Abstract
Anthocyanins are a family of water-soluble vacuolar pigments present in almost all flowering plants. The chemistry, biosynthesis and functions of these flavonoids have been intensively studied, in part due to their benefit for human health. Given that they are efficient antioxidants, intense research has been devoted to studying their possible roles against damage caused by reactive oxygen species (ROS). However, the redox homeostasis established between antioxidants and ROS is important for plant growth and development. On the one hand, high levels of ROS can damage DNA, proteins, and lipids, on the other, they are also required for cell signaling, plant development and stress responses. Thus, a balance is needed in which antioxidants can remove excessive ROS, while not precluding ROS from triggering important cellular signaling cascades. In this article, we discuss how anthocyanins and ROS interact and how a deeper understanding of the balance between them could help improve plant productivity, nutritional value, and resistance to stress, while simultaneously maintaining proper cellular function and plant growth.
Key message
The balance between anthocyanins and ROS could be manipulated to improve plant productivity, nutritional value, and resistance to stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anthocyanins are polyphenols of the flavonoid class of specialized plant metabolites (Smeriglio et al. 2016). They consist of anthocyanidin skeletons with carbohydrate side chains. It is currently unclear which selective pressures have caused anthocyanins to become almost ubiquitous among plants (Wen et al. 2020). Indeed, the presence of red–violet pigments in all land plants is considered a prime example of convergent evolution (Piatkowski et al. 2020). The anthocyanin biosynthetic pathway did not evolve until the emergence of seed plants, while the liverworts, mosses, lycophytes, and ferns only have “anthocyanin-like” red–violet pigments (Piatkowski et al. 2020). Specifically, the emergence of the flavanone-3-hydroxylase enzyme (F3H) was the key event in the production of the flavonoid backbone and the enormous variety of flavonoids and anthocyanins in seed plants, possibly originating from an ancestor transitional form of a flavone synthase I (FNS I), which is widely distributed in more basal land plants (Li et al. 2020; Piatkowski et al. 2020). In terms of function, the roles of anthocyanin in plants as a photo-attenuator (Zheng et al. 2021) metal–chelator (Macar et al. 2020; Xie et al. 2018) and antioxidant (Trojak and Skowron 2017), have been extensively demonstrated. Another factor that supports the importance of these pigments to the plant is the considerable energetic cost of their biosynthesis, with each phenol ring having an ΔrH° ≅ 165 kJ (Parks et al. 1954). The anthocyanin molecule has a C15 (C6-C3-C6) structure, where each C6 is a phenol ring. Thus, each anthocyanin molecule requires an investment of more than 330 kJ to construct.
There is growing interest in elevating the levels of anthocyanins in edible plants for dietary purposes (Butelli et al. 2008; Gonzali and Perata 2020), mainly due to their role as scavengers of reactive oxygen species (ROS). ROS can trigger degradation of proteins, lipid peroxidation and DNA damage (Smirnoff and Arnaud 2019), often leading to cell senescence and death (Cheung and Vousden 2022). On the other hand, an equilibrium between antioxidants and ROS is essential for plant development despite their opposite functions (Schippers et al. 2016). In this article, we provide a brief overview of anthocyanins and ROS and discuss how knowledge about the balance between the levels of these two classes of chemicals can be harnessed for agricultural benefits.
Anthocyanins
The term “anthocyanins” means “blue flowers”, however they can be found in other plant organs and display shades of pink, red, purple and blue depending on the chemical structure, intravacuolar pH, co-pigments and interacting metal ions (Kallam et al. 2017). Here, we summarize the current knowledge of anthocyanin chemistry, their biosynthesis and metabolism, and finally their function as antioxidants.
Anthocyanins are water-soluble flavonoids composed of three elements: an anthocyanidin backbone decorated with sugar moieties and, occasionally, acyl-conjugates (Sasaki et al. 2014; Stommel et al. 2009). Basically, an anthocyanidin is a structure composed of two benzyl rings connected by a heterocyclic ring. Anthocyanidin glycosylation enhances water solubility and acylation increases stability (Mazza et al. 2004; Smeriglio et al. 2016). Changes in vacuolar pH alter the color of anthocyanins due to variation in the resonance structure produced by electronic transitions (Smeriglio et al. 2016; Wrolstad et al. 2005). Resonance also helps explain the redox properties of anthocyanins, which are relevant for their antioxidant capacity (Smeriglio et al. 2016). The biosynthesis of anthocyanins is increased by environmental stresses such as high irradiance, cold, drought and nutrient deficiency, especially nitrogen and phosphorus (Chalker-Scott 1999; Nakabayashi et al. 2014; Oren-Shamir 2009). Anthocyanin degradation seems to also be stimulated under stress, and at least under drought, increases have been reported in the activity of vacuolar β-glucosidase, the key enzyme involved in the first step of anthocyanin degradation (Wang et al. 2011). By the same token, stress-responsive hormones, like abscisic acid (ABA) and jasmonates (JA), also induce anthocyanins accumulation (Cotado et al. 2018; Li et al. 2019a, b; Zhou et al. 2009). A convergence point between abiotic stress and anthocyanins biosynthesis is that both are activated by ROS accumulation (Nakabayashi et al. 2014). Despite these findings, the cost-benefit ratio to produce these pigments remains unclear (Lo Piccolo et al. 2020). Interestingly, in the Caryophyllales order anthocyanins are absent, and the alkaloid betacyanins are the replacement pigment taking over their function (Jain and Gould 2015; Stafford 1994). Their accumulation also depends on environmental factors but anthocyanins and betacyanins never occur together in the same plant and little is known about the molecular basis of this mutual exclusion (Sakuta 2014). Phylogenetic analyses suggests that this is a legacy of the single and early origin of betacyanin pigmentation in the evolutionary history of the order, which was lost in the anthocyanin lineages (Brockington et al. 2015; Polturak and Aharoni 2018).
The chemical structure of flavonoids has a direct impact on their ability to scavenge different types of ROS (Dimitrić Marković et al. 2014). Zhang et al. (2015) demonstrated that the presence of OH groups in the B-ring influences the antioxidant activity of the molecule (Fig. 1). Glycosylation decreases the scavenger activity compared to the original aglycones structures, thus minimizing their proton donating and metal chelating abilities (Zhao et al. 2014). Other B-ring substitutes can alter the antioxidant activity. Among the six types of natural anthocyanidins, pelargonidin has four OH groups and showed the lowest antioxidant activity whereas delphinidin, which has six OH groups, showed the highest activity (Rahman et al. 2006).
Molecular structure of anthocyanins. The C6-C3-C6 show seven substitution positions that are enumerated (R3, R4, R5, R6, R7, R3’, R4’ and R5’). The color and antioxidant capacity of anthocyanins depend directly on these positions. *Coloration derived from the flowers that contain the compound, as Catharanthus roseus, Primula rosea and Petunia sp
Biochemically, anthocyanins have been described as compounds that prevent the oxidation by scavenging free radicals and reducing the oxidative stress, given that anthocyanins act as H-atom donor or as single electron transfer (Einbond et al. 2004). Also, the main structure of anthocyanins, the anthocyanin chalcones and the quinoidal base with double bonds conjugated to the keto group are efficient antioxidants (Smeriglio et al. 2016). The antioxidant capacity of anthocyanins has already been demonstrated in planta. In Arabidopsis, flavonol and anthocyanin over-accumulators, such as transgenic lines overexpressing ANTHOCYANIN PIGMENT 1 (PAP1/MYB75) or PRODUCTION OF FLAVONOL GLYCOSIDES 1 (MYB12/PFG1) showed higher free radical scavenger activities than the wild type (Nakabayashi et al. 2014). Corroborating this increased antioxidant capacity, these cyanic lines showed three-fold higher survival rates in methyl viologen-induced oxidative stress compared to wild-type plants (Nakabayashi et al. 2014). On the flip side, Arabidopsis mutants deficient in anthocyanins, such as transparent testa (tt), showed greater levels of endogenous ROS and lower antioxidant capacity under high-irradiance stress (Xu et al. 2017).
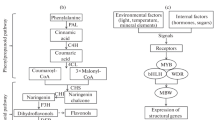
The anthocyanin biosynthesis pathway is highly conserved in plants (Chen et al. 2019) (Fig. 2). The expression of ‘early biosynthesis genes’ (EBGs: CHS, CHI, F3H and F3’H) are regulated directly by R2R3-MYB transcription factors while the ‘late biosynthesis genes’ (LBGs: DFR, LDOX, ANS and UF3GT) and membrane transporters (TT12 and ABCC) are controlled by the MBW (MYB-bHLH-WD40) transcriptional activation complex (Chaves-Silva et al. 2018; Chen et al. 2019). Many studies demonstrated that R2R3-MYB transcription factors play critical roles against biotic and abiotic stresses (Ai et al. 2018; He et al. 2020; Wei et al. 2017). A simplistic model is that while stresses disrupt ROS scavenging mechanisms and promotes cell damage by oxidative stress (Miller et al. 2010; Xu and Rothstein 2018), they also trigger anthocyanin biosynthesis for increased antioxidant protection (Chalker-Scott 1999). However, the regulation of the biosynthesis of anthocyanins and phenylpropanoids in general is complex and the effect of the MBW complex can be either positive (Broeckling et al. 2016; Li et al. 2020) or negative (Dong et al. 2020; Xiang et al. 2019), depending on the transcription factors participating in its composition (Chaves-Silva et al. 2018).
Reactive oxygen species (ROS)
Molecular oxygen (O2) is a stable molecule with two unpaired electrons with parallel spin in a degenerated π orbital. As a consequence, O2 accepts one electron at a time, which generates ROS (Gill and Tuteja 2010) (Table 1). Some organelles have high rates of oxygen reduction, making them highly vulnerable to oxidative stress, such as mitochondria due to respiration, chloroplasts due to photosynthesis, and peroxisomes and glyoxysomes due to photorespiration and fatty acid β-oxidation, respectively (Daiber and Münzel 2015; Zurbriggen et al. 2010). The various types of ROS are produced through different pathways in each organelle and their concentrations possibly help the cell to recognize regions of physiological disturbance during stressful conditions (Singh et al. 2019). In mitochondria, the first ROS produced is the radical superoxide (O2·−), which is quickly dismutated into O2 and hydrogen peroxide (H2O2) by the iron superoxide dismutase (Fe-SOD). In the chloroplasts, the first ROS produced is singlet oxygen (1O2) due to light excitation of chlorophylls, which generates lasting chlorophyll triplet excited states that are quenched by carotenoids (Cejudo et al. 2021). Additionally, in an aqueous environment such as that within the cell, the presence of redox metals such as iron (Fe2+) and copper (Cu2+) allows for the occurrence of the Fenton reaction, which generates hydroxyl radicals (·OH) (Winterbourn 1995).
Singlet oxygen (1O2) is a non-radical ROS that does not have an extra unpaired electron. Given that 1O2 has no spin constraints, it can interact almost indiscriminately with other biological molecules, including nucleic acids, proteins, and lipids (Dmitrieva et al. 2020; Yang et al. 2019). The first 1O2 targets are covalent double bonds, such as those in polyunsaturated fatty acids (e.g., oleic acid, linoleic acid) (Hajimohammadi et al. 2018) and some amino acids (e.g., histidine, cysteine, and methionine). Among the two types of lipid peroxidation (Girotti 1985), type I is triggered by free radicals with high redox potential, such as ·OH, whereas type II is mediated by 1O2 (nonradical) and is the main cause of photosynthetic tissue death due to photooxidative damage (Mor et al. 2014). Although 1O2 lifespan is very short (~ 40 ns), it is long enough to allow it to diffuse out of the chloroplast, interfere with signaling cascades in the cytosol and reach the cell wall, plasma membrane, and tonoplast (Fischer et al. 2013).
Superoxide (O2·−) and hydrogen peroxide (H2O2) are generated through common pathways, such as the electron transport chains of the mitochondrion and chloroplast, and the lipid β-oxidation pathway. Unlike singlet oxygen, superoxide is much less reactive and acts as both oxidant and reductant with great voracity for iron-sulphur clusters commonly present in enzymes (Fisher et al. 2016). It may also be the major prompter of tryptophan degradation pathway in peptides and proteins under stress (Carroll et al. 2018). Superoxide is, however, unlikely to act as a direct cellular signal because of its high instability and inability to diffuse through the membrane (Yang et al. 2019), even though there is convincing evidence that superoxide and other ROS could act as indirect signals by means of protein oxidative damage (Møller et al. 2011).
By contrast, hydrogen peroxide (H2O2) has particular properties for signaling, such as (i) a relatively long half-life (1 ms) (Mittler and Zilinskas 1991); (ii) the ability to diffuse through aquaporins embedded in membranes over great distances within the cell (Bienert et al. 2007); (iii) the ability of inhibiting important enzymes by oxidation of heme-thiol ligand to—SOH (Albertolle and Peter Guengerich 2018); (iv) mild and specific reactivity; and (v) the ability to be produced by several metabolic reactions via the reduction of other ROS, and to generate other ROS through the Fenton reaction (Sharma et al. 2012). H2O2 interacts with cysteine residues in proteins, which can lead to changes in the redox state of some polypeptides and trigger cell signaling cascades for cell proliferation, differentiation, and programmed cell death (Mittler 2017). The chloroplast is a major source of H2O2 in the cell and, therefore, H2O2 is an important signal during photosynthesis (Cejudo et al. 2021; Pilon et al. 2011).
Anthocyanins biosynthesis, transport and ROS production in different compartments of a standard plant cell. CHS chalcone synthase; CHI chalcone isomerase; F3H flavanone 3-dioxygenase; F3′5′H flavonoid 3′,5′ hydroxylase; F3′H flavanone 3′-hydroxylase; DFR dihydroflavonol 4-reductase; ANS anthocyanidin synthase; 3GT anthocyanidin 3-O-glucosyltransferase; GSTs glutathione S-transferases; MATE Multidrug and toxic compound extrusion; ABC ATP-binding cassette transporters; SOD superoxide dismutase
Interactions between anthocyanins and ROS
Numerous abiotic stresses and even regular metabolism can promote an increase in ROS production (Hasanuzzaman et al. 2020; Huang et al. 2019). The balance between ROS production and elimination must be strictly maintained in the cell at any moment to avoid lethal damage. One way to achieve this homeostasis is through the counterbalance with antioxidants, such as flavonoids, of which anthocyanins may constitute a significant portion. A key issue is the potential for physical interaction between anthocyanins and ROS, which has hitherto not been addressed in-depth (except for flavonoids in general) (Agati et al. 2020, 2021; Ferreyra et al. 2021). Indeed, there is evidence showing in vitro antioxidant activity as previously discussed, and usually the lack of flavonols biosynthesis is associated with ROS homeostasis imbalance (Chapman and Muday 2021; Muhlemann et al. 2018; Silva-Navas et al. 2016). Nevertheless, the mechanism by which anthocyanins, and not flavonols, interacts with ROS to promote their scavenging is poorly understood.
Anthocyanins are synthesized in the cytosol and transported for storage in the vacuole via three different pathways: glutathione S-transferases (GSTs) (Sun et al. 2012), multidrug and toxic compound extrusion (MATE) (Gomez et al. 2009) and ATP-binding cassette (ABC) transporters (Behrens et al. 2019). The following scenario is proposed. The cytosol flavonol pool may be regulated and replenished by anthocyanins stored in the vacuole, and when ROS levels increase, the flavonol pool is depleted. Vacuolar glucosidase then breaks down the anthocyanins (Wang et al. 2011) and aglycones are transferred from the vacuole to help regulate ROS homeostasis in the cytosol, either by immediate interaction as a H-donor and single electron transfer or by the activity of enzymes such as peroxidases (Liu et al. 2018; Oren-Shamir 2009; Tena et al., 2020). However, this is quite unclear, even though the association between flavonoids (flavonols and anthocyanins) content and ROS level is known to be antagonistic.
As discussed previously, flavonoid biosynthesis is energetically costly and finely controlled as a direct or indirect response to changes in ROS content. Therefore, the biotechnological manipulation of anthocyanin content in crops (e.g. Zhang et al. 2014) must be carefully balanced with the normal function of ROS signaling. To explore this notion, we briefly consider key points of intersection between anthocyanins and ROS in plant development, namely in the processes of seed germination, root development, pollen tube growth and senescence.
Seed germination
Germination starts with tissue hydration and an increase in respiration that leads to a rise in ROS production by respiration burst oxidative homologue (RBOH) proteins (Moles et al. 2019; Muller et al. 2009). The process elevates the content of several ROS, including H2O2, O2·− and ·OH·H2O2 disturbs seed dormancy in multiple ways, especially by enhancing ABA catabolism and promoting biosynthesis of gibberellins (GAs) (Liu et al. 2010; Wang et al. 2019). Interestingly, anthocyanins affect germination by counteracting ROS. Decreased anthocyanin synthesis led to a decrease in seed dormancy (McCarty et al. 1989; Zhao et al. 2019). On the other hand, stress that intensifies ROS production above a certain threshold that underpins breakage of seed dormancy, can impair germination and seedling establishment rather than favouring it (Jeevan Kumar et al. 2015). Differential dormancy exists in seeds with different colors in the same species (Zhao et al. 2019). The Leymus chinensis transcription factor LcbHLH92 is negatively correlated with anthocyanin/proanthocyanidin-specific genes, and its overexpression in Arabidopsis significantly inhibited the transcript levels of DFR and ANS genes in leaves and seeds, resulting in yellow seeds with higher germination rates compared to the wild-type brown seeds (Zhao et al. 2019).
Root development
Besides their obvious functions, roots are also a frontline stress-sensing organ that relays information to the shoot (Burko et al. 2020; Glanz-Idan et al. 2020). For instance, accumulation of heavy metals promotes radical superoxide (O2·−), hydroxyl radicals (·OH) and hydrogen peroxide (H2O2) production, leading to toxicity symptoms (Asati et al. 2016). In chickpea (Cicer arietinum) roots under hypoxia, anthocyanin biosynthesis was associated with an increase in ROS (Nazari et al. 2019). Nonetheless, ROS are also important for root development. The tomato (Solanum lycopersicum) mutant anthocyanin reduced (are) is a knockout for the FLAVONOID 3-HYDROXYLASE (F3H) gene. It accumulates low anthocyanin levels and concomitantly shows higher ROS content in roots and increased root hair formation compared to the wild type (Maloney et al. 2014). The influence of flavonols and anthocyanins on lateral root development may be facilitated by their inhibition of auxin transported since decreased flavonols content in the are mutant resulted in greater auxin flux away from the maturation zone (Maloney et al. 2014).
Another possibility is that plants may use flavonoids as carbon skeleton reserves, suggesting that phenolics in general, including anthocyanins, may be a carbon sink for excess photosynthetic carbon (Lo Piccolo et al. 2020). This is directly related with anthocyanin accumulation in leaves, but the role of anthocyanin accumulation in roots for energy storage is yet to be elucidated. In Arabidopsis, the anthocyanin precursor kaempferol acts by reducing superoxide and ROS concentration, i.e., as a negative regulator of ROS-stimulated lateral root emergence (Chapman and Muday 2021). Furthermore, flavonoids are related to root light avoidance in plants, that upon perceiving light, flavonols are directed to the lighted region in the elongation zone, which changes the internal gradient of superoxide and auxin in the copula, promoting root bending to avoid light (Silva-Navas et al. 2016).
Pollen tube growth
Sexual reproduction of plants requires long-distance growth of the pollen tube for fertilization of the female gametophyte. Recent reports suggest that this process is regulated by antagonic interactions between flavonols and ROS (O2·− and ·OH) and hydrogen peroxide (H2O2), whereby flavonols play an important role in keeping ROS homeostasis, preventing it from reaching the inhibitory level during heat stress (Chen et al. 2021; Muhlemann et al. 2018). Additionally, the anthocyanin reduced (are) tomato mutant has reduced flavonol accumulation in pollen grains and tubes, associated with impaired pollen viability, germination, tube growth, and tube integrity, resulting in reduced seed set (Muhlemann et al. 2018). These effects were reverted by antioxidant treatment and revealed that flavonol metabolites regulate plant sexual reproduction at both normal and elevated temperatures by maintaining ROS homeostasis.
Senescence
Senescence is a highly regulated ontogenetic process that leads to ROS accumulation and ultimately cell death (Thomas et al. 2003). In leaves, it is accompanied by degradation of chlorophylls and anthocyanin synthesis (Merzlyak et al. 2008; Vangelisti et al. 2020). During senescence, carbon export from source to sink is reduced, leading to hexose accumulation (Chen et al. 2022). This increase in sugar content induces the biosynthesis of phenylpropanoids, including anthocyanins (Nardozza et al. 2020). A proposed mechanism states anthocyanin biosynthesis represents an alternative carbon sink that limits sugar hyperaccumulation to avoid feedback inhibition of the photosynthetic machinery, including a reduction of ribulose-1,5-biphosphate carboxylase–oxygenase (Rubisco) expression (Lastdrager et al. 2014). The induction of anthocyanin accumulation by ROS signaling during senescence should also account for the negative effect of flavonoids on auxin transport. A number of synthetic compounds have been shown to block the process of auxin transport by inhibition of the auxin efflux carrier complex. These synthetic auxin transport inhibitors may act by mimicking endogenous molecules. Flavonoids have been suggested to be auxin transport inhibitors based on their in vitro activity (Brown et al., 2001). The hypothesis that flavonoids regulate auxin transport in vivo was tested in Arabidopsis by comparing wild-type (WT) and transparent testa 4 (tt4) plants with a mutation in the gene encoding the first enzyme in flavonoid biosynthesis, chalcone synthase. In a comparison between tt4 and WT plants, phenotypic differences were observed, including three times as many secondary inflorescence stems, reduced plant height, decreased stem diameter, and increased secondary root development. Growth of WT Arabidopsis plants on naringenin, a biosynthetic precursor to those flavonoids with auxin transport inhibitor activity in vitro, leads to a reduction in root growth and gravitropism, similar to the effects of synthetic auxin transport inhibitors. Analyses of auxin transport in the inflorescence and hypocotyl of independent tt4 alleles indicate that auxin transport is elevated in plants with a tt4 mutation. In hypocotyls of tt4, this elevated transport is reversed when flavonoids are synthesized by growth of plants on the flavonoid precursor, naringenin. These results are consistent with a role for flavonoids as endogenous regulators of auxin transport (Brown et al. 2001), since this hormone delays senescence by acting antagonistically to ethylene (Guo et al. 2021).
Concluding remarks
Anthocyanins play an important role in the induction of morpho-physiological modifications to facilitate plant adaptation to environmental stresses. High anthocyanin content provides protection against excessive irradiance by both attenuation of light absorption by the leaf and ROS-scavenging activity. However, the relative importance of anthocyanins in these responses remains controversial, mainly because of their vacuolar localization, which is spatially removed from the primary sites of ROS production in other organelles. It is also clear that stress conditions, either biotic or abiotic, lead to high ROS accumulation. ROS can react with and damage cellular components, but they also participate in cell signaling cascades. Many general stress response genes are regulated by signaling pathways that uses ROS as indirect messengers and different ROS formed in the same cell compartment can result in different intermediate signaling molecules that regulate specific gene clusters, leading to a direct response related to the stress condition at play.
It is essential, therefore, to assess the impact of anthocyanins (instead of flavonols in general) accumulation on abiotic stress resistances. In many instances, anthocyanin biosynthesis is induced as a response to attacks of pathogens (e.g., (Kangatharalingam et al. 2002) and insects (Li et al. 2019a, b; Schaefer and Rolshausen 2006). Even though a relationship between anthocyanin accumulation and abiotic stress appears to exist, the underlying mechanisms of this response and the impact on the redox status of the plant still need to be systematically explored.
The impact of anthocyanin overaccumulation on crop yield has not been fully established. Some attention and systematic analyses assessing how anthocyanins and other antioxidants may affect cell signaling and plant phenotypes will be helpful to enable breeding pipelines to create anthocyanin-enhanced crop varieties without adverse effects (e.g. low germination rates, undesirable developmental phenotypes, lower photosynthesis rates) (Sestari et al. 2014). In tomato, a promoter replacement line overexpressing ANTHOCYANIN1 (ANT1) showed reduced leaf thickness and impaired side branching, with a concomitant penalty in fruit yield (Cerqueira et al. 2022). A more in-depth assessment of the impact of anthocyanin accumulation on yield comparing cyanic and isogenic non-cyanic genotypes within a redox physiology perspective could be revealing both at the fundamental and applied levels and may lead to greater understanding as to the subtle interplay of this team of rivals.
Data availability
All data supporting the findings of the present study are available within the paper.
References
Agati G, Brunetti C, Fini A, Gori A, Guidi L, Landi M, Sebastiani F, Tattini M (2020) Are flavonoids effective antioxidants in plants? Twenty years of our investigation. Antioxidants 9:1–17. https://doi.org/10.3390/antiox9111098
Agati G, Guidi L, Landi M, Tattini M (2021) Anthocyanins in photoprotection: knowing the actors in play to solve this complex ecophysiological issue. New Phytol 232:2228–2235. https://doi.org/10.1111/nph.17648
Ai TN, Naing AH, Yun BW, Lim SH, Kim CK (2018) Overexpression of rsmyb1 enhances anthocyanin accumulation and heavy metal stress tolerance in transgenic petunia. Front Plant Sci 9:1–15. https://doi.org/10.3389/fpls.2018.01388
Albertolle ME, Peter Guengerich F (2018) The relationships between cytochromes P450 and H2O2: production, reaction, and inhibition. J Inorg Biochem 186:228–234. https://doi.org/10.1016/j.jinorgbio.2018.05.014
Asati A, Pichhode M, Nikhil K (2016) Effect of heavy metals on plant growth: an overview. Int J Appl Innov Eng Manag 5:56–66
Behrens CE, Smith KE, Iancu CV, Choe J, Dean JV (2019) Transport of anthocyanins and other flavonoids by the arabidopsis ATP-binding cassette transporter AtABCC2. Sci Rep 9:1–15. https://doi.org/10.1038/s41598-018-37504-8
Bienert GP, Møller ALB, Kristiansen KA, Schulz A, Møller IM, Schjoerring JK, Jahn TP (2007) Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J Biol Chem 282:1183–1192. https://doi.org/10.1074/jbc.M603761200
Brockington SF, Yang Y, Gandia-Herrero F, Covshoff S, Hibberd JM, Sage RF, Wong GKS, Moore MJ, Smith SA (2015) Lineage-specific gene radiations underlie the evolution of novel betalain pigmentation in Caryophyllales. New Phytol 207:1170–1180. https://doi.org/10.1111/nph.13441
Broeckling BE, Watson RA, Steinwand B, Bush DR (2016) Intronic sequence regulates sugar-dependent expression of Arabidopsis thaliana production of anthocyanin Pigment-1/MYB75. PLoS One 11:1–21. https://doi.org/10.1371/journal.pone.0156673
Brown DE (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126:524–535. https://doi.org/10.1104/pp.126.2.524
Burko Y, Gaillochet C, Seluzicki A, Chory J, Busch W (2020) Local HY5 activity mediates hypocotyl growth and shoot-to-root communication. Plant Commun 1:100078. https://doi.org/10.1016/j.xplc.2020.100078
Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EGWM, Hall RD, Bovy AG, Luo J, Martin C (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26:1301–1308. https://doi.org/10.1038/nbt.1506
Carroll L, Pattison DI, Davies JB, Anderson RF, Lopez-Alarcon C, Davies MJ (2018) Superoxide radicals react with peptide-derived tryptophan radicals with very high rate constants to give hydroperoxides as major products. Free Radic Biol Med 118:126–136. https://doi.org/10.1016/j.freeradbiomed.2018.02.033
Cejudo FJ, González MC, Pérez-Ruiz JM (2021) Redox regulation of chloroplast metabolism. Plant Physiol 186:9–21. https://doi.org/10.1093/plphys/kiaa062
Cerqueira JVA, Zhu F, Mendes K, Nunes-Nesi A, Martins SCV, Benedito V, Fernie AR, Zsögön A (2022) Promoter replacement of ANTHOCYANIN1 induces anthocyanin accumulation and triggers shade avoidance response through developmental, physiological and metabolic reprogramming in tomato. Hortic Res 10:uhac254. https://doi.org/10.1093/hr/uhac254
Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9. https://doi.org/10.1111/j.1751-1097.1999.tb01944.x
Chapman JM, Muday GK (2021) Flavonols modulate lateral root emergence by scavenging reactive oxygen species in Arabidopsis thaliana. J Biol Chem 296:100222. https://doi.org/10.1074/jbc.RA120.014543
Chaves-Silva S, Santos AL, dos, Chalfun-Júnior A, Zhao J, Peres LEP, Benedito VA (2018) Understanding the genetic regulation of anthocyanin biosynthesis in plants—tools for breeding purple varieties of fruits and vegetables. Phytochemistry 153:11–27. https://doi.org/10.1016/j.phytochem.2018.05.013
Chen L, Hu B, Qin Y, Hu G, Zhao J (2019) Advance of the negative regulation of anthocyanin biosynthesis by MYB transcription factors. Plant Physiol Biochem 136:178–187. https://doi.org/10.1016/j.plaphy.2019.01.024
Chen W, Xiao Z, Wang Y, Wang J, Zhai R, Lin-Wang K, Espley R, Ma F, Li P (2021) Competition between anthocyanin and kaempferol glycosides biosynthesis affects pollen tube growth and seed set of Malus. Hortic Res. https://doi.org/10.1038/s41438-021-00609-9
Chen, Yanbo, Wu P, Zhang C, Guo Y, Liao B, Chen, Yaping, Li M, Wu G, Wang Y, Jiang H (2022) Ectopic expression of JcCPL1, 2, and 4 affects epidermal cell differentiation, Anthocyanin biosynthesis and leaf senescence in Arabidopsis thaliana. Int J Mol Sci. https://doi.org/10.3390/ijms23041924
Cheung EC, Vousden KH (2022) The role of ROS in tumour development and progression. Nat Rev Cancer 22:280–297. https://doi.org/10.1038/s41568-021-00435-0
Cotado A, Müller M, Morales M, Munné-Bosch S (2018) Linking jasmonates with pigment accumulation and photoprotection in a high-mountain endemic plant, Saxifraga longifolia. Environ Exp Bot 154:56–65. https://doi.org/10.1016/j.envexpbot.2017.12.018
Daiber A, Münzel T (2015) Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxid Redox Signal 23:899–942. https://doi.org/10.1089/ars.2015.6376
Dimitrić Marković JM, Amić D, Lučić B, Marković ZS (2014) Oxidation of kaempferol and its iron(III) complex by DPPH radicals: spectroscopic and theoretical study. Monatshefte Chem 145:557–563. https://doi.org/10.1007/s00706-013-1135-z
Dmitrieva VA, Tyutereva EV, Voitsekhovskaja OV (2020) Singlet oxygen in plants: generation, detection, and signaling roles. Int J Mol Sci. https://doi.org/10.3390/ijms21093237
Dong NQ, Sun Y, Guo T, Shi CL, Zhang YM, Kan Y, Xiang YH, Zhang H, Yang YB, Li YC, Zhao HY, Yu HX, Lu ZQ, Wang Y, Ye WW, Shan JX, Lin HX (2020) UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat Commun 11:1–16. https://doi.org/10.1038/s41467-020-16403-5
Dumanović J, Nepovimova E, Natić M, Kuča K, Jaćević V (2021) The significance of reactive oxygen species and antioxidant defense system in plants: a concise overview. Plant Sci, Front. https://doi.org/10.3389/fpls.2020.552969
Einbond LS, Reynertson KA, Luo X-D, Basile MJ, Kennelly EJ (2004) Anthocyanin antioxidants from edible fruits. Food Chem 84:23–28. https://doi.org/10.1016/S0308-8146(03)00162-6
Ferreyra MLF, Serra P, Casati P (2021) Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol Plant 173:736–749. https://doi.org/10.1111/ppl.13543
Fischer BB, Hideg É, Krieger-Liszkay A (2013) Production, detection, and signaling of singlet oxygen in photosynthetic organisms. Antioxid Redox Signal 18:2145–2162. https://doi.org/10.1089/ars.2012.5124
Fisher B, Yarmolinsky D, Abdel-Ghany S, Pilon M, Pilon-Smits EA, Sagi M, Van Hoewyk D (2016) Superoxide generated from the glutathione-mediated reduction of selenite damages the iron-sulfur cluster of chloroplastic ferredoxin. Plant Physiol Biochem 106:228–235. https://doi.org/10.1016/j.plaphy.2016.05.004
Fujii J, Homma T, Osaki T (2022) Superoxide radicals in the execution of cell death. Antioxidants. https://doi.org/10.3390/antiox11030501
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930. https://doi.org/10.1016/j.plaphy.2010.08.016
Girotti AW (1985) Mechanisms of lipid peroxidation. J Free Radic Biol Med 1:87–95. https://doi.org/10.1016/0748-5514(85)90011-X
Glanz-Idan N, Tarkowski P, Turečková V, Wolf S (2020) Root-shoot communication in tomato plants: Cytokinin as a signal molecule modulating leaf photosynthetic activity. J Exp Bot 71:247–257. https://doi.org/10.1093/jxb/erz399
Gomez C, Terrier N, Torregrosa L, Vialet S, Alexandre FL, Verriès C, Souquet JM, Mazauric JP, Klein M, Cheynier V, Ageorges A (2009) Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters1[W][OA]. Plant Physiol 150:402–415. https://doi.org/10.1104/pp.109.135624
Gonzali S, Perata P (2020) Anthocyanins from purple tomatoes as novel antioxidants to promote human health. Antioxidants 9:1–17. https://doi.org/10.3390/antiox9101017
Guo Y, Ren G, Zhang K, Li Z, Miao Y, Guo H (2021) Leaf senescence: progression, regulation, and application. Mol Hortic 1:1–25. https://doi.org/10.1186/s43897-021-00006-9
Hajimohammadi M, Sereshk AV, Schwarzinger C, Knör G (2018) Suppressing effect of 2-nitrobenzaldehyde on singlet oxygen generation, fatty acid photooxidation, and dye-sensitizer degradation. Antioxidants. https://doi.org/10.3390/antiox7120194
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Al Mahmud J, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:1–52. https://doi.org/10.3390/antiox9080681
He J, Liu Yuqiang, Yuan D, Duan M, Liu Yanling, Shen Z, Yang C, Qiu Z, Liu D, Wen P, Huang J, Fan D, Xiao S, Xin Y, Chen X, Jiang L, Wang H, Yuan L, Wan J (2020) An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc Natl Acad Sci U S A 117:271–277. https://doi.org/10.1073/pnas.1902771116
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:1–10. https://doi.org/10.3389/fpls.2019.00800
Jain G, Gould KS (2015) Are betalain pigments the functional homologues of anthocyanins in plants? Environ Exp Bot 119:48–53. https://doi.org/10.1016/j.envexpbot.2015.06.002
Jeevan Kumar SP, Rajendra Prasad S, Banerjee R, Thammineni C (2015) Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann Bot 116:663–668. https://doi.org/10.1093/aob/mcv098
Kallam K, Appelhagen I, Luo J, Albert N, Zhang H, Deroles S, Hill L, Findlay K, Andersen ØM, Davies K, Martin C (2017) Aromatic decoration determines the formation of anthocyanic vacuolar inclusions. Curr Biol 27:945–957. https://doi.org/10.1016/j.cub.2017.02.027
Kangatharalingam N, Pierce ML, Bayles MB, Essenberg M (2002) Epidermal anthocyanin production as an indicator of bacterial blight resistance in cotton. Physiol Mol Plant Pathol 61:189–195. https://doi.org/10.1006/pmpp.2002.0434
Lastdrager J, Hanson J, Smeekens S (2014) Sugar signals and the control of plant growth and development. J Exp Bot 65:799–807. https://doi.org/10.1093/jxb/ert474
Li G, Zhao J, Qin B, Yin Y, An W, Mu Z, Cao Y (2019a) ABA mediates development-dependent anthocyanin biosynthesis and fruit coloration in Lycium plants. BMC Plant Biol 19:1–13. https://doi.org/10.1186/s12870-019-1931-7
Li X, Ouyang X, Zhang Z, He L, Wang Y, Li Y, Zhao J, Chen Z, Wang C, Ding L, Pei Y, Xiao Y (2019b) Over-expression of the red plant gene R1 enhances anthocyanin production and resistance to bollworm and spider mite in cotton. Mol Genet Genomics 294:469–478. https://doi.org/10.1007/s00438-018-1525-3
Li DD, Ni R, Wang PP, Zhang XS, Wang PY, Zhu TT, Sun CJ, Liu CJ, Lou HX, Cheng AX (2020) Molecular basis for chemical evolution of flavones to flavonols and anthocyanins in land plants. Plant Physiol 184:1731–1743. https://doi.org/10.1104/pp.20.01185
Liu Y, Ye N, Liu R, Chen M, Zhang J (2010) H2O2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. J Exp Bot 61:2979–2990. https://doi.org/10.1093/jxb/erq125
Liu Y, Tikunov Y, Schouten RE, Marcelis LFM, Visser RGF, Bovy A (2018) Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: a review. Front Chem. https://doi.org/10.3389/fchem.2018.00052
Lo Piccolo E, Landi M, Massai R, Remorini D, Guidi L (2020) Girled-induced anthocyanin accumulation in red-leafed Prunus cerasifera: effect on photosynthesis, photoprotection and sugar metabolism. Plant Sci 294:110456. https://doi.org/10.1016/j.plantsci.2020.110456
Macar O, Kalefetoğlu Macar T, Çavuşoğlu K, Yalçın E (2020) Protective effects of anthocyanin-rich bilberry (Vaccinium myrtillus L.) extract against copper(II) chloride toxicity. Environ Sci Pollut Res 27:1428–1435. https://doi.org/10.1007/s11356-019-06781-9
Maloney GS, DiNapoli KT, Muday GK (2014) The anthocyanin reduced tomato mutant demonstrates the role of flavonols in tomato lateral root and root hair development. Plant Physiol 166:614–631. https://doi.org/10.1104/pp.114.240507
Mazza G, Cacace JE, Kay CD (2004) Methods of analysis for anthocyanins in plants and biological fluids. J AOAC Int 87:129–145. https://doi.org/10.1093/jaoac/87.1.129
McCarty DR, Carson CB, Stinard PS, Robertson DS (1989) Molecular analysis of viviparous-1: an abscisic acid-insensitive mutant of Maize. Plant Cell 1:523. https://doi.org/10.2307/3868973
Merzlyak MN, Chivkunova OB, Solovchenko AE, Naqvi KR (2008) Light absorption by anthocyanins in juvenile, stressed, and senescing leaves. J Exp Bot 59:3903–3911. https://doi.org/10.1093/jxb/ern230
Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R (2010) Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ 33:453–467. https://doi.org/10.1111/j.1365-3040.2009.02041.x
Mittler R (2017) ROS are good. Trends Plant Sci 22:11–19. https://doi.org/10.1016/j.tplants.2016.08.002
Mittler R, Zilinskas BA (1991) Purification and characterization of pea cytosolic ascorbate peroxidase. Plant Physiol 97:962–968. https://doi.org/10.1104/pp.97.3.962
Moles TM, Guglielminetti L, Huarancca Reyes T (2019) Differential effects of sodium chloride on germination and post-germination stages of two tomato genotypes. Sci Hortic. https://doi.org/10.1016/j.scienta.2019.108730
Møller IM, Rogowska-Wrzesinska A, Rao RSP (2011) Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective. J Proteom 74:2228–2242. https://doi.org/10.1016/j.jprot.2011.05.004
Mor A, Koh E, Weiner L, Rosenwasser S, Sibony-Benyamini H, Fluhr R (2014) Singlet oxygen signatures are detected independent of light or chloroplasts in response to multiple stresses. Plant Physiol 165:249–261. https://doi.org/10.1104/pp.114.236380
Muhlemann JK, Younts TLB, Muday GK (2018) Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc Natl Acad Sci USA 115:E11188–E11197. https://doi.org/10.1073/pnas.1811492115
Muller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Leubner-Metzger G (2009) In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol 150:1855–1865. https://doi.org/10.1104/pp.109.139204
Nakabayashi R, Yonekura-Sakakibara K, Urano K, Suzuki M, Yamada Y, Nishizawa T, Matsuda F, Kojima M, Sakakibara H, Shinozaki K, Michael AJ, Tohge T, Yamazaki M, Saito K (2014) Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J 77:367–379. https://doi.org/10.1111/tpj.12388
Nardozza S, Boldingh HL, Kashuba MP, Feil R, Jones D, Thrimawithana AH, Ireland HS, Philippe M, Wohlers MW, McGhie TK, Montefiori M, Lunn JE, Allan AC, Richardson AC (2020) Carbon starvation reduces carbohydrate and anthocyanin accumulation in red-fleshed fruit via trehalose 6-phosphate and MYB27. Plant Cell Environ 43:819–835. https://doi.org/10.1111/pce.13699
Nazari M, Mostajeran A, Zarinkamar F (2019) Strong effect of recovery period between hypoxia events on roots of chickpea (Cicer arietinum L). Rhizosphere 11:100163. https://doi.org/10.1016/j.rhisph.2019.100163
Oren-Shamir M (2009) Does anthocyanin degradation play a significant role in determining pigment concentration in plants? Plant Sci 177:310–316. https://doi.org/10.1016/j.plantsci.2009.06.015
Parks GS, Manchester KE, Vaughan LM (1954) Heats of combustion and formation of some alcohols, phenols, and ketones. J Chem Phys 22:2089–2090. https://doi.org/10.1063/1.1740005
Piatkowski BT, Imwattana K, Tripp EA, Weston DJ, Healey A, Schmutz J, Shaw AJ (2020) Phylogenomics reveals convergent evolution of red–violet coloration in land plants and the origins of the anthocyanin biosynthetic pathway. Mol Phylogenet Evol 151:106904. https://doi.org/10.1016/j.ympev.2020.106904
Pilon M, Ravet K, Tapken W (2011) The biogenesis and physiological function of chloroplast superoxide dismutases. Biochim Biophys Acta Bioenerg 1807:989–998. https://doi.org/10.1016/j.bbabio.2010.11.002
Polturak G, Aharoni A (2018) La Vie en Rose: Biosynthesis, sources, and applications of betalain pigments. Mol Plant 11:7–22. https://doi.org/10.1016/j.molp.2017.10.008
Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, Squadrito GL, Davies KJA (2006) Free radical biology and medicine: it’s a gas, man! Am J Physiol Regul Integr Comp Physiol. https://doi.org/10.1152/ajpregu.00614.2005
Rahman MM, Ichiyanagi T, Komiyama T, Hatano Y, Konishi T (2006) Superoxide radical- and peroxynitrite-scavenging activity of anthocyanins; structure–activity relationship and their synergism. Free Radic Res 40:993–1002. https://doi.org/10.1080/10715760600815322
Sakuta M (2014) Diversity in plant red pigments: anthocyanins and betacyanins. Plant Biotechnol Rep 8:37–48. https://doi.org/10.1007/s11816-013-0294-z
Sasaki N, Nishizaki Y, Ozeki Y, Miyahara T (2014) The role of acyl-glucose in anthocyanin modifications. Molecules 19:18747–18766. https://doi.org/10.3390/molecules191118747
Schaefer HM, Rolshausen G (2006) Plants on red alert: do insects pay attention? BioEssays 28:65–71. https://doi.org/10.1002/bies.20340
Schippers JHM, Foyer CH, van Dongen JT (2016) Redox regulation in shoot growth, SAM maintenance and flowering. Curr Opin Plant Biol 29:121–128. https://doi.org/10.1016/j.pbi.2015.11.009
Sestari I, Zsögön A, Rehder GG, Teixeira L, de Hassimotto L, Purgatto NMA, Benedito E, Peres VA, L.E.P (2014) Near-isogenic lines enhancing ascorbic acid, anthocyanin and carotenoid content in tomato (Solanum lycopersicum L. cv Micro-Tom) as a tool to produce nutrient-rich fruits. Sci Hortic 175:111–120. https://doi.org/10.1016/j.scienta.2014.06.010
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. https://doi.org/10.1155/2012/217037
Silva-Navas J, Moreno-Risueno MA, Manzano C, Téllez-Robledo B, Navarro-Neila S, Carrasco V, Pollmann S, Gallego FJ, Del Pozo JC (2016) Flavonols mediate root phototropism and growth through regulation of proliferation-to-differentiation transition. Plant Cell 28:1372–1387. https://doi.org/10.1105/tpc.15.00857
Singh A, Kumar A, Yadav S, Singh IK (2019) Reactive oxygen species-mediated signaling during abiotic stress. Plant Gene 18:100173. https://doi.org/10.1016/j.plgene.2019.100173
Smeriglio A, Barreca D, Bellocco E, Trombetta D (2016) Chemistry, pharmacology and health benefits of anthocyanins. Phytother Res 1286:1265–1286. https://doi.org/10.1002/ptr.5642
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221:1197–1214. https://doi.org/10.1111/nph.15488
Stafford HA (1994) Anthocyanins and betalains: evolution of the mutually exclusive pathways. Plant Sci 101:91–98. https://doi.org/10.1016/0168-9452(94)90244-5
Stommel JR, Lightbourn GJ, Winkel BS, Griesbach RJ (2009) Transcription factor families regulate the anthocyanin biosynthetic pathway in capsicum annuum. J Am Soc Hortic Sci 134:244–251. https://doi.org/10.21273/jashs.134.2.244
Sun Y, Li H, Huang JR (2012) Arabidopsis TT19 functions as a carrier to transport anthocyanin from the cytosol to tonoplasts. Mol Plant 5:387–400. https://doi.org/10.1093/mp/ssr110
Tena N, Martín J, Asuero AG (2020) State of the art of anthocyanins: antioxidant activity, sources, bioavailability, and therapeutic effect in human health. Antioxidants 9:451. https://doi.org/10.3390/antiox9050451
Thomas H, Ougham HJ, Wagstaff C, Stead AD (2003) Defining senescence and death. J Exp Bot 54:1127–1132. https://doi.org/10.1093/jxb/erg133
Trojak M, Skowron E (2017) Role of anthocyanins in high-light stress response. World Sci News 81:145–163
Vangelisti A, Guidi L, Cavallini A, Natali L, Lo Piccolo E, Landi M, Lorenzini G, Malorgio F, Massai R, Nali C, Pellegrini E, Rallo G, Remorini D, Vernieri P, Giordani T (2020) Red versus green leaves: transcriptomic comparison of foliar senescence between two Prunus cerasifera genotypes. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-58878-8
Wang PT, Liu H, Hua HJ, Wang L, Song CP (2011) A vacuole localized β-glucosidase contributes to drought tolerance in Arabidopsis. Chin Sci Bull 56:3538–3546. https://doi.org/10.1007/s11434-011-4802-7
Wang X, Guo C, Peng J, Li C, Wan F, Zhang S, Zhou Y, Yan Y, Qi L, Sun K, Yang S, Gong Z, Li J (2019) ABRE-BINDING FACTORS play a role in the feedback regulation of ABA signaling by mediating rapid ABA induction of ABA co-receptor genes. New Phytol 221:341–355. https://doi.org/10.1111/nph.15345
Wei H, Zhao H, Su T, Bausewein A, Greiner S, Harms K, Rausch T (2017) Chicory R2R3-MYB transcription factors CiMYB5 and CiMYB3 regulate fructan 1-exohydrolase expression in response to abiotic stress and hormonal cues. J Exp Bot 68:4323–4338. https://doi.org/10.1093/jxb/erx210
Wen W, Alseekh S, Fernie AR (2020) Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr Opin Plant Biol 55:100–108. https://doi.org/10.1016/j.pbi.2020.04.004
Winterbourn CC (1995) Toxicity of iron and hydrogen peroxide: the fenton reaction. Toxicol Lett. https://doi.org/10.1016/0378-4274(95)03532-X
Wrolstad RE, Durst RW, Lee J (2005) Tracking color and pigment changes in anthocyanin products. Trends Food Sci Technol 16:423–428. https://doi.org/10.1016/j.tifs.2005.03.019
Xiang L, Liu X, Li H, Yin X, Grierson D, Li F, Chen K (2019) CmMYB#7, an R3 MYB transcription factor, acts as a negative regulator of anthocyanin biosynthesis in chrysanthemum. J Exp Bot 70:3111–3123. https://doi.org/10.1093/jxb/erz121
Xie Y, Zhu X, Li Y, Wang C (2018) Analysis of the pH-Dependent Fe(III) Ion Chelating activity of anthocyanin extracted from black soybean [Glycine max (L.) Merr.] Coats. J Agric Food Chem 66:1131–1139. https://doi.org/10.1021/acs.jafc.7b04719
Xu Z, Rothstein SJ (2018) ROS-Induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal Behav 13:e1451708. https://doi.org/10.1080/15592324.2018.1451708
Xu Z, Mahmood K, Rothstein SJ (2017) ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in arabidopsis. Plant Cell Physiol 58:1364–1377. https://doi.org/10.1093/pcp/pcx073
Yang Z, Qian J, Yu A, Pan B (2019) Singlet oxygen mediated iron-based Fenton-like catalysis under nanoconfinement. Proc Natl Acad Sci USA 116:6659–6664. https://doi.org/10.1073/pnas.1819382116
Zhang Y, Butelli E, Martin C (2014) Engineering anthocyanin biosynthesis in plants. Curr Opin Plant Biol 19:81–90. https://doi.org/10.1016/j.pbi.2014.05.011
Zhang Y, De Stefano R, Robine M, Butelli E, Bulling K, Hill L, Rejzek M, Martin C, Schoonbeek HJ (2015) Different reactive oxygen species scavenging properties of flavonoids determine their abilities to extend the shelf life of tomato. Plant Physiol 169:1568–1583. https://doi.org/10.1104/pp.15.00346
Zhao CL, Chen ZJ, Bai XS, Ding C, Long TJ, Wei FG, Miao KR (2014) Structure-activity relationships of anthocyanidin glycosylation. Mol Divers 18:687–700. https://doi.org/10.1007/s11030-014-9520-z
Zhao P, Li X, Jia J, Yuan G, Chen S, Qi D, Cheng L, Liu G (2019) BHLH92 from sheepgrass acts as a negative regulator of anthocyanin/proanthocyandin accumulation and influences seed dormancy. J Exp Bot 70:269–284. https://doi.org/10.1093/jxb/ery335
Zheng XT, Yu ZC, Tang JW, Cai ML, Chen YL, Yang CW, Chow WS, Peng CL (2021) The major photoprotective role of anthocyanins in leaves of Arabidopsis thaliana under long-term high light treatment: antioxidant or light attenuator? Photosynth Res 149:25–40. https://doi.org/10.1007/s11120-020-00761-8
Zhou X, Hua D, Chen Z, Zhou Z, Gong Z (2009) Elongator mediates ABA responses, oxidative stress resistance and anthocyanin biosynthesis in Arabidopsis. Plant J 60:79–90. https://doi.org/10.1111/j.1365-313X.2009.03931.x
Zurbriggen MD, Carrillo N, Hajirezaei MR (2010) ROS signaling in the hypersensitive response: when, where and what for? Plant Signal Behav 5:393–396. https://doi.org/10.4161/psb.5.4.10793
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was partly funded by the National Institute of Science and Technology in Plant Physiology under Stress Conditions (INCT, Plant Stress Physiology 406455/2022-8) and the Minas Gerais State Research Support Foundation (FAPEMIG, Brazil, APQ-01942-22). AZ was supported by a CAPES/Alexander von Humboldt Foundation Experienced Researcher Fellowship (88881.472837/2019-01). FZ was supported by the Major Special Projects and Key R&D Projects in Yunnan Province (202102AE090020 and 202102AE090054).
Author information
Authors and Affiliations
Contributions
All authors contributed equally to drafting, writing and revising the manuscript.
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cerqueira, J.V.A., de Andrade, M.T., Rafael, D.D. et al. Anthocyanins and reactive oxygen species: a team of rivals regulating plant development?. Plant Mol Biol 112, 213–223 (2023). https://doi.org/10.1007/s11103-023-01362-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-023-01362-4