion of seed storage protein trafficking and the reduction of total protein contents. Repressing ER-stress might increase total protein of TPC7 seeds, leading to increase of yield of TPC7 protein per kilogram of host tissue. Therapeutic and industrially important proteins have been produced in plants, with varying levels of expression. A few proteins and peptides, such as Zera, hydrophobins and elastin-like polypeptides, are utilized as PB-inducing fusions for high-level production and purification of recombinant proteins in plants (Conley et al. 2011). Thus, the TPC7 body may be a promising reservoir for high-level production and sequestering storage of recombinant proteins.
References
Campana R, Vrtala S, Maderegger B, Dall’Antonia Y, Zafred D, Blatt K, Herrmann H, Focke-Tejkl M, Swoboda I, Scheiblhofer S, Gieras A, Neubauer A, Keller W, Valent P, Thalhamer J, Spitzauer S, Valenta R (2011) Altered IgE epitope presentation: a model for hypoallergenic activity revealed for Bet v 1 trimer. Mol Immunol 48:431–441
Conley AJ, Joensuu JJ, Richman A, Menassa R (2011) Protein body-inducing fusions for high-level production and purification of recombinant proteins in plants. Plant Biotechnol J 9:419–433
Ferreira F, Ebner C, Kramer B, Casari G, Briza P, Kungl AJ, Grimm R, Jahn-Schmid B, Breiteneder H, Kraft D, Breitenbach M, Rheinberger HJ, Scheiner O (1998) Modulation of IgE reactivity of allergens by site-directed mutagenesis: potential use of hypoallergenic variants for immunotherapy. FASEB J 12:231–242
Friedl-Hajek R, Radauer C, O’Riordain G, Hoffmann-Sommergruber K, Leberl K, Scheiner O, Breiteneder H (1999) New Bet v 1 isoforms including a naturally occurring truncated form of the protein derived from Austrian birch pollen. Mol Immunol 36:639–645
Geli MI, Torrent M, Ludevid D (1994) Two structural domains mediate two sequential events in [gamma]-Zein Targeting: protein endoplasmic reticulum retention and protein body formation. Plant Cell 6:1911–1922
Kaul S, Scheurer S, Danz N, Schicktanz S, Vieths S, Hoffmann A (2003) Monoclonal IgE antibodies against birch pollen allergens: novel tools for biological characterization and standardization of allergens. J Allergy Clin Immunol 111:1262–1268
Kawagoe Y, Suzuki K, Tasaki M, Yasuda H, Akagi K, Katoh E, Nishizawa NK, Ogawa M, Takaiwa F (2005) The critical role of disulfide bond formation in protein sorting in the endosperm of rice. Plant Cell 17:1141–1153
Krebitz M, Wiedermann U, Essl D, Steinkellner H, Wagner B, Turpen TH, Ebner C, Scheiner O, Breiteneder H (2000) Rapid production of the major birch pollen allergen Bet v 1 in Nicotiana benthamiana plants and its immunological in vitro and in vivo characterization. FASEB J 14:1279–1288
Kudo K, Ohta M, Yang L, Wakasa Y, Takahashi S, Takaiwa F (2013) ER stress response induced by the production of human IL-7 in rice endosperm cells. Plant Mol Biol 81:461–475
Kumamaru T, Ogawa M, Satoh H, Okita TW (2007) Protein body biogenesis in cereal endosperms. In: Olsen OA (ed) Endosperm. Springer, Berlin, pp 141–158
Lascombe MB, Grégoire C, Poncet P, Tavares GA, Rosinski-Chupin I, Rabillon J, Goubran-Botros H, Mazié JC, David B, Alzari PM (2000) Crystal structure of the allergen Equ c 1. A dimeric lipocalin with restricted IgE-reactive epitopes. J Biol Chem 275:21572–21577
Lending CR, Larkins BA (1989) Changes in the zein composition of protein bodies during maize endosperm development. Plant Cell 1:1011–1023
Liu F, Li B, Tung EJ, Grundke-Iqbal I, Iqbal K, Gong CX (2007) Site-specific effects of tau phosphorylation on its microtubule assembly activity and self-aggregation. Eur J Neurosci 26:3429–3436
Maleki SJ, Kopper RA, Shin DS, Park CW, Compadre CM, Sampson H, Burks AW, Bannon GA (2000) Structure of the major peanut allergen Ara h 1 may protect IgE-binding epitopes from degradation. J Immunol 164:5844–5849
Müntz K (1998) Deposition of storage proteins. Plant Mol Biol 38:77–99
Nicholson L, Gonzalez-Melendi P, van Dolleweerd C, Tuck H, Perrin Y, Ma JK, Fischer R, Christou P, Stoger E (2005) A recombinant multimeric immunoglobulin expressed in rice shows assembly-dependent subcellular localization in endosperm cells. Plant Biotechnol J 3:115–127
Oono Y, Wakasa Y, Hirose S, Yang L, Sakuta C, Takaiwa F (2010) Analysis of ER stress in developing rice endosperm accumulating beta-amyloid peptide. Plant Biotechnol J 8:691–718
Pompa A, Vitale A (2006) Retention of a bean phaseolin/maize gamma-Zein fusion in the endoplasmic reticulum depends on disulfide bond formation. Plant Cell 18:2608–2621
Qu LQ, Takaiwa F (2004) Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol J 2:113–125
Saito Y, Shigemitsu T, Yamasaki R, Sasou A, Goto F, Kishida K, Kuroda M, Tanaka K, Morita S, Satoh S, Masumura T (2012) Formation mechanism of the internal structure of type I protein bodies in rice endosperm: relationship between the localization of prolamin species and the expression of individual genes. Plant J 70:1043–1055
Sato H, Kato T, Arawaka S (2013) The role of Ser129 phosphorylation of α-synuclein in neurodegeneration of Parkinson’s disease: a review of in vivo models. Rev Neurosci 24:115–123
Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow EM (1999) Phosphorylation that detaches tau protein from microtubules (Ser262, Ser214) also protects it against aggregation into Alzheimer paired helical filaments. Biochemistry 38:3549–3558
Simmonds DH (1978) Structure, composition and biochemistry of cereal grains. In: Pomeranz Y (ed) Cereals ‘78: better nutrition for the world’s millions. Am Assoc Cereal Chem, St Paul, pp 105–137
Suzuki K, Kaminuma O, Yang L, Takai T, Mori A, Umezu-Goto M, Ohtomo T, Ohmachi Y, Noda Y, Hirose S, Okumura K, Ogawa H, Takada K, Hirasawa M, Hiroi T, Takaiwa F (2011) Prevention of allergic asthma by vaccination with transgenic rice seed expressing mite allergen: induction of allergen-specific oral tolerance without bystander suppression. Plant Biotechnol J 9:982–990
Takaiwa F, Ogawa M, Okita TW (1999) Rice glutelins. In: Shewry PR, Casey R (eds) Seed proteins. Kluwer, Dordrecht, pp 401–425
Takaiwa F, Yang L, Yasuda H (2008) Health-promoting transgenic rice: application of rice seeds as a direct delivery system for bioactive peptides in human health. In: Hirano HY (ed) Biotechnology in agriculture and forestry. Springer, Heidelberg, pp 357–373
Takaiwa F, Hirose S, Takagi H, Yang L, Wakasa Y (2009) Deposition of a recombinant peptide in ER-derived protein bodies by retention with cysteine-rich prolamins in transgenic rice seed. Planta 229:1147–1158
Torrent M, Llompart B, Lasserre-Ramassamy S, Llop-Tous I, Bastida M, Marzabal P, Westerholm-Parvinen A, Saloheimo M, Heifetz PB, Ludevid MD (2009a) Eukaryotic protein production in designed storage organelles. BMC Biol 7:5
Torrent M, Llop-Tous I, Ludevid MD (2009b) Protein body induction: a new tool to produce and recover recombinant proteins in plants. Methods Mol Biol 483:193–208
Torres E, Gonzalez-Melendi P, Stöger E, Shaw P, Twyman RM, Nicholson L, Vaquero C, Fischer R, Christou P, Perrin Y (2001) Native and artificial reticuloplasmins co-accumulate in distinct domains of the endoplasmic reticulum and in post-endoplasmic reticulum compartments. Plant Physiol 127:1212–1223
Verdino P, Westritschnig K, Valenta R, Keller W (2002) The crossreactive calcium-binding pollen allergen, Phl p 7, reveals a novel dimer assembly. EMBO J 21:5007–5016
Vieths S, Frank E, Scheurer S, Meyer HE, Hrazdina G, Haustein D (1998) Characterization of a new IgE-binding 35-kDa protein from birch pollen with cross-reacting homologues in various plant foods. Scand J Immunol 47:263–272
Vitale A, Ceriotti A (2004) Protein quality control mechanisms and protein storage in the endoplasmic reticulum. A conflict of interests? Plant Physiol 136:3420–3426
Wakasa Y, Yang L, Hirose S, Takaiwa F (2009) Expression of unprocessed glutelin precursor alters polymerization without affecting trafficking and accumulation. J Exp Bot 60:3503–3511
Wakasa Y, Yasuda H, Oono Y, Kawakatsu T, Hirose S, Takahashi H, Hayashi S, Yang L, Takaiwa F (2011) Expression of ER quality control-related genes in response to changes in BiP1 levels in developing rice endosperm. Plant J 65:675–689
Wakasa Y, Hayashi S, Takaiwa F (2012) Expression of OsBiP4 and OsBiP5 is highly correlated with the endoplasmic reticulum stress response in rice. Planta 236:1519–1527
Wakasa Y, Takagi H, Hirose S, Yang L, Saeki M, Nishimura T, Kaminuma O, Hiroi T, Takaiwa F (2013) Oral immunotherapy with transgenic rice seed containing destructed Japanese cedar pollen allergens, Cry j 1 and Cry j 2, against Japanese cedar pollinosis. Plant Biotechnol J 11:66–67
Wallner M, Stöcklinger A, Thalhamer T, Bohle B, Vogel L, Briza P, Breiteneder H, Vieths S, Hartl A, Mari A, Ebner C, Lackner P, Hammerl P, Thalhamer J, Ferreira F (2007) Allergy multivaccines created by DNA shuffling of tree pollen allergens. Allergy Clin Immunol 120:374–380
Wang S, Takahashi H, Kajiura H, Kawakatsu T, Fujiyama K, Takaiwa F (2013) Transgenic rice seeds accumulating recombinant hypoallergenic birch pollen allergen Bet v 1 generate giant protein bodies. Plant Cell Physiol 54:917–933
Yang L, Hirose S, Suzuki K, Hiroi T, Takaiwa F (2012a) Expression of hypoallergenic Der f 2 derivatives with altered intramolecular disulphide bonds induces the formation of novel ER-derived protein bodies in transgenic rice seeds. J Exp Bot 63:2947–2959
Yang L, Hirose S, Takahashi H, Kawakatsu T, Takaiwa F (2012b) Recombinant protein yield is enhanced by specific suppression of endogenous seed proteins at the same deposit site in transgenic rice seed. Plant Biotechnol J 10:1035–1045
Yasuda H, Tada Y, Hayashi Y, Jomori T, Takaiwa F (2005) Expression of the small peptide GLP-1 in transgenic plants. Transgenic Res 14:677–684
Yasuda H, Hirose S, Kawakatsu T, Wakasa Y, Takaiwa F (2009) Overexpression of BiP has inhibitory effects on the accumulation of seed storage proteins in endosperm cells of rice. Plant Cell Physiol 50:1532–1543
Acknowledgments
The authors thank Ms. M. Utsuno, Ms. Y. Ikemoto, Ms. H. Yajima and Ms. Y. Suzuki for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogo, Y., Takahashi, H., Wang, S. et al. Generation mechanism of novel, huge protein bodies containing wild type or hypoallergenic derivatives of birch pollen allergen Bet v 1 in rice endosperm. Plant Mol Biol 86, 111–123 (2014). https://doi.org/10.1007/s11103-014-0216-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-014-0216-7