Abstract
miR828 in Arabidopsis triggers the cleavage of Trans-Acting SiRNA Gene 4 (TAS4) transcripts and production of small interfering RNAs (ta-siRNAs). One siRNA, TAS4-siRNA81(−), targets a set of MYB transcription factors including PAP1, PAP2, and MYB113 which regulate the anthocyanin biosynthesis pathway. Interestingly, miR828 also targets MYB113, suggesting a close relationship between these MYBs, miR828, and TAS4, but their evolutionary origins are unknown. We found that PAP1, PAP2, and TAS4 expression is induced specifically by exogenous treatment with sucrose and glucose in seedlings. The induction is attenuated in abscisic acid (ABA) pathway mutants, especially in abi3-1 and abi5-1 for PAP1 or PAP2, while no such effect is observed for TAS4. PAP1 is under regulation by TAS4, demonstrated by the accumulation of PAP1 transcripts and anthocyanin in ta-siRNA biogenesis pathway mutants. TAS4-siR81(−) expression is induced by physiological concentrations of Suc and Glc and in pap1-D, an activation-tagged line, indicating a feedback regulatory loop exists between PAP1 and TAS4. Bioinformatic analysis revealed MIR828 homologues in dicots and gymnosperms, but only in one basal monocot, whereas TAS4 is only found in dicots. Consistent with this observation, PAP1, PAP2, and MYB113 dicot paralogs show peptide and nucleotide footprints for the TAS4-siR81(−) binding site, providing evidence for purifying selection in contrast to monocots. Extended sequence similarities between MIR828, MYBs, and TAS4 support an inverted duplication model for the evolution of MIR828 from an ancestral gymnosperm MYB gene and subsequent formation of TAS4 by duplication of the miR828* arm. We obtained evidence by modified 5′-RACE for a MYB mRNA cleavage product guided by miR828 in Pinus resinosa. Taken together, our results suggest that regulation of anthocyanin biosynthesis by TAS4 and miR828 in higher plants is evolutionarily significant and consistent with the evolution of TAS4 since the dicot—monocot divergence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trans-Acting SiRNA (TAS) genes are small interfering RNA (siRNA)-generating loci that regulate target gene expression in trans (Peragine et al. 2004; Vazquez et al. 2004; Allen et al. 2005). The production of trans-acting siRNAs (ta-siRNAs) from TAS loci depends on microRNA (miRNA)-directed cleavage of their transcripts by ARGONAUTE (AGO)-containing RNA-Induced Silencing Complexes (RISCs), which sets the phase for 21-nt siRNA production by RNA-DEPENDENT RNA POLYMERASE 6 (RDR6) in collaboration with SUPPRESSOR OF GENE SILENCING 3 (SGS3), DICER-LIKE 4 (DCL4), DOUBLE-STRANDED RNA BINDING PROTEIN 4 (DRB4), and HUA ENHANCER 1 (HEN1), a small RNA (sRNA) methyltransferase (Peragine et al. 2004; Vazquez et al. 2004; Allen et al. 2005; Yoshikawa et al. 2005). Arabidopsis thaliana has eight TAS loci from four families, TAS1-4 (Allen et al. 2005; Rajagopalan et al. 2006). TAS1 and TAS2 transcripts are subject to miR173-directed cleavage in association with AGO1 to generate siRNAs targeting several transcripts of pentatricopeptide repeat-containing genes and others with unknown function (Allen et al. 2005; Montgomery et al. 2008b). TAS3 transcript, on the other hand, is cleaved through the specific interaction of miR390 with AGO7 (Adenot et al. 2006; Fahlgren et al. 2006; Garcia et al. 2006; Montgomery et al. 2008a). Interestingly, an autoregulatory network has been found involving miR390, TAS3, and ta-siRNA targets AUXIN RESPONSE FACTORS 2 (ARF2), ARF3, and ARF4 (Yoon et al. 2010; Marin et al. 2010). TAS3-derived siRNAs (ta-siARFs) inhibit ARF2/3/4 expression, while ARF4 downregulates miR390 accumulation in contrast to the upregulation of miR390 by ARF3 in response to auxin. The outcome of this complex feedback loop is a fine-tuning of lateral root growth dependent on the auxin receptor TRANSPORT INHIBITOR RESPONSE 1 (TIR1; a target of miR393), which directs transcriptional regulation in response to localized auxin fluxes (Yoon et al. 2010; Marin et al. 2010).
Regulatory networks of sRNAs, including miRNAs and ta-siRNAs, modulate their targets’ expression in response to primary (N, P, K) and secondary (S, Mg, Ca) macronutrient condition changes in the cell and/or environment. For example, low sulfate induces miR395 expression, which decreases the mRNA level for its targets ATP SULFURYLASE 1 (APS1) and several other genes in the sulfate assimilation pathway (Jones-Rhoades and Bartel 2004; Kawashima et al. 2009). The induction of miR395 is modulated by SULFUR LIMITATION 1 (SLIM1), a putative transcription factor in the same pathway, although the expression domain for such induction does not correlate with one of its targets, SULFATE TRANSPORTER 2;1 (SULTR2;1)/ARABIDOPSIS SULFATE TRANSPORTER 68 (AST68) (Kawashima et al. 2009). Other examples include phosphate (Pi) starvation, which up-regulates miR399b/c/f expression and down-regulates their common target PHOSPHATE 2 (PHO2)/UBIQUITIN-CONJUGATING ENZYME 24 (UBC24) (Fujii et al. 2005; Chiou et al. 2006). Transgenic Arabidopsis plants over-expressing MIR399 accumulate five to six times more Pi in shoots than wild type. Intriguingly, a non-coding RNA, INDUCED BY PHOSPHATE STARVATION 1 (IPS1) can sequester miR399 by base-pairing through a mechanism termed “target mimicry” and thereby up-regulate PHO2 expression level to help translocate over-accumulated Pi in shoots (Franco-Zorrilla et al. 2007). Deep sequencing techniques have uncovered miRNAs such as miR398, miR778, miR827, and miR2111 responsive to Pi deficiency (Pant et al. 2009; Hsieh et al. 2009). Several members of the miR169 family and miR398a are repressed by nitrogen (N) limitation (Pant et al. 2009). Another nutrient-responsive example for miRNAs comes from the report that exogenous sucrose (Suc) treatment increases levels of miR398 in a dose-dependent, but not time-dependent manner, probably by activating the transcription of MIR398c (Dugas and Bartel 2008). miR398 reduces the expression of its targets, including COPPER SUPEROXIDE DISMUTASE 1 (CSD1) and CSD2 at both mRNA and protein levels.
PRODUCTION OF ANTHOCYANIN PIGMENT 1 (PAP1)/MYB DOMAIN PROTEIN 75 (MYB75) and PAP2/MYB90 encode transcription factors that regulate expression of anthocyanin biosynthetic genes in vegetative tissues. They might be involved in regulating leaf senescence because for both of these processes, sugars can be triggers (Pourtau et al. 2006; Borevitz et al. 2000; Teng et al. 2005; Solfanelli et al. 2006; Gonzalez et al. 2008). In this study, we report that TAS4 and its targets PAP1 and PAP2 are responsive to Suc. Part of the response is impaired in ABA insensitive 3 (abi3) and abi5 mutants. PAP1 and TAS4 expression appear to involve in an autoregulatory loop, as evidenced by the over-accumulation of PAP1 transcript levels and anthocyanin in ta-siRNA pathway mutants, and the up-regulation of TAS4-siR81(−) in pap1-D, an activation-tagged transgenic line. We also performed bioinformatic analysis and uncovered the existence of miR828 in gymnosperms and angiosperms, whereas TAS4 only is found in dicots. The cleavage by miR828 was mapped on one MYB transcript from Pinus resinosa by 5′-RACE. Finally, sequence alignments suggest an inverted duplication model for MIR828 and TAS4 evolution.
Materials and methods
Plant materials and growth conditions
Arabidopsis (Arabidopsis thaliana ecotype Columbia) wild-type and mutant plants were grown as previously described (Luo et al. 2009). The accessions used in this study are listed as follows: Ler-0 [CS20], abi1-1 [CS22], abi2-1 [CS23], abi3-1 [CS24], abi5-1 [CS8105], aba1-1 [CS21], Col-0 [CS60,000], abi4-103 [CS3838], hen1-1 [CS6583], dcl4-2 [CS6954], drb4 [SALK_113384c], rdr6-15, sgs3-14 [SALK_001394], tas4 [SALK_066997], mir828 [SALK_097788], hyl1-2 [SALK_064863], hst-6 [CS24279], hst-7 [CS24280], and pap1-D [CS3884] (Borevitz et al. 2000; Alonso et al. 2003).
For the treatment with sugars, 3-day-old Arabidopsis seedlings were grown on filter papers supplemented with Murashige and Skoog standard medium (MS medium, one-half strength, control). Half of the samples were transferred to new filter papers supplemented with Suc, glucose (Glc) or mannitol solutions at a concentration of 100 mM and harvested by freezing in liquid N2 at various time points up to 24 h, or subjected to the treatment of different sugars for 12 h with a series of concentrations ranging from 0 to 100 mM.
Taxus globosa (Mexican yew) and Pinus resinosa (red pine) plants were purchased from Forrest Farm(Williams, OR) and Heronswood Nursery (Warminster, PA), respectively, and total RNA was extracted from green needles as described (Chang et al. 1993) for Rapid Amplification of cDNA Ends (RACE) experiments.
RNA preparation and detection
Total RNA was isolated using Trizol regent (Invitrogen, Carlsbad CA). Northern blots and sRNA blots were performed as described (Xie et al. 2005). High molecular weight RNA was precipitated from total RNA with 2 M LiCl followed by centrifugation (13,000 rpm, 15 min). The supernatant was added to three volumes 100% ethanol to precipitate low molecular weight RNA. Ten μg total RNA or 20 μg low molecular weight RNA was loaded in each lane for formaldehyde-agarose or PAGE gel electrophoresis, respectively. For Northern blots, probes were prepared from agarose gel-purified PAP1 cDNA from Arabidopsis cDNA library amplified using primers “PAP1_atgF” and “PAP1_tagR” and radio-labeled with α-32P-dCTP by a random primer labeling kit (Takara, Shiga Japan). To check equal loading, the membrane blot was stripped and re-probed with antisense γ-32P-labelled oligonucleotides for miR160, 5S rRNA, and/or U6 small nuclear RNA (snRNA). RNA blots were scanned using a Storm 860 PhosphorImager (GE Healthcare, Piscataway NJ). mRNA or sRNA signals were quantified using the ImageQuant TL software (v2003, GE Healthcare). Specifically, we divided the TAS4-siR81(−), PAP1, and control (5S rRNA, miR160, or U6 snRNA) band areas into nine vertical subsections of equal area per lane. The paired subsections for signals of a given lane were integrated separately after subtracting representative background fields flanking the test and control bands. A ratio of TAS4-siR81(−) or PAP1 mRNA signals to various controls was calculated for these independent sections. The average of four to six uniform ratios across the band was calculated after discarding subsections that contained artifacts identified visually and attributed to gel or blotting processes. Modified RACE experiments were performed according to the manufacturer’s specification (Invitrogen). Cloned cDNAs encoding MYB homologues obtained from RACE experiments on P. resinosa and T. globosa were submitted to GenBank (accession numbers HQ997774 and HQ997775, respectively). Probe and primer sequences are listed in Supplementary Table S1.
Real-time RT-PCR
RNA was extracted from seedlings grown on 0.5× MS medium (control) or on the same medium with 100 mM sugars added for a series of time points as described in Figure legends. Total RNA was subjected to DNase I treatment (Promega, Madison WI) after extraction by Trizol solution (Invitrogen). Five micrograms of each sample were reverse-transcribed into cDNA with Oligo dT primers (Promega) by Moloney Murine Leukemia Virus Reverse Transcriptase (Promega) for 1 h at 42°C. Quantitative real-time PCR (qRT-PCR) assay was performed with the Absolute SYBR Green qPCR Mixes (Thermo Scientific) on the ABI Prism 7,300 sequence detection system (Applied Biosystems, Carlsbad CA). Oligonucleotides were synthesized by Sigma–Aldrich (St. Louis, MO). ACTIN8 primer pairs were used for internal control on aliquots of cDNA. Relative quantitation for gene expression was done using the comparative CT method as described in the ABI Prism 7300 Sequence Detection System User Bulletin (Applied Biosystems).
Anthocyanin quantitation
Extraction and quantification of anthocyanin from Arabidopsis seedling was performed as described (Teng et al. 2005; Solfanelli et al. 2006) with minor modifications. In brief, 10–20 three day-old seedlings were placed in a microcentrifuge tube and centrifuged briefly to allow surface liquid to be pipetted off. The samples were weighed twice on an analytical balance to obtain an average fresh weight of tissue. One mL of extraction buffer (1% [v/v] hydrochloric acid in methanol) was added followed by incubation at 4°C for 24 h. Extracts were centrifuged (15 min at 13,000 rpm) and the absorbance of the supernatant was determined at 530 and 657 nm in a BioMate 5 spectrophotometer (Thermo Spectronic). Relative anthocyanin units are defined as equal to one absorbance unit [A 530 − (1/4 × A 657 )] × 1,000] per gram fresh material in one mL of extraction buffer. Mean values were obtained from three biological replicates.
Bioinformatic analysis
Expressed Sequence Tags (ESTs) and protein sequences were obtained by BLASTing from GenBank (www.ncbi.nlm.nih.gov). The alignment was performed with the Vector NTI software package (Invitrogen, Version 9) or T-Coffee (www.tcoffee.org). Secondary structures of RNAs were predicted using MFOLD (Zuker 2003).
Results
Sugar induction of PAP1 and TAS4 expression
PAP1 and PAP2 are predicted targets of TAS4-siR81(−) (Fig. 1a) (Rajagopalan et al. 2006). Using qRT-PCR, PAP1 expression was assayed in response to sugars. In a time-course treatment with exogenous sugars of Col-0 seedlings, PAP1 expression was induced by Suc and Glc up to ~5- and 14-fold, respectively, whereas PAP1 was not induced by the non-metabolizable sugar mannitol used as a control (Fig. 1b). PAP2 showed a similar but lower induction than PAP1 by Suc treatment (~6-fold less than PAP1; see Fig. 1c, the rows for “Ler-0” and “Col-0”).
qRT-PCR shows temporal induction of PAP1, PAP2 and TAS4 by Suc and Glc, and crosstalk with ABA signaling. Panel a schematic of PAP1 and PAP2 genes with qRT-PCR primer pairs mapped below (F1, R1 for PAP1; F2, R2 for PAP2, respectively). The base pairing between TAS4-siR81(−) with PAP1 or PAP2 is shown underneath. Panel b Time course of Suc, Glc, or mannitol treatments of Col-0 seedling at a concentration of 100 mM up to 24 h. Each treatment is represented by a column of colored boxes, and each time point is indicated by an individual row. For Suc treatment of Arabidopsis seedlings, 3-day-old seedlings were grown on filter papers supplemented with Murashige and Skoog (MS) standard medium (½ strength, control). Data (average transcript level from three technical replicates) were visualized using BAR HeatMapper Plus software (http://bar.utoronto.ca/ntools/cgi-bin/ntools_heatmapper_plus.cgi). Data are represented as fold change (unity = control) after normalization to ACTIN8 expression. Effects of different sugars on gene expression range from pale yellow (low) to deep red (high). The experiment was performed twice with similar results. Panels c, d 100 mM Suc response of ABA mutant genotypes treated for 24 h. The expression data for each gene is represented by a column of colored boxes, while each genotype assayed is indicated by an individual row
Abscisic acid (ABA) signaling synergizes with sugar and induces anthocyanin accumulation in early seedling development (Rolland et al. 2006; Finkelstein et al. 2002). Several sugar-insensitive mutants were isolated as allelic to ABA synthesis (aba) and ABA insensitive (abi) mutants. For example, sucrose insensitive 10 (sis10) was cloned in a forward genetic screen and shown to be allelic to ABI3, encoding a B3 domain transcription factor that confers sugar and ABA sensitivity and regulates anthocyanin production (Parcy et al. 1997; Huang et al. 2008). sucrose uncoupled 6 (sun6), sugar-insensitive 5 (sis5), glucose insensitive 6 (gin6), and impaired sucrose induction 3 (isi3) are mutant alleles of ABI4, which encodes an APETALA2 domain transcription factor (Huijser et al. 2000; Laby et al. 2000; Arenas-Huertero et al. 2000; Rook et al. 2001). To measure the effects of sugar induction on PAP1 and TAS4, qRT-PCR assays were performed on samples from mutants in ABA biosynthesis and signaling pathways (Fig. 1c, d). Interestingly, in abi1-1, abi2-1, abi4-103, and aba1-1 mutants the induction of PAP1 and PAP2 by Suc was significantly reduced (~2- to 8-fold) compared to wild type Ler-0 or Col-0, although still effectively Suc-responsive (Fig. 1c). This indicated the positive effect of ABA signaling and biosynthesis on PAP1/PAP2 responses to Suc. In abi3-1 and abi5-1 mutants, PAP1 expression upon Suc treatment was severely decreased compared to wild type (~21- and 47-fold less, respectively). In addition, PAP2 barely responded to Suc treatment in abi3-1 and abi5-1 mutants. These results are generally consistent with the sugar-insensitive phenotypes associated with abi3, abi4 and abi5 mutants (Bossi et al. 2009). Interestingly, the expression of MYB82, a PAP1 paralog which has a predicted but un-validated miR828 complementary site (Rajagopalan et al. 2006) (data not shown), did not respond to Suc in wild type or mutants. However, TAS4 expression was increased two to threefold by Suc in the abi1-1, abi3-1 and abi5-1 mutants in comparison to Ler-0, suggesting its expression is independent of the ABA signaling pathway or subject to secondary effects (Fig. 1d).
sRNA blots showed that TAS4-siR81(−) was induced strongly in a dose-dependent manner by exogenous Suc treatment for 12 h (Fig. 2a). The expression of TAS4-siR81(−) was induced by physiological concentrations of 6.25 mM Suc or 12.5 mM Glc (Jang and Sheen 1994) relative to a corresponding control (2.1-, and 1.8-fold higher than mannitol control, respectively). Clear signals corresponding to TAS4-siR81(−) were detected for samples treated with 25 mM Suc (2.6-fold higher than that in samples treated by mannitol), with maximum signal intensities observed for samples treated with 100 mM Suc for 12 h (14-fold higher than mannitol control). Increasing Glc concentrations had similar effects as Suc on TAS4-siR81(−) expression (~3- to 6-fold induction after 12 h), while the non-metabolized osmolyte mannitol (a negative control) had a very weak effect, indicating that TAS4-siRNA81(−) induction is primarily due to metabolizable sugars and that the mannitol effect observed at high concentrations may be an osmotic stress-related response (Fig. 2a, data not shown). As the basis for quantifying endogenous sRNA abundance, miR160 and 5S rRNA expression were shown to be independent of sugar treatments, which supports the specificity of Suc and Glc induction for TAS4-siR81(−) expression (Fig. 2a, b). The response of TAS4-siR81(−) to Suc or Glc was also transient, reaching a peak at 12 h (~14- and 18-fold induction by Suc and Glc, respectively) with subsequent declines in abundance at 24 h (~6- and 4-fold induction by Suc or Glc, respectively, Fig. 2b), suggesting a homeostatic mechanism involving the expression of TAS4.
Physiological concentrations of Suc and Glc induce expression of TAS4-siR81(−). Panel a 3-day-old Col-0 wild-type seedlings were grown on filter papers supplemented with MS medium (½ strength, control) and then subjected to treatment with different sugars in series of concentrations ranging from 0 to 100 mM for 12 h. Panel b time-course experiment from 3 to 24 h treatments with 100 mM Suc, Glc or mannitol. As loading controls, probes for 5S rRNA and miR160 were hybridized to the same membrane. Band intensities for TAS4-siR81(−) are shown normalized to that of miR160 below each lane (±standard error of mean) and graphically as ‘effect wedges’ above the treatment headers. The relative abundances for TAS4-siR81(−) are presented as the ratio of normalized abundance from Suc or Glc treatments to that from respective mannitol controls (set to unity). A representative result from three experiments is shown
An autoregulatory feedback loop involving PAP1 and TAS4 regulates anthocyanin production
PAP1 is predicted to carry a functional TAS4-siR81(−) target site (Rajagopalan et al. 2006; Hsieh et al. 2009). Its regulation by RISC is supported by qRT-PCR experiments showing up-regulation in mir828 and tas4 T-DNA insertion mutants (Hsieh et al. 2009). We further examined the genetic requirements of PAP1 induction in ta-siRNA pathway mutants, namely dcl4-2, rdr6-15, sgs3-14, and hyl1-2, in response to Suc. Fig. 3a (arrow) shows that PAP1 mRNA was elevated from 2.6- to 10.7-fold in these mutant seedlings in response to treatment with sucrose for 12 h, as well as in miR828 and tas4 mutants (11.4- and 8.1-fold increases, respectively). In pap1-D, a dominant activation-tagged transgenic line, PAP1 expression was elevated compared to wild type (5.8-fold induction). Interestingly, there was a band of size ~450 nt (asterisk in Fig. 3a) presumed to be the TAS4-siR81(−)-directed 3′ cleavage product of PAP1 mRNA, based on similar phenomena observed for many miRNA targets (Souret et al. 2004). The cleavage product was just barely visible in Col-0, dcl4-2, and hst-7. The accumulation of both PAP1 mRNAs and its 3′ cleavage product in pap1-D suggests that increased PAP1 mRNA levels may enhance post-transcriptional regulation of itself by TAS4-siR81(−). A sRNA blot confirmed that TAS4-siR81(−) expression was below detection levels in wild type and all ta-siRNA pathway mutants assayed, but significantly increased in pap1-D (Fig. 3b). Taken together, these results suggest that an autoregulatory feedback loop involving PAP1 and TAS4-siR81(−) operates on and coordinates TAS4 expression. Supporting this notion, two putative PAP1-binding motifs (C/T)(A/C)NCCACNN(G/T) were found within the 2,000 nt region upstream of the TAS4 transcription start site (Fig. S1A), according to PAP1 cis-regulatory elements functionally characterized by transient assays in protoplasts (Dare et al. 2008).
a negative feedback regulatory loop involving with PAP1 and TAS4-siR81(−). Panel a 3-day-old Col-0 wild-type seedlings were grown on filter papers supplemented with ½ strength MS medium (control) and then subjected to treatment with 100 mM sucrose for 12 h. The arrow indicates a band corresponding to the full length mRNA for PAP1, and the star shows a signal with the correct predicted size of the TAS4-siR81(−)-mediated 3′ cleavage product of PAP1. Total RNA (10 μg) was loaded for each sample and stained with ethidium bromide before blotting to confirm equal loadings. The relative abundance for PAP1 is presented below the gel as the ratio of band intensities for each mutant versus that from wild type Col-0. Panel b sRNA blot analysis for TAS4-siR81(−) expression in ta-siRNA pathway mutants, a mir828 T-DNA insertion mutant, and a pap1-D over-expressing activation-tagged transgenic line. Low molecular weight RNA (20 μg) was loaded for each lane. The same membrane was re-hybridized with a probe against U6 snRNA to show equal loading. The experiment was repeated twice with similar results
dcl4-2 and drb4-1 mutants over-accumulate anthocyanin in leaves and flowers of plants older than 6 weeks (Nakazawa et al. 2007). To find out the effect of Suc treatments, we assayed the accumulation of anthocyanin in various ta-siRNA pathway mutants (Fig. 4). With the exception of pap1-D, untreated 3 day-old mutant seedlings did not accumulate significantly different amounts of anthocyanins than their corresponding wild types (Fig. 4, blue bars). After 12 h Suc treatment, all mutants displayed increased accumulation of anthocyanin compared to their non-treated seedlings (Fig. 4, red bars), consistent with previous findings (Nakazawa et al. 2007). Like untreated pap1-D mutant results, pap1-D seedlings had the highest anthocyanin accumulation after treatment, with hyl1-2 mutants also accumulating significantly higher amounts of anthocyanins compared to wild type Col-0 (Fig. 4, asterisks). All other tested ta-siRNA pathway mutants accumulated higher amounts of anthocyanin than wild types. These results suggest that the release of PAP1 repression by loss of TAS4-siR81(−) (Fig. 3a) in the mutants could be responsible for increased anthocyanin under Suc stimulus conditions (Fig. 4).
Sucrose treatment induces anthocyanin accumulation in ta-siRNA pathway mutants. Three-day-old Arabidopsis seedlings were grown on filter papers supplemented with MS medium (½ strength), half of which were transferred to new filter papers supplemented with 100 mM Suc for 12 h and the rest treated with H2O. Data from one of two representative experiments is shown. Error bars are standard errors of mean (n = 3 biological replicates). Asterisks indicate significantly higher anthocyanin than wild type control (P < 0.06, one-sided Student’s t-test, equal variance assumed)
Evolution of TAS4 and its regulator miR828
Bioinformatic analysis of ESTs in land plants demonstrated the existence of TAS4 in dicots, such as Euphorbia esula, Actinidia chinensis, and Vitis vinifera (Fig. 5; data not shown). The TAS4 orthologs bear conserved miR828 binding sites, whereas a less-conserved TAS4-siR81(−) complementary site is located downstream by a constant distance of four 21-nt phases (Fig. 5 black lines), despite the sequence divergence in the intervening region. These data clearly show that a “selective sweep” has acted over evolutionary time on miR828 and TAS4-siR81(−) sequences to maintain the function of TAS4 in these species. Supporting evidence was found by alignment of sequences for PAP1/PAP2/MYB113 orthologs which show the peptide footprint for miR828 binding sites is generally conserved for both dicot and monocot plants (Fig. 6), while that for TAS4-siR81(−) binding sites is specific for most dicots only (Fig. 7). DNA sequence alignment revealed a MYB-like gene in Fagopyrum as potential target for miR828, based on sequence similarity with miR828 complementary site in Arabidopsis MYB113 (Fig. S2A). MYBA6 in Vitis is also predicted as TAS4 target (compare Fig. 5 with Fig. S2B; data not shown). These observations support purifying selection for miR828 and TAS4 regulation on individual MYB targets in different dicot species as shown initially in Arabidopsis (Rajagopalan et al. 2006).
Sequence alignment of TAS4 paralogs in dicot plants. Sequences were obtained by BLASTing TAS4/AT3G25795 (n.t. 870–980) against the GenBank experimental plant EST database. Alignments were color-coded based on the confidence of the local alignment of T-Coffee (yellow < brown < red, www.tcoffee.org). The putative miR828 binding site and TAS4-siR81(−)-generating site are labeled with black lines. Asterisks show residues identical for the given position. Abbreviations correspond to species are listed as follows with TAS4 paralog (GenBank accession numbers). Ath, Arabidopsis thaliana; Tca, Theobroma cacao (CU512683.1); Ees, Euphorbia esula (DV114602.1); Ptr, Populus tremula (DN495932.1); Mdo, Malus domestica (CN490819.1); Ach, Actinidia chinensis (FG511890.1); Mgu, Mimulus guttatus (DV209191.1), and Vvi, Vitis vinifera (EC986896.1). Con consensus, the same nucleotide on one position is represented by asterisk
Amino acid sequence alignment of miR828 complementary sites in PAP1 orthologs from diverse flowering plant genera. Sequences were obtained by BLASTing the Arabidopsis PAP1 sequence to the GenBank protein database (www.ncbi.nlm.nih.gov). The alignment was done by Vector NTI (Invitrogen, version 9). A cartoon for MYB ortholog conserved domain structure is shown above the alignment. The miR828 complimentary sites are labeled by a bracket and the conserved residues are shaded
Amino acid sequence alignment of TAS4-siR81(−) complementary sites in PAP1 orthologs from diverse flowering plant genera. See legend of Fig. 6 for details of methods
By searching plant EST databases, MIR828 orthologs with extensive base pairing to form hairpins were found in a variety of dicot species, including A. lyrata, E. esula, V. vinifera, and gymnosperms Picea glauca (spruce) and Pinus contorta (lodgepole pine) (Fig. 8a and Figs. S3–S5). The candidate MIR828s share significant similarity for mature miR828 and flanking regions, suggesting an ancient origin of MIR828 (Fig. 8a). Interestingly, genomic sequences with great similarity to the miR828 orthologs were found in Trillium camschatcense (Fig. 8a “Tca”), a basal monocot species. The T. camschatcense miR828-like sequence would form an extensive hairpin (a hallmark of miRNA precursors) if expressed (Fig. S6). In contrast to most monocots (which have characteristic narrow, thick, hard leaves with parallel venation and tiny, wind-dispersed seeds released from dry capsules), Trillium possesses broad, thin, soft leaves, net venation, and fleshy fruits (Givnish et al. 2006). This phylogenetic relationship suggests a plausible hypothesis that MIR828 was lost early in the monocot lineage and plays some important roles in gymnosperm and dicot physiology. Remarkably, the gymnosperm P. glauca predicted pri-miR828 transcript carries two miR828 sites on a polycistronic precursor (Fig. S3), while all analyzed dicot pri-miR828s have one (Fig. S4, data not shown). Two predicted alternative secondary structures with similar delta-G free energies form “good” hairpin structure which could generate mature miR828 from either of these candidate loci (Fig. S3).
Evolution and function of MIR828 and TAS4 evidenced by sequence alignment and modified 5’-RACE validation of MYB endonucleolytic cleavage in a gymnosperm. Panel a Sequence alignment for MIR828 genes from dicot, monocot and gymnosperm species. Alignments were color-coded based on the confidence of the local alignment of T-Coffee (yellow < brown < red). The predicted mature miR828 sites are labeled with a black line. Asterisks show consensus (con) nucleotides identical for the given position. Abbreviations correspond to species listed as follows (with accession numbers from GenBank). Arabidopsis MIR828 sequences are from miRBase. In Picea glauca, since there are two miR828 sequences on one long precursor, sequences spanning the 5’ mature miR828 is used for alignment. Ath, A. thaliana; Aly, Arabidopsis lyrata; Tca, Trillium camschatcense (AB250300.1); Pgl, Picea glauca (CO236109.1); Pco, Pinus contorta (GT251244.1). Panel b extended sequence alignment of candidate P. contorta MIR828 gene and three predicted MYB targets from P. contorta. The GenBank accession numbers for MYB targets are shown. MIR828 5’ arm (−) is the reverse complement sequence for the strand where mature miR828 locates. MIR828 3’ arm (+) is the strand where miR828* maps. The location corresponding to mature miR828 is labeled by a black line. Panel c sequence alignment for MIR828 gene and TAS4 in A. thaliana showing homologies suggestive of a common evolutionary lineage. The miR828* site on the MIR828 3′ arm (+) and the miR828 and siR81(−) complementary sites on TAS4 are indicated by black lines. Panel d 5′-RACE clones establish cleavage of a P. resinosa MYB target mRNA by miR828. All 14 clones sequenced mapped to the predicted miR828-cleavage site, based on the closely related P. contorta miR828
Sequence comparison among MIR828, MYBs and TAS4 revealed some clues for a monophyletic origin. By DNA sequence alignment, extended similarities were found across the reverse strand of the P. contorta 5′ arm of MIR828 precursor, the sense strand of the 3′ arm, and three predicted cognate MYB targets (Fig. 8b black line). Similarly, when the A. thaliana TAS4 sequence is aligned with the arm for miR828* and its downstream sequences (presumably pri-miR828 sequence), they show extensive conservation, including and beyond the miR828 binding site (i.e. miR828*) and the region for the 3′ end of TAS4 (Fig. 8c). Our data suggests an inverted duplication model for the evolution of MIR828 and TAS4 (see below).
To search for experimental evidence supporting our hypothesis for TAS4 origin, a RACE assay was performed on RNA samples from ancient land plants, including T. globosa and P. resinosa. Using the conserved nucleotide sequence footprint found within miR828 binding sites for TAS4 paralogs in dicot plants (Fig. 5), a degenerate primer was designed as described (Axtell and Bartel 2005). However, we were unsuccessful to clone any TAS4 sequences (data not shown). Interestingly, MYB-like genes were cloned from these experiments which had plausible miR828 complementary sites (data not shown). Follow-up 5′-RACE experiments resulted in validation of cleaved products for the P. resinosa MYB gene at the putative miR828 binding site (Fig. 8d). Consistent with our model, no remnant TAS4-siR81(−) complementary site was found within this MYB cDNA sequence (GenBank accession no. HQ997774). These data support the existence of miR828 and a regulatory role in gymnosperms.
Discussion
PAP1 and TAS4 respond to endogenous sugar signals
Based on the presented data, we propose a working model for the autoregulatory feedback loop involving PAP1 and TAS4 (Fig. 9). PAP1/MYB75 expression is induced by exogenous treatment of physiological concentrations of Suc and Glc in Arabidopsis seedlings. Suc may be transported into the nucleus by Suc transporter(s), which activates Suc-induced transcription factors that bind to the promoter of PAP1 and activate its transcription (orange arrows). The elevated expression of PAP1 may bind to the promoter of TAS4 via PAP1 cis-elements and promote the transcription of TAS4. TAS4 may also respond to sugar stimulus through a signaling pathway in which PAP1 is involved. The subsequent increased expression of TAS4 will produce more TAS4-siR81(−) by the guidance of miR828 through RISC-mediated cleavage, which then reduces the PAP1 transcript level by the same mechanism (Fig. 9, scissors). The proper regulation of PAP1 expression level by the autoregulatory feedback loop would give plants a means to monitor changes in nutrient and/or environmental conditions. Interestingly, PAP1 cis-regulatory elements are also found in the putative promoters for MIR828 and PAP1 itself (Fig. S1B&C), one of which may locate within the 3′-UTR of FOREVER YOUNG (FEY, AT4G27760), a gene upstream of MIR828 (AT4G27765). This could suggest a complex transcriptional regulation by PAP1 on TAS4, MIR828, and itself.
A working model for the feedback regulatory loop involving PAP1/MYB75 and TAS4 in response to sugars in Arabidopsis. PAP1 expression is induced by Suc, Glc or other stimulus. PAP1 may regulate TAS4 expression presumably by binding to the PAP1 cis-regulatory elements in TAS4 promoter and transactivate its transcription. Alternatively, TAS4 expression may directly respond to sugar stimulus through a signaling pathway involving PAP1. Increased TAS4 transcript abundance generates more TAS4-siR81(−) through the ta-siRNA pathway, which then down-regulates PAP1, PAP2 and MYB113 expression levels. miR828 controls MYB113 expression by guiding MYB113 transcripts into RISC. At the same time, miR828 also promotes TAS4 cleavage and routes its cleaved product into ta-siRNA pathways for TAS4-siR81(−) biogenesis, which reinforces the feedback loop involving PAP1 and TAS4, as well as the regulatory network on PAP2 and MYB113 by TAS4. It is not clear whether PAP1 regulates MIR828 transcription, or whether miR828 can down-regulate the expression level of MYB82, a putative miR828 target
Sugar sensing and signaling pathways have been tightly linked with Pi bioavailability in the root responding to Pi starvation (Hammond and White 2008). Arabidopsis plants accumulate starch and sugars in the leaves when treated with low Pi (Lundmark et al. 2010). Several phosphate starvation-responsive genes are sugar-inducible, including PURPLE ACID PHOSPHATASE 17 (PAP17/ACP5), RIBONUCLEASE 1 (RNS1), and INDUCED BY PHOSPHATE STARVATION 1 (IPS1). On the other hand, some hexokinase-independent sugar-sensing genes, for example β-AMYLASE (β-AMY) and CHALCONE SYNTHASE (CHS), are induced by Pi starvation in detached leaf assays as well (Muller et al. 2005). Interestingly, PAP1 expression is triggered by Suc treatment and Pi starvation to similar levels (4- and 3.5-fold, respectively) in a leaf transcriptome profiling study (Muller et al. 2007). miR828 and TAS4-siR81(−) expression respond to Pi deficiency in the shoots of Col-0 as shown by sRNA deep sequencing and Northern blot (Hsieh et al. 2009). However, this finding was not observed by other groups using either RT-PCR, sRNA sequencing, or locked nucleic acid-based microarrays (Pant et al. 2009; Lundmark et al. 2010).
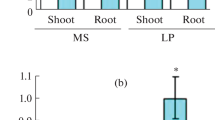
It has been shown that Suc synthesis increases in the leaves of Pi-deficient Arabidopsis, bean, barley, spinach and soybean plants, although some variation may exist (Hammond and White 2008). Suc in the shoot can also be translocated to the root via phloem as the causal intermediary signal, supported by the evidence that Suc concentrations in the root of Pi-starved soybean plants are higher than that in Pi-replete plants (Fredeen et al. 1989; Ciereszko et al. 1996), but not in Arabidopsis (Ciereszko et al. 2001). In addition, genetic screens identified a Pi-deficient mutant, pho3, with reduced root acid phosphatase activity under low Pi conditions (Zakhleniuk et al. 2001). PHO3 is allelic to SUC2, a Suc transporter for phloem loading (Lloyd and Zakhleniuk 2004). The pho3 mutants accumulate high levels of Suc and other carbohydrates because of its inability to translocate them to the roots. Strikingly, PAP1 and PAP2 expression is significantly increased in pho3 mutants based on transcriptome profiling (Lloyd and Zakhleniuk 2004). Taken together, we propose that the up-regulation of TAS4-siR81(−) and miR828 in Pi deficiency could be the consequence of accumulation of Suc and/or Glc in the shoots. In line with this, TAS4-siR81(−) and miR828 are found in shoots, but not roots, of Col-0 seedlings under Pi starvation (Hsieh et al. 2009).
Evolution of TAS and MIRNA genes
We mapped the cleavage site on a MYB target guided by miR828 in P. resinosa, providing direct evidence for miR828 function in gymnosperms. Although P. resinosa miR828 was not found in this study, its paralogs were predicted in closely related P. contorta and P. glauca species with the same mature miR828 (Fig. 8a). It may indicate the conservation for miR828 sequence and for its regulation of MYB targets in gymnosperms. Interestingly, the regulation of MYB expression in dicots may be different from that in gymnosperms. PAP1/PAP2/MYB113 in Arabidopsis all carry TAS4-siR81(−) binding site, and MYB113 is targeted by miR828 as well, which was confirmed by 5′-RACE (Rajagopalan et al. 2006). PAP1 and/or PAP2 are expressed more abundantly and widely than MYB113. For example, PAP1 expression is induced by a variety of stress conditions such as heat, drought, chilling, N deficiency, and ABA in addition to sugars, whereby anthocyanin is accumulated [www.genevestigator.com (Hruz et al. 2008), data not shown]. The common availability of TAS4-siR81(−) binding sites in these MYBs could point out a more important role for TAS4 regulation of them in dicots. miR828 may function as an upstream riboregulator for MYBs, in which it fine-tunes TAS4 expression, whereas the downstream TAS4-derived siRNAs control MYB transcript levels. How miR828 and TAS4 coordinates MYB expression in response to different physiological conditions becomes a critical question to answer.
Although the modes for generating ta-siRNAs and their functions in gene regulation and plant development have been extensively studied, little is known about the molecular evolution of TAS genes. The fact that miR828 and TAS4-siR81(−) regulate the same set of target genes provides a good case for phylogenetic analysis. From our bioinformatic approaches and RACE assays, TAS4 paralogs are only found in dicot plants, while miR828 and its target orthologs are extant in gymnosperms and dicotyledonous plants, suggesting a more ancient origin for MIR828. The extended homologies of cognate MIR828 with its targets in P. contorta, and for TAS4 and MIR828 3′ arm with miR828* in Arabidopsis (Fig. 8b, c) may provide hints for an evolution pathway from MIR828 to TAS4. Our hypothesis is that MYB sequences underwent inverted duplication in a common ancestor of gymnosperms and dicots, from which MIR828 came into being. Subsequently, a duplication event may have occurred on the 3′ arm of miR828 to give two miR828* sequences. Such events could give birth to a proto TAS4 gene, which would be captured by the ta-siRNA pathway(s). Superimposed evolutionary constraints may have driven it towards a role as a regulator of MYB gene expression. From the MIR828-like DNA sequence in T. camschatcense (Fig. 8a), we suggest MIR828 sequences died early in the monocot lineage. The question of why we couldn’t find any monocot or gymnosperm TAS4 is unanswered, but may be related to evolution of specialized MYB functions with implications for homeostatic feedback regulation of environmental signals and the dicot radiation.
Abbreviations
- TAS :
-
Trans-Acting SiRNA Gene
- miRNA:
-
microRNA
- Suc:
-
Sucrose
- Glc:
-
Glucose
- PAP1:
-
Production of Anthocyanin Pigment1
- qRT-PCR:
-
Quantitative real-time Polymerase Chain Reaction
- ABA:
-
Abscisic acid
- sRNA:
-
Small RNA
References
Adenot X, Elmayan T, Lauressergues D, Boutet S, Bouche N, Gasciolli V, Vaucheret H (2006) DRB4-dependent TAS3 trans-acting siRNAs control leaf morphology through AGO7. Curr Biol 16:927–932
Allen E, Xie Z, Gustafson AM, Carrington JC (2005) MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121:207–221
Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, Gadrinab C, Heller C, Jeske A, Koesema E, Meyers CC, Parker H, Prednis L, Ansari Y, Choy N, Deen H, Geralt M, Hazari N, Hom E, Karnes M, Mulholland C, Ndubaku R, Schmidt I, Guzman P, Aguilar-Henonin L, Schmid M, Weigel D, Carter DE, Marchand T, Risseeuw E, Brogden D, Zeko A, Crosby WL, Berry CC, Ecker JR (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:653–657
Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14:2085–2096
Axtell MJ, Bartel DP (2005) Antiquity of microRNAs and their targets in land plants. Plant Cell 17:1658–1673
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12:2383–2394
Bossi F, Cordoba E, Dupre P, Mendoza MS, Roman CS, Leon P (2009) The Arabidopsis ABA-Insensitive (ABI) 4 factor acts as a central transcription activator of the expression of its own gene, and for the induction of ABI5 and SBE2.2 genes during sugar signaling. Plant J 59:359–374
Chang S, Puryear J, Cairney J (1993) A simple and efficient method for isolating RNA from pine trees. Plant Mol Biol Rep 11:113–116
Chiou TJ, Aung K, Lin SI, Wu CC, Chiang SF, Su CL (2006) Regulation of phosphate homeostasis by microRNA in Arabidopsis. Plant Cell 18:412–421
Ciereszko IGA, Mikulska M, Rychter AM (1996) Assimilate translocation in bean plants (Phaseolus vulgaris l.) during phosphate deficiency. J Plant Physiol 149:343–348
Ciereszko I, Johansson H, Hurry V, Kleczkowski LA (2001) Phosphate status affects the gene expression, protein content and enzymatic activity of UDP-glucose pyrophosphorylase in wild-type and pho mutants of Arabidopsis. Planta 212:598–605
Dare AP, Schaffer RJ, Lin-Wang K, Allan AC, Hellens RP (2008) Identification of a cis-regulatory element by transient analysis of co-ordinately regulated genes. Plant Methods 4:17
Dugas DV, Bartel B (2008) Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol Biol 67:403–417
Fahlgren N, Montgomery TA, Howell MD, Allen E, Dvorak SK, Alexander AL, Carrington JC (2006) Regulation of auxin response Factor3 by TAS3 ta-siRNA affects developmental timing and patterning in Arabidopsis. Curr Biol 16:939–944
Finkelstein RR, Gampala SS, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell 14(Suppl):S15–S45
Franco-Zorrilla JM, Valli A, Todesco M, Mateos I, Puga MI, Rubio-Somoza I, Leyva A, Weigel D, Garcia JA, Paz-Ares J (2007) Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet 39:1033–1037
Fredeen AL, Rao IM, Terry N (1989) Influence of phosphorus nutrition on growth and carbon partitioning in Glycine max. Plant Physiol 89:225–230
Fujii H, Chiou TJ, Lin SI, Aung K, Zhu JK (2005) A miRNA involved in phosphate-starvation response in Arabidopsis. Curr Biol 15:2038–2043
Garcia D, Collier SA, Byrne ME, Martienssen RA (2006) Specification of leaf polarity in Arabidopsis via the trans-acting siRNA pathway. Curr Biol 16:933–938
Givnish TJ, Pires JC, Graham SW, Mcpherson MA, Prince LM, Patterson TB, Rai HS, Roalson EH, Evans TM, Hahn WJ, Millam KC, Meerow AW, Molvray M, Kores PJ, O’brien HE, Hall JC, Kress WJ, Sytsma KJ (2006) Phylogenetic relationships of monocots based on the highly informative plastid gene ndhF: evidence for widespread concerted convergence. Aliso 22:28–51
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/BHLH/MYB transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827
Hammond JP, White PJ (2008) Sucrose transport in the phloem: integrating root responses to phosphorus starvation. J Exp Bot 59:93–109
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27:297–300
Hruz T, Laule O, Szabo G, Wessendorp F, Bleuler S, Oertle L, Widmayer P, Gruissem W, Zimmermann P (2008) Genevestigator v3: A reference expression database for the meta-analysis of transcriptomes. Adv Bioinform 2008:420747
Hsieh LC, Lin SI, Shih AC, Chen JW, Lin WY, Tseng CY, Li WH, Chiou TJ (2009) Uncovering small RNA-mediated responses to phosphate deficiency in Arabidopsis by deep sequencing. Plant Physiol 151:2120–2132
Huang Y, Li CY, Biddle KD, Gibson SI (2008) Identification, cloning and characterization of sis7 and sis10 sugar-insensitive mutants of Arabidopsis. BMC Plant Biol 8:104
Huijser C, Kortstee A, Pego J, Weisbeek P, Wisman E, Smeekens S (2000) The Arabidopsis SUCROSE UNCOUPLED-6 gene is identical to ABSCISIC ACID INSENSITIVE-4: involvement of abscisic acid in sugar responses. Plant J 23:577–585
Jang JC, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6:1665–1679
Jones-Rhoades MW, Bartel DP (2004) Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol Cell 14:787–799
Kawashima CG, Yoshimoto N, Maruyama-Nakashita A, Tsuchiya YN, Saito K, Takahashi H, Dalmay T (2009) Sulphur starvation induces the expression of microRNA-395 and one of its target genes but in different cell types. Plant J 57:313–321
Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23:587–596
Lloyd JC, Zakhleniuk OV (2004) Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot 55:1221–1230
Lundmark M, Korner CJ, Nielsen TH (2010) Global analysis of microRNA in Arabidopsis in response to phosphate starvation as studied by locked nucleic acid-based microarrays. Physiol Plant 140:57–68
Luo QJ, Samanta MP, Koksal F, Janda J, Galbraith DW, Richardson CR, Ou-Yang F, Rock CD (2009) Evidence for antisense transcription associated with microRNA target mRNAs in Arabidopsis. PLoS Genet 5(4):e1000457
Marin E, Jouannet V, Herz A, Lokerse AS, Weijers D, Vaucheret H, Nussaume L, Crespi MD, Maizel A (2010) miR390, Arabidopsis TAS3 tasiRNAs, and their auxin response factor targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell 22:1104–1117
Montgomery TA, Howell MD, Cuperus JT, Li D, Hansen JE, Alexander AL, Chapman EJ, Fahlgren N, Allen E, Carrington JC (2008a) Specificity of Argonaute7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133:128–141
Montgomery TA, Yoo SJ, Fahlgren N, Gilbert SD, Howell MD, Sullivan CM, Alexander A, Nguyen G, Allen E, Ahn JH, Carrington JC (2008b) AGO1-miR173 complex initiates phased siRNA formation in plants. Proc Natl Acad Sci USA 105:20055–20062
Muller R, Nilsson L, Nielsen LK, Nielsen TH (2005) Interaction between phosphate starvation signalling and hexokinase-independent sugar sensing in Arabidopsis leaves. Physiol Plant 124:81–90
Muller R, Morant M, Jarmer H, Nilsson L, Nielsen TH (2007) Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiol 143:156–171
Nakazawa Y, Hiraguri A, Moriyama H, Fukuhara T (2007) The dsRNA-binding protein DRB4 interacts with the Dicer-like protein DCL4 in vivo and functions in the trans-acting siRNA pathway. Plant Mol Biol 63:777–785
Pant BD, Musialak-Lange M, Nuc P, May P, Buhtz A, Kehr J, Walther D, Scheible WR (2009) Identification of nutrient-responsive Arabidopsis and rapeseed microRNAs by comprehensive real-time polymerase chain reaction profiling and small RNA sequencing. Plant Physiol 150:1541–1555
Parcy F, Valon C, Kohara A, Misera S, Giraudat J (1997) The ABSCISIC ACID-INSENSITIVE3, FUSCA3, and LEAFY COTYLEDON1 loci act in concert to control multiple aspects of Arabidopsis seed development. Plant Cell 9:1265–1277
Peragine A, Yoshikawa M, Wu G, Albrecht HL, Poethig RS (2004) SGS3 and SGS2/SDE1/RDR6 are required for juvenile development and the production of trans-acting siRNAs in Arabidopsis. Genes Dev 18:2368–2379
Pourtau N, Jennings R, Pelzer E, Pallas J, Wingler A (2006) Effect of sugar-induced senescence on gene expression and implications for the regulation of senescence in Arabidopsis. Planta 224:556–568
Rajagopalan R, Vaucheret H, Trejo J, Bartel DP (2006) A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev 20:3407–3425
Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Ann Rev Plant Biol 57:675–709
Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW (2001) Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J 26:421–433
Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P (2006) Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol 140:637–646
Souret FF, Kastenmayer JP, Green PJ (2004) AtXRN4 degrades mRNA in Arabidopsis and its substrates include selected miRNA targets. Mol Cell 15:173–183
Teng S, Keurentjes J, Bentsink L, Koornneef M, Smeekens S (2005) Sucrose-specific induction of anthocyanin biosynthesis in Arabidopsis requires the MYB75/PAP1 gene. Plant Physiol 139:1840–1852
Vazquez F, Vaucheret H, Rajagopalan R, Lepers C, Gasciolli V, Mallory AC, Hilbert JL, Bartel DP, Crete P (2004) Endogenous trans-acting siRNAs regulate the accumulation of Arabidopsis mRNAs. Mol Cell 16:69–79
Xie Z, Allen E, Wilken A, Carrington JC (2005) DICER-like 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc Natl Acad Sci USA 102:12984–12989
Yoon EK, Yang JH, Lim J, Kim SH, Kim SK, Lee WS (2010) Auxin regulation of the microRNA390-dependent transacting small interfering RNA pathway in Arabidopsis lateral root development. Nucleic Acids Res 38:1382–1391
Yoshikawa M, Peragine A, Park MY, Poethig RS (2005) A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev 19:2164–2175
Zakhleniuk OV, Raines CA, Lloyd JC (2001) pho3: A phosphorus-deficient mutant of Arabidopsis thaliana (L. Heynh). Planta 212:529–534
Zuker M (2003) MFold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31:3406–3415
Acknowledgments
The authors thank the Arabidopsis Biological Resource Center at Ohio State University for seeds, the greenhouse staff in the Department of Biological Sciences at Texas Tech University, Ruth Finkelstein for the abi4-103 mutant seeds, Zhixin Xie for the rdr6-15 and dcl4-2 mutant seeds, Xuemei Chen for the hen1-1 mutant seeds, and Hong-Liang Zhu for discussion and technical support. This work was supported by the National Institutes of Health (R21GM077245 to C.D.R.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2011_9778_MOESM1_ESM.docx
Fig. S1. Predicted PAP1 cis-regulatory elements (C/T)(A/C)NCCACNN(G/T) in TAS4, MIR828 and PAP1 promoters. Putative promoter sequences for 2,000 bp upstream of the transcription start sites for TAS4, MIR828 and PAP1 (Panels A, B, and C, respectively) were extracted from the TAIR website (www.arabidopsis.org). TATA and CAAT boxes are predicted by the Plant Cis-acting Regulatory DNA Elements Database (PLACE) (Higo et al. 1999), and PAP1 cis-regulatory elements are found manually according to a consensus sequence functionally characterized as described (Dare et al. 2008). The cis-regulatory elements within the putative MIR828 promoter locate in the 3′-untranslated region (3′-UTR) of its upstream gene At4g27750. The annotation for color labels is as follows: white letters in uppercase with blue color background, stop codon; orange letters in uppercase, exon; red letters in lowercase, 3′-UTR; purple letters in lowercase, intron; black letters in lowercase, intergenic regions; white letters in lowercase with red color background, PAP1 cis-regulatory elements; green color, TATA box; yellow color, CAAT box. (DOCX 20 kb)
11103_2011_9778_MOESM2_ESM.tif
Fig. S2. Sequence alignments of MYB family members from dicot and monocot species. The full length cDNA sequences were aligned with T-Coffee and part of the alignments is shown. Panel A: alignment for sequences spanning miR828 complementary site. Panel B: alignment for sequences spanning TAS4-siR81(−) complementary site. Abbreviations correspond to species are listed as follows. Ath, Arabidopsis thaliana; Vvi, Vitis vinifera; Ghy, Gerbera hybrid cultivar; Iba, Ipomoea batatas; Fcy, Fagopyrum cymosum. (TIFF 221 kb)
11103_2011_9778_MOESM3_ESM.tif
Fig. S3. Alternate hairpin structures of the Picea glauca (spruce) pri-miR828 predicted by MFOLD, showing only one “good” DCL substrate per structure. Green color shows mature miR828; red and blue lines indicate putative alternate secondary structures for the same pri-miR828. (TIFF 156 kb)
11103_2011_9778_MOESM4_ESM.tif
Fig. S4. Predicted secondary structure of Euphorbia esula pri-miR828 by MFOLD. The predicted mature miR828 sequences are in green. (TIFF 136 kb)
11103_2011_9778_MOESM5_ESM.tif
Fig. S5. Predicted secondary structure of Pinus contorta pri-miR828 by MFOLD. The predicted mature miR828 sequences are in green. (TIFF 111 kb)
11103_2011_9778_MOESM6_ESM.tif
Fig. S6. Predicted secondary structure of Trillium camschatcense MIR828 gene by MFOLD. The predicted mature miR828 sequences are in green. (TIFF 99 kb)
Rights and permissions
About this article
Cite this article
Luo, QJ., Mittal, A., Jia, F. et al. An autoregulatory feedback loop involving PAP1 and TAS4 in response to sugars in Arabidopsis. Plant Mol Biol 80, 117–129 (2012). https://doi.org/10.1007/s11103-011-9778-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-011-9778-9