Abstract
In the last few years, the role of reactive oxygen species as signaling molecules has emerged, and not only as damage-related roles. Here, we analyzed how root hydraulic properties were modified by different hydrogen peroxide (H2O2) concentrations applied exogenously to the root medium. Two different experimental setups were employed: Phaseolus vulgaris plants growing in hydroponic or in potted soils. In both experimental setups, we found an increase of root hydraulic conductance (L) in response to H2O2 application for the first time. Twenty millimolar was the threshold concentration of H2O2 for observing an effect on L in the soil experiment, while in the hydroponic experiment, a positive effect on L was observed at 0.25 mM H2O2. In the hydroponic experiment, a correlation between increased L and plasma membrane aquaporin amount and their root localization was observed. These findings provide new insights to study how several environmental factors modify L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Plants are exposed to several environmental changes which, in some circumstances, may cause stress. Many environmental stresses induce similar plant responses, such as tissue dehydration or oxidative damage. These two stress effects are caused by soil water limitation (Aroca et al. 2003), extreme temperatures (Aroca et al. 2003; Wahid et al. 2007), soil salinity (Tattini and Traversi 2008), or flooding (Sairam et al. 2008). Plant tissue dehydration is caused by the imbalance between water loss by leaf transpiration and water uptake by roots. In fact, upon exposure to different stresses, plants reduce their stomatal aperture in order to minimize their water loss (Yang et al. 2005; Schachtman and Goodger 2008). At the same time, root water uptake capacity under different environmental stress conditions is related to tolerance to such stresses (Marulanda et al. 2003; Aroca et al. 2005; del Martínez-Ballesta et al. 2006).
Root water uptake is determined, in part, by root hydraulic conductance (L), which is ultimately regulated by plasma membrane aquaporins activity (Kaldenhoff et al. 1998; Javot et al. 2003). Aquaporins are membrane-intrinsic proteins that facilitate the flow of water and other small and neutral solutes through membranes following an osmotic gradient (Maurel 2007; Maurel et al. 2008). In plants, the aquaporin family comprises around 30 different proteins, at least in Arabidopsis, maize, and rice (Chaumont et al. 2001; Johanson et al. 2001; Sakurai et al. 2005). Plant aquaporins are subdivided into four subgroups based on their amino acid sequence similarity: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin-like intrinsic proteins (NIPs), and small and basic intrinsic proteins (SIPs). PIPs are further subdivided into PIP1 and PIP2 proteins, with PIP2 proteins being more active in terms of water transport capacity (Chaumont et al. 2000; Sakurai et al. 2005). Apart from their different subcellular localization, each aquaporin protein differs in its solute transport selectivity (Katsuhara et al. 2008).
L generally decreases under abiotic stresses conditions (Aroca et al. 2005, 2006; del Martínez-Ballesta et al. 2006; Boursiac et al. 2008). However, a maize cold tolerant genotype and a bean cultivar increased their L after some days of cold exposure (Vernieri et al. 2001; Aroca et al. 2005). The decrease of L by different abiotic stresses, such as cold or salt, has been linked to the production of reactive oxygen species (ROS) under such conditions (Ktitorova et al. 2002; Lee et al. 2004; Aroca et al. 2005; Rhee et al. 2007; Boursiac et al. 2008). In fact, when exogenous hydrogen peroxide (H2O2) treatment, commonly used to simulate ROS effects in plants (Desikan et al. 2001; Wan and Liu 2008), is added to solution surrounding roots, a decrease of L is commonly observed (Ktitorova et al. 2002; Lee et al. 2004; Aroca et al. 2005; Rhee et al. 2007; Boursiac et al. 2008). However, in some studies, exogenous H2O2 caused a slight non significant increase of L (Aroca et al. 2005; Boursiac et al. 2008). At the same time, the effect of exogenous application of abscisic acid (ABA) on the root-free exuded sap flow rate is modified by exogenous co-application of catalase (H2O2-scavenging enzyme) or ascorbate (a potent and widespread antioxidant) in Phaseolus vulgaris plants, with the effect of antioxidants being different as the exogenous ABA concentration applied rose (Aroca 2006).
The above cited results indicate a possible role of ROS in regulating root hydraulic properties, decreasing or increasing L depending on the environmental circumstances. This interpretation fits the dual role of ROS in plants, since they can act as damaging or signaling molecules, depending on the environmental conditions (Hung et al. 2005; Miller et al. 2008; Van Breusegem et al. 2008). In fact, it is well documented how ROS act as signaling molecules during ABA-induced stomatal closure (Pei and Kuchitsu 2005; Wang and Song 2008). At the same time, several mechanisms have been proposed to explain the diminution of L caused by ROS. Ye and Steudle (2006) proposed that H2O2, or most likely, hydroxyl radical (OH•) could cause conformational changes on aquaporins, avoiding water transport. Boursiac et al. (2008) found an internalization of plasma membrane vesicles containing a high amount of PIP proteins after H2O2 addition to Arabidopsis roots, which could cause a diminution of the plasma membrane hydraulic conductivity. The studies of Ye and Steudle (2006) and Boursiac et al. (2008) were carried out in a relatively short period of time, from 15 to 120 min. This time frame fits well with the initial decrease of L observed after plant exposure to several stresses (Aroca et al. 2001; del Martínez-Ballesta et al. 2006; Boursiac et al. 2008). However, this time frame is very short when compared to that required by bean plants to increase their L upon exposure to cold (1 day; Vernieri et al. 2001), or after ABA application (1 day; Aroca 2006), or to that needed by a maize cold-tolerant genotype to increase L upon exposure to cold (3 days; Aroca et al. 2001, 2005).
Most of the plants in nature are associated with arbuscular mycorrhizal (AM) fungi (Gai et al. 2006). By this association, the fungus obtains carbon compounds from the plant and a niche to complete its life cycle, while the plant obtains access to nutrient and water resources from the fungus, which is inaccessible to the roots (Harrison 2005). At the same time, by the AM symbiosis, the host plant enhances their tolerance to several biotic and abiotic stresses (Wu et al. 2006; Aroca et al. 2007; Liu et al. 2007). This enhancement is, in part, caused by the modulation of the antioxidant capacity of the host plant (Wu et al. 2006), as well as L and PIP aquaporin abundance (Aroca et al. 2007). Hence, it is possible that the different responses of L and aquaporins to different abiotic stresses between AM and non AM roots (Porcel et al. 2006; Aroca et al. 2007) could be mediated by the different regulation of root cells redox status by the AM symbiosis (Schützendübel and Polle 2002; Wu et al. 2006). Therefore, experiments were carried out with both hydroponic and soil-growing plants, allowing the establishment and development of the AM symbiosis in the second grown medium.
In the present research, we investigated how different exogenous concentrations of H2O2 affect L in bean plants during different times of exposure (from 1 to 24 h). Also, L and PIP aquaporin transcriptional regulation and protein abundance were also studied, as well as their root localization. We found, for the first time, an increase of L in response to specific exogenous H2O2 concentrations, which correlated with an increase in PIP2 aquaporin amount in the cells from the water-absorbing zone of the roots. These results point out a possible involvement of ROS in the increase of L observed in response to some environmental stresses.
Materials and methods
Biological materials and experimental design
Hydroponic experiments
Seeds of Phaseolus vulgaris L. cv. Borlotto were soaked for 3 min in pure ethanol and then rinsed three times with distilled water. Seeds were sown in wet sepiolite (a clay mineral) and, after 9 days, seedlings were transferred to aerate 9-l containers filled with 80% nutrient solution (Aroca 2006). The nutrient solution was replaced every four days. Plants were grown in a controlled growth chamber at 23:21°C (day:night), in a photoperiod of 16:8 h (day:night), with a photosynthetic photon flux density of 300 μmol m−2 s−1. After 2 weeks of hydroponic growth, the roots were exposed to a final concentration of 0.25, 0.5, 1, or 5 mM H2O2. Root samples and L measurements were taken 1, 6, or 24 h after the H2O2 addition in both treated and non treated plants. Samples for molecular and biochemical analysis were immersed in liquid nitrogen and stored at −80°C until use. The other measurements were immediately carried out after removing the roots from the solution.
Soil pot experiments
Bean seeds were treated and germinated as described above, and then transplanted to 500-ml pots filled with a sterilized mixture of sand and soil (1:1, v/v). Half of the pots were inoculated with the AM fungus Glomus intraradices (Schenk and Smith) isolate BEG 123. For details about the soil characteristics and inoculation procedure, see Porcel et al. (2006). Five weeks after being transplanted, the soil roots were exposed to a final concentration of 0.25, 0.5, 1, 5, 10, or 20 mM H2O2 by adding 20 ml of appropriate dilution from a concentrated solution to the pots. Root samples and L measurements were taken 1, 6, and 24 h after H2O2 addition in both treated and non treated plants. Samples for molecular and biochemical analysis were immersed in liquid nitrogen and stored at −80°C until use. Mycorrhizal development inside the roots was measured after clearing washed roots in 10% KOH and staining with 0.05% trypan blue in lactic acid (w/v), according to Philips and Hayman (1970). The extent of mycorrhizal root colonization was calculated according to the gridline intersect method (Giovannetti and Mosse 1980).
Root hydraulic properties measurements
Root hydraulic conductance (L) was measured as described previously by Aroca et al. (2001, 2006). Briefly, plants growing hydroponically or in pots (the latter immersed in aerated solution) were cut with a razor blade just below the cotyledons. A pipette connected to a silicon tube was attached to the stem. The liquid exuded in the first 15 min was discarded to avoid phloem contaminations. The exudates of the following 2 h were collected and weighed. The osmolarities of the exuded sap were determined using a cryoscopic osmometer (Osmomat 030, Gonotec Gmbh, Berlin, Germany). L was calculated as: L = J v/ΔΨs, where J v is the exuded sap flow rate and ΔΨs is the osmotic potential gradient between the exuded sap and the solution. L was measured in 15 plants from hydroponic pots and nine from soil pots.
Reactive oxygen species determination
Hydrogen peroxide contents of root tissues from soil pot plants were determined by Patterson’s method (Patterson et al. 1984), with slight modifications as described previously by Aroca et al. (2003). One gram of root fresh weight from four different plants was homogenized in a cold mortar with 5 ml 5% (w/v) TCA containing 0.1 g of activated charcoal and 1% (w/v) PVPP. The homogenate was centrifuged at 18,000g for 10 min. The supernatant was filtered through a Millipore filter (0.22 μm). A volume of 1.2 ml of 100 mM potassium phosphate buffer (pH = 8.4) and 0.6 ml of the colorimetric reagent were added to 130 μl of the supernatant. The colorimetric reagent was freshly made by mixing 1:1 (v/v) 0.6 mM potassium titanium oxalate and 0.6 mM 4-2 (2-pyridylazo)resorcinol (disodium salt). The samples were incubated at 45°C for 1 h and the absorbance at 508 nm was recorded. The same procedure was followed to measure the H2O2 content in the hydroponic solution after 1, 6, or 24 h of H2O2 application.
The probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) was used to detect ROS in hydroponic roots, both from untreated or treated with 1 mM H2O2 plants (Benabdellah et al. 2009). Roots were incubated with 10 μM H2DCF-DA in PBS (137 mM NaCl, 2.7 mM KCl, 1.4 mM Na2HPO4, pH = 7.4). The probe can cross the root plasma membrane freely, and is then cleaved to its impermeable counterpart, dichlorofluorescein (H2DCF), by endogenous esterases. H2DCF functions as a reporter of ROS content. After 30 min, the medium was replaced with fresh PBS and observations were made under the fluorescence microscope. The excitation and the emission wavelengths were 450–490 and 520 nm (green), respectively.
Root electrolyte leakage
Root electrolyte leakage was measured in hydroponic culture plants as an index of membrane root injury as previously described by Aroca et al. (2005). Briefly, entire root systems from 14 plants were placed individually in tubes containing 50 ml of distilled water. After 3 h of incubation at room temperature in smooth agitation, the conductivity of the solution was measured (conductivity meter, COND 510, XS Instrumentation) and is referred to as C 0. Then, the tubes were placed at −80°C for 1 h and incubated again at room temperature for 2 h. The conductivity of the solution at this time was referred to as C T. The conductivity of distilled water before the root immersion was measured and referred to as C W. The percentage of electrolyte leakage was calculated as follows: EL = [(C 0 − C W)/(C T − C W)] × 100.
PIP expression analyses
Expression analyses of the six PIP genes described up to now in P. vulgaris were carried out by quantitative real-time RT-PCR (Aroca et al. 2007; Zhou et al. 2007). Total RNA was isolated from P. vulgaris roots by a phenol/chloroform extraction method followed by precipitation with LiCl (Kay et al. 1987). DNase treatment of total RNA was performed according to Promega’s recommendations. Total RNA (2.5 μg) was reversed transcribed to first strand cDNA using AMV-RT enzyme and oligo(dT)15 primer (Promega, Madison, WI, USA). Quantitative real-time RT-PCR was carried out by using an iCycler (Bio-Rad, Hercules, CA, USA), as described by Porcel et al. (2006). The PIP genes analyzed were: PvPIP1;1 (Acc. No. U97023), PvPIP1;2 (Acc. No. AY995196), PvPIP1;3 (Acc. No. DQ855475), PvPIP2;1 (Acc. No. AY995195), PvPIP2;2 (Acc. No. EF624001), and PvPIP2;3 (Acc. No. EF624002). Specific primers for PvPIP1;2, PvPIP2;2, and PvPIP2;3 were the same as those described by Aroca et al. (2007) and Zhou et al. (2007). The PCR mix and PCR program were as described by Porcel et al. (2006), except annealing temperature, which was switched to 58°C. The primers for PvPIP1;1 were forward: ATTAGGTGGTGCATGTGTCG, and reverse: CAGCCGTACATCACACATAGAG. Primers for PvPIP1;3 were forward: GTGGGTAAAAGAACCAGACG, and reverse: CACATCGCTCATGGTCATC. Primers for PvPIP2;1 were forward: CTCCAACAAGGCTATGGTGT, and reverse: CCCACAACAACTGTAGCTGA. Primers were designed to amplify a fragment of the 3′ untranslated region of each gene that is specific for each of them. Standardization was carried out by measuring the expression of the P. vulgaris actin gene in each sample by using P. vulgaris actin-specific primers (Wen et al. 2005). The relative abundances of transcription were calculated by using the 2−ΔΔ c t method (Livak and Schmittgen 2001). Analyses were carried out in three independent RNA samples from three different root samples. Analyses were repeated three times for each RNA sample.
Western blots
Microsomes were isolated from P. vulgaris roots as described by Daniels et al. (1994). Briefly, roots were homogenized in grinding buffer (100 mM Tris–HCl pH = 7.5, 1 mM EDTA, 12% (w/v) sucrose, 0.2 mM aminoethylbenzenesulfonylfluoride, 2 μg ml−1 aprotinin, 1 μg ml−1 leupeptin), and the supernatant was collected after centrifugation at 15,000g for 10 min. The supernatant was filtered through a single layer of cheesecloth and centrifuged again at 100,000g for 2 h. The pellet was resuspended in 40 μl of grinding buffer plus 0.1% (w/v) SDS. Ten micrograms of protein were loaded in each line after incubating for 30 min at 37°C in the presence of denaturing buffer (20 mM Tris–HCl pH = 8.6, 1% (w/v) SDS, 0.3% (v/v) β-mercaptoethanol, 8% (v/v) glycerol, 0.2% (w/v) bromophenol-blue). Proteins were transferred to a PVDF membrane at 100 mA for 1 h. The membrane was blocked for 2 h at room temperature with 5% (w/v) non fat milk in Tris-buffered-saline (TBS) with 0.05% Tween 20. After that, the membrane was incubated overnight at 4°C with the corresponding antibody dilution (1:2,000 for PIP1 and PIP2 antibodies, and 1:1,000 for phosphorylated PIP2 antibody). Goat anti-rabbit Ig coupled to horseradish peroxidase (Sigma) was used as a secondary antibody at a dilution of 1:20,000. The signal was developed using a chemiluminescent substrate (West-Pico, Super Signal, Pierce, Rockford, IL, USA). PIP1 and PIP2 antibodies were kindly provided by Dr. Maurel (INRA, Montpelier, France), and were raised in rabbit against a 42-amino acid N-terminal peptide of AtPIP1;1 or 17-amino acid C-terminal peptide of AtPIP2;1, respectively (Santoni et al. 2003). Phosphorylated PIP2 antibody was kindly provided by Dr. Hermann (Donald Danforth Plant Science Center, St. Louis, MO, USA) and raised against the most conserved sequence among all PIP2 proteins in diverse species with a possible phosphorylation site (Aroca et al. 2005). These antibodies were used previously with P. vulgaris root microsomes (Aroca et al. 2006, 2007). Microsomes were isolated from two different root samples. Western blots were developed on both microsome samples of each treatment without significant differences between them.
PIP2 immunolocalization
PIP2 proteins were localized using the AtPIP2;1 antibody used for Western blots. Roots were fixed for 30 min in freshly prepared 4% (w/v) paraformaldehyde (Sigma) PBS solution, washed two times with PBS for 45 min, and digested at 37°C for 30 min in a mixture of 2.5% (w/v) pectolyase (Sigma) and 2.5% (w/v) cellulose (Sigma) dissolved in PBS in order to facilitate antibody permeation. For thin-section analyses, the roots were cut into 5-mm pieces and fixed in 4% (w/v) formaldehyde in PBS for 2 h at 4°C. Samples were washed twice in PBS and dehydrated through a graded ethanol series and infiltrated in 1:1 100% ethanol and LR White (London Resin Co.) overnight and 100% resin for 24 h. Serial sections, 1-μm thick, were obtained by means of an Ultracut R Ultramicrotome (Leica, Bensheim, Germany).
Sections and roots pieces were incubated with the anti-AtPIP2;1 diluted 1:2,000 in PBS, containing 0.1% w/v bovine serum albumin. Incubation was done in a moist chamber for 90 min at RT. Slides were then washed twice for 10 min in PBS and incubated for 90 min with secondary antibody, goat anti-rabbit IgG–FITC conjugate (Molecular Probes) diluted to 1:1,000 in the same way as the first antibody. Incubation was performed at the same temperature as with the first antibody in darkness. After washing for 10 min in PBS, the sections and root pieces were mounted under a cover slip using p-phenylenediamine anti-fade mounting. Fluorescence was examined with a Nikon Eclipse inverted microscope equipped with epifluorescence and appropriate filter sets (peak excitation, 590 nm; peak emission, 617 nm). Photographs were taken with a DS-Fi1 Nikon camera.
Results
We first tested how the H2O2 levels were maintained during the experiment in the hydroponic solution. After 1 h of adding 0.25 or 1 mM H2O2, the H2O2 content was decreased by almost 50%. After 6 h of addition, H2O2 was only detected at 10% of the initial values, and after 24 h, the H2O2 content was almost undetected in both treatments (data not shown).
Root hydraulic properties
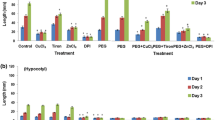
Root hydraulic conductance (L) was measured in roots exuding under atmospheric pressure. Under these conditions, water only goes through roots thanks to the osmotic gradient between root solution and root xylem. Hence, water only flowed through the cell-to-cell pathway according to Steudle’s model (Steudle and Peterson 1998). Under hydroponic culture, the application of 5 mM exogenous H2O2 reduced root water flux (J v ) and L without any recovery after 24 h (Fig. 1a, c). At the same time, the 5 mM H2O2 application caused an increase in the osmotic gradient between the nutrient solution and exuded sap (ΔΨs) after 1 h of exposure (Fig. 1b). A total of 1 mM H2O2 had similar effects to 5 mM in J v and L, but at 1 mM concentration, both parameters recovered after 24 h of exposure (Fig. 1a, c). The two lowest concentrations of H2O2 used (0.25 and 0.5 mM) caused an increase of J v and L after 24 h of exposure (Fig. 1a, c). However, 0.25 mM H2O2 exposure caused an increase of L after 6 h, and a decrease of ΔΨs after 6 and 24 h (Fig. 1b, c). In summary, lower H2O2 concentrations caused an increase of L, more pronounced after 24 h, while higher concentrations caused an inhibition of L, beginning just after 1 h. However, a recovery of L was observed in 1 mM treated roots after 24 h.
Root-free exuded sap (J
v
, a), osmotic potential difference between exuded sap and nutrient solution (ΔΨs, b), and root hydraulic conductance (L, c) of Phaseolus vulgaris plants untreated (□), or treated with 0.25 mM ( ), 0.5 mM (■), 1 mM (
), 0.5 mM (■), 1 mM ( ), or 5 mM (
), or 5 mM ( ) H2O2 for 1, 6, or 24 h, corresponding to 3, 8, or 2 h after the lights turned on. The asterisks indicate significant difference (P < 0.05) with the corresponding untreated root after ANOVA and Fisher LSD analysis. The bars represent mean ± SD (n = 15)
) H2O2 for 1, 6, or 24 h, corresponding to 3, 8, or 2 h after the lights turned on. The asterisks indicate significant difference (P < 0.05) with the corresponding untreated root after ANOVA and Fisher LSD analysis. The bars represent mean ± SD (n = 15)
In the plants growing in soil, only the highest H2O2 concentration used (20 mM) had a significant effect on J v or L, and only after 24 h. Therefore, we only show these results in Fig. 2. Interestingly, 20 mM H2O2 exposure up to 24 h caused an increase of L in both AM and non AM plants, but only caused a significant increase of J v in non AM plants (Fig. 2). Thus, the increase of L by H2O2 in AM plants was caused by slight non significant increases or decreases of J v and ΔΨs, respectively (Fig. 2). In other words, the effect of H2O2 was more pronounced in non AM plants.
Root-free exuded sap (J v , a), osmotic potential difference between exuded sap and nutrient solution (ΔΨs, b), and root hydraulic conductance (L, c) of P. vulgaris plants inoculated or not inoculated with the AM fungus Glomus intraradices, and exposed (black columns) or not (white columns) to 20 mM H2O2 for 24 h. The different letters indicate significant differences (P < 0.05) among treatments after ANOVA and Fisher LSD analysis. The bars represent mean ± SD (n = 9)
Hydrogen peroxide levels in soil roots
It is well documented that AM and non AM roots differ in their capacity to remove ROS (Wu et al. 2006), thus, we evaluated root H2O2 levels in soil-growing roots in order to clarify the different response of J v and L to H2O2 in AM and non AM plants. Just after 1 h of exogenous H2O2 application, the roots of non AM plants presented 2.5 times higher concentrations of H2O2 than non AM untreated roots, and this difference remained during 6 h of exposure (Fig. 3a). On the contrary, AM roots only increased their H2O2 contents after 6 h of exposure to H2O2 (Fig. 3a). After 24 h of exposure, both kinds of plants returned their root H2O2 content to untreated values (Fig. 3a). Therefore, the roots of non AM plants responded faster and were more prolonged to H2O2 exposure than the roots of AM plants.
a Hydrogen peroxide levels of P. vulgaris roots inoculated (white columns) or not inoculated (black columns) with the AM fungus G. intraradices after 1, 6, or 24 h of exposure to 20 mM H2O2. The data are expressed as a percentage of the untreated roots. The asterisks indicate significant difference (P < 0.05) with the corresponding untreated root after ANOVA and Fisher LSD analysis. The bars represent mean ± SD (n = 4). b Relative mRNA expression of different PIP genes of P. vulgaris roots inoculated (white columns) or not inoculated (black columns) with the AM fungus G. intraradices after 24 h of exposure to 20 mM H2O2. The asterisks indicate significant difference (P < 0.05) with the corresponding untreated root after ANOVA and Fisher LSD analysis. The bars represent mean ± SD (n = 3). c Western blot analysis of microsomes from P. vulgaris roots inoculated or not with the AM fungus G. intraradices and treated or not with 20 mM H2O2. The antibodies used were against AtPIP1;1 (PIP1), AtPIP2;1 (PIP2), or phosphorylated PIP2s at Ser 121 (PhPIP2). The last picture represents the coomassie brilliant blue line of each microsome treatment
PIP expression and abundance in soil roots
Since J v and L are determined, in part, by PIP aquaporin activity (Kaldenhoff et al. 1998; Javot et al. 2003), we analyzed PIP gene expression and protein abundance in AM and non AM roots after 24 h of exposure to 20 mM H2O2. H2O2 exposure caused an increase of PvPIP2;2 and PvPIP2;3 gene expression in AM roots, while it decreased PvPIP1;1, PvPIP1;2, PvPIP2;1, and PvPIP2;2 gene expression in non AM roots (Fig. 3b). Thus, the effect of H2O2 exposure on PIP gene expression was opposite in both kinds of roots.
PIP1s and PIP2s protein abundance and phosphorylation state at Ser 121 (123) of PIP2s proteins were analyzed by Western blots using antibodies against a 42-amino acid N-terminal peptide of AtPIP1;1, a 17-amino acid C-terminal peptide of AtPIP2;1 (Santoni et al. 2003), and an 8-amino acid peptide surrounding Ser 113 of Triticum aestivum PIP2 (Aroca et al. 2005). These same antibodies have been successfully used previously with microsomes from P. vulgaris roots (Aroca et al. 2006, 2007). The three antibodies presented the same signaling motif. Without H2O2 treatment, no signal was detected in AM roots (Fig. 3c). After H2O2 treatment, no changes in any of the signal amounts was observed in non AM roots, but a significant increase was observed in AM roots (Fig. 3c). So, a certain correlation between an increase of L and PIP expression and abundance was observed in AM roots, although this correlation was not found in non AM roots. Since PIPs protein abundance changes along the root axes, and between different kinds of root cells (Hachez et al. 2006), we decided to focus on hydroponic experiments.
Electrolyte leakage and ROS contents in hydroponic roots
One of the causes of decreasing L by 1 or 5 mM H2O2 treatments could be membrane damage. This can be estimated by measuring the root electrolyte leakage (EL) (Aroca et al. 2005; Aroca 2006). So, we decided to measure the EL in roots of untreated or treated with 0.25 or 1 mM H2O2 plants, selecting the minimal concentrations of H2O2 that caused an increase and a decrease in L, respectively (Fig. 1c). EL only increased after 1 and 6 h of root exposure to 1 mM H2O2, falling back to control values after 24 h (Fig. 4). The same pattern of EL was observed in root ROS content visualized by H2DCF-DA probe in the roots treated with 1 mM H2O2 (Fig. 5). The ROS content increased after 1 and 6 h of root exposure to 1 mM H2O2, but returned back to control levels after 24 h. Therefore, a correlation between an increase of EL and ROS and a decrease of L was found in roots exposed to 1 mM H2O2. At the same time, the same roots recovered their L values, while decreasing their EL and ROS levels.
Electrolyte leakage (EL) of roots grown in hydroponic soil after 1, 6, or 24 h of exposure to 0 (white columns), 0.25 (grey columns), or 1 mM (black columns) H2O2. The asterisks indicate significant difference (P < 0.05) with the corresponding untreated root after ANOVA and Fisher LSD analysis. The bars represent mean ± SD (n = 14)
PIP gene expression and abundance in hydroponic roots
As previously commented, the expression analysis of PIP genes was carried out in untreated roots or roots treated with 0.25 or 1 mM H2O2. In untreated roots, PvPIP1;1 and PvPIP1;2 genes did not change their expression during the day (Fig. 6a, c). By contrast, PvPIP1;3, PvPIP2;1, and PvPIP2;3 genes decreased their expression after 6 h (8 h after dawn) of H2O2 application (Fig. 6b, e, f). PvPIP1;3 and PvPIP2;3 genes recovered to 1-h (3 h after dawn) expression values after 24 h (2 h after dawn) of H2O2 treatment. On the contrary, PvPIP2;1 gene expression retained 6-h values after 24 h of H2O2 treatment (Fig. 6b, e, f). Finally, PvPIP2;2 gene expression was lower after 24 h of H2O2 treatment than after 1 or 6 h of treatment (Fig. 6d).
Relative mRNA expression of PvPIP1;1 (a), PvPIP1;2 (c), PvPIP1;3 (e), PvPIP2;1 (b), PvPIP2;2 (d), and PvPIP2;3 (f) of roots grown in hydroponic soil after 1, 6, or 24 h of exposure to 0 (white columns), 0.25 (grey columns), or 1 mM (black columns) H2O2. The different letters indicate significant differences (P < 0.05) among treatments after ANOVA and Fisher LSD analysis. The bars represent mean ± SD (n = 3)
After 1 h of exposure of roots to 0.25 mM H2O2, PvPIP1;1 and PvPIP1;3 genes increased their expression, while the expression of PvPIP2;3 gene was decreased (Fig. 4a, e, f). At the same time, the expression of PvPIP2;2 gene also decreased after 6 h of root exposure to 0.25 mM H2O2 (Fig. 6d). On the other hand, root exposure to 1 mM H2O2 caused an increase of PvPIP1;2 and PvPIP1;3 gene expression after 1 h of exposure (Fig. 6c, e), while after 24 h of exposure, an increase in expression was observed in PvPIP1;1 and PvPIP2;1 genes (Fig. 6a, b). By contrast, after 6 h of root exposure to 1 mM H2O2, a decrease in the expression of PvPIP1;1, PvPIP1;2, and PvPIP2;2 genes was observed (Fig. 6a, c, d). No more significant changes on PIPs gene expression by H2O2 treatments were detected.
Thereafter, PIPs protein abundance and phosphorylation at Ser 121 was analyzed by Western blots as described above (Fig. 7). No signal was detected with any of the antibodies used in untreated or in 1 mM H2O2 treated root microsomes. However, a strong signal was detected after 1 h of root exposure to 0.25 mM H2O2 with the three antibodies used. Minor signal was detected after 6 h and, again, a strong signal was detected after 24 h of root exposure to 0.25 mM H2O2. However, the signal at 24 h was slightly lower than that observed at 1 h. It is worthy to mention that these strong signals were detected in the lanes where less protein was loaded according to coomassie staining.
Western blot analysis of microsomes from P. vulgaris roots grown in hydroponic soil after 1, 6, or 24 h of exposure to 0, 0.25, or 1 mM H2O2. The antibodies used were against AtPIP1;1 (PIP1), AtPIP2;1 (PIP2) or phosphorylated PIP2s at Ser 121 (PhPIP2). The last line represents the coomassie brilliant blue line of each microsome treatment
A correlation between PIP1 protein abundance and PvPIP1;1 and PvPIP1;3 gene expression was observed after at least 1 h of exposure to 0.25 mM H2O2 (Figs. 6a, e and 7), but no correlation was found for PIP2 proteins. So, we decided to analyze PIP2 genes expression pattern at shorter times (10, 20, and 40 min), but no significant changes by 0.25 mM H2O2 treatment on PIP2 genes expression were observed (data not shown).
At the same time, PIPs protein abundance and phosphorylation state increased after 1 and 24 h of root exposure to 0.25 mM H2O2 (Fig. 7), but L only increased after 24 h (Fig. 1c). Therefore, we decided to carry out the immunolocalization of PIP2 proteins in untreated, 1 and 24 h 0.25 mM H2O2 treated roots (Fig. 8). PIP2 was selected instead of PIP1 because PIP2 proteins usually exhibit more water transport activity than PIP1 proteins (Chaumont et al. 2000; Sakurai et al. 2005). Immunolocalization was first carried out in root tips and at 2 cm from tips, since this is the zone where roots are thought to become more active in terms of water uptake (Hukin et al. 2002; Hachez et al. 2006). After 1 and 24 h of root exposure to 0.25 mM H2O2, PIP2 protein abundance increased in the root tips; the increase at 2 cm above the root tips was only detected after 24 h of exposure (Fig. 8). To observe some differences in the cellular-type localization of PIP2 protein between 1 and 24 h 0.25 mM H2O2 treated roots, 1-μm sections were carried out at 2 cm above the root tips. The results indicated a general increase of PIP2 protein amount in all kinds of root cells, being more pronounced in stele cells and in the cortical cells surrounding the stele, after 24 h of exposure. A much weaker response was observed after 1 h of exposure (Fig. 8). A very weak signal was detected when PIP2 antibody was omitted (data not shown).
Immunolocalization of PIP2 proteins in intact roots or in transversal sections of untreated roots exposed to 0.25 mM H2O2 for 1 or 24 h. The antibody used was against AtPIP2;1. More red fluorescence means more PIP2 protein amount. Each fluorescence picture is accompanied by its corresponding bright-field picture
Discussion
The fact that, after 24 h of adding H2O2, we could almost not detect H2O2 in the hydroponic solution is in agreement with the half-time of H2O2 in culture medium, ranging from 4 to 15 min when the concentration applied was between 0.1 and 0.2 mM (Dringen and Hamprecht 1997). However, an increase of J v and L was observed here after 24 h of adding 0.25 mM H2O2. Similarly, Moskova et al. (2009) found that pea leaves sprayed with 2.5 mM H2O2 24 h before being subjected to the herbicide paraquat were more resistant to it than non pretreated leaves. At the same time, P. vulgaris plants needed 24 h to increase their J v and L upon exposure to different ABA concentrations (Aroca 2006). Therefore, it seems that J v and L need a certain period of time to increase their values after the environmental conditions change. It is also known that H2O2 (or other ROS) are intermediate signals that also activate other signals (ion channels, protein kinases, and phosphatases, etc.) before the functional proteins and physiological processes are activated (Van Breusegem et al. 2008; Wang and Song 2008).
Here, an increase of J v and L caused by the exogenous addition of H2O2 was found for the first time. Such an increase was found in both sets of experiments, hydroponic and soil-growing plants. In the hydroponic experiment, when the H2O2 concentration added was equal to or higher than 1 mM, a decrease on J v and L was observed, as found by other researchers (Ktitorova et al. 2002; Lee et al. 2004; Aroca et al. 2005; Rhee et al. 2007; Boursiac et al. 2008) using different plant species and H2O2 concentrations. It is possible that our finding was related to the plant species (cultivar) used. The same P. vulgaris cultivar that has been used here was reported to increase its L after 1 day of exposure to 5°C (Vernieri et al. 2001). Since cold stress causes a rise in ROS generation (Hung et al. 2005), it is possible that such ROS were the cause of the L increase. On the other hand, Aroca et al. (2005) found that the same concentration of H2O2 (100 μM) applied for 1 h had an opposite effect on L depending on the maize cultivar analyzed. Indeed, the cold-tolerant cultivar showed a slight non significant increase of L, while the cold-sensitive cultivar showed a strong decrease of L. At the same time, Boursiac et al. (2008) linked the reduction of L in Arabidopsis thaliana caused by salinity or salicylic acid with a generation of ROS, indicating a sensitivity of A. thaliana to both treatments. Hence, the results found here open the possibility to consider ROS as signaling molecules for increasing L under some environmental stresses, as considered previously for other physiological processes, such as stomatal movements (Pei and Kuchitsu 2005; Wang and Song 2008). We consider ROS and not only H2O2 because other ROS molecules such as OH• could be generated by Fenton reaction under our experimental conditions (Ye and Steudle 2006).
Soil experiment
The pot soil experiment was carried out since most of the plants in nature establish a symbiosis with AM fungi (Gai et al. 2006), by which the fungus obtains carbon compounds and a protective niche, and the plant obtains mineral nutrients (especially phosphorous) and water resources which are not available for the roots (Harrison 2005). At the same time, AM symbiosis confers to the host plant more tolerance to several abiotic stresses, partly due to the higher capacity of AM plants to take water up and to remove ROS (Ruiz-Lozano 2003; Wu et al. 2006). In this study, the percentage of root length colonization by the AM fungi was 21%. Here, soil-growing plants’ L was less than half the average value for hydroponic plants. This difference in L values could be caused by the different age of the plants (Ruggiero et al. 2003), since soil-growing plants were 3 weeks older than hydroponic plants, or by the different morphology of their roots. In fact, Zimmermann and Steudle (1998) found that hydroponic maize roots’ L, measured as we did in the present research, was higher than that of aeroponic ones (roots growing in a mist culture) because of a development of exodermis in aeroponic roots. Similar development of exodermis could occur in soil-growing roots, but not in the hydroponic roots in our experimental setup.
Besides the above-mentioned differences between hydroponic and soil growing roots, we found an increase of L and J v by H2O2 application in both kinds of media. These two results reinforce the validation of our findings. The fact that we needed 20 mM H2O2 in soil-growing roots to obtain the same effect as with 0.25 mM in hydroponic roots can be explained because H2O2 in soil had a slow rate of diffusion or because H2O2 in soil also reacted with several molecules besides roots.
The more pronounced increase of L in non AM roots than in AM roots could be caused, indeed, by different levels of H2O2 inside the roots (lower levels in AM roots). In fact, it is commonly found that there is a higher antioxidant capacity in AM roots than in non AM roots (Ruiz-Lozano 2003; Wu et al. 2006). At the same time, a different PIPs gene expression behavior between AM and non AM roots has been extensively found under various environmental conditions (Ouziad et al. 2006; Porcel et al. 2006; Aroca et al. 2007). Here, the patterns of PIP gene expression in AM and non AM roots exposed to H2O2 changed in opposite directions. While in AM roots two out of six PIP genes increased their expression, in non AM roots, four out of six PIP genes showed a decrease of their expression. In AM roots, correlation between an increase of PvPIP2;2 and PvPIP2;3 gene expression and an increase in PIP2 protein abundance was observed. However, no correlation was found between PIP1 gene expression and protein abundance in both kinds of roots, or between PIP2s gene expression and protein abundance in non AM roots. This lack of correlation between PIP gene expression and PIP protein abundance has been seen previously (Aroca et al. 2005; Sakurai et al. 2005; Hachez et al. 2008), and can be explained in different ways. Here, we only analyze the gene expression of three genes out of a potential family of five to eight genes, based on studies in other plant species (Chaumont et al. 2001; Johanson et al. 2001; Sakurai et al. 2005). At the same time, post-transcriptional regulation or different protein degradation could be taking place. A similar lack of correlation was found between PIP protein amount and the increase of L observed in non AM roots by the H2O2 treatment. This apparent inconsistency could be caused by a different subcellular localization of PIP proteins (Zelazny et al. 2007), or because the antibodies used here did not recognize some of the PIP genes involved in the observed L increase (Aroca et al. 2006). To further continue with the analysis of H2O2 effects in PIP behavior, we focused on the hydroponic experiment.
Hydroponic experiment
We found a decrease of L at 1 and 5 mM H2O2 application, which recovered after 24 h of exposure only in 1 mM H2O2 treated roots. The behavior of L rising and dropping was inversely matched by the values of EL and ROS contents in the roots, but was independent of PIP gene expression. Therefore, L behavior at 1 mM could be related to membrane damage caused by H2O2 application and a subsequent recovery of such damage (Aroca et al. 2005; Aroca 2006). However, a direct blockage of aquaporins (Ye and Steudle 2006) by ROS or a membrane internalization of PIP proteins (Boursiac et al. 2005, 2008) cannot be excluded.
However, the most interesting finding of the present research was the increase of L caused by 0.25 and 0.5 mM H2O2 exposure. Such an increase of L correlated with an increase of PIP protein abundance, as shown by Western blots. However, an increase of PIP protein abundance was observed after 1 h of exposure to 0.25 mM H2O2 without any increase of L. Therefore, we decided to localize where this PIP protein accumulation was taking place along the root. At both time points (1 and 24 h), an increase of PIP2 protein abundance was observed in he root tips. Since ROS can be involved in promoting root tip growth (Monshausen et al. 2007), and root tip growth needs an active water influx inside the growing cells (Nonami 1998), it is possible that such an accumulation of PIP2 proteins in root tips after H2O2 addition was a consequence of the root growth promotion by H2O2 application.
On the contrary, when we looked at 2 cm above root tip, where it is the water-absorbing zone of the root is thought to be located (Hukin et al. 2002; Hachez et al. 2006), an increase in PIP2 protein amount was only detected after 24 h of exposure to 0.25 mM H2O2. This different root localization between 1 and 24 h of exposure to 0.25 mM H2O2 correlated with the different behavior of L. At the same time, the highest PIP2 protein amount was localized in the stele cells after 24 h of H2O2 application, most probably favoring the flow of water to the xylem. This different localization of PIP2 protein between the two kinds of roots could also explain the results of the Western blots and their apparent discrepancy with L results.
Zhou et al. (2007) characterized the water transport capacity of PvPIP1;1, PvPIP2;2, and PvPIP2;3, and only the last one was able to transport water in oocytes experiments. Here, no relationship between an increase of L and an increase of any of the PvPIP genes analyzed was found. Thus, it is possible that other PvPIP genes, not yet identified, were involved in such an increase of L. To ascertain which specific PIP aquaporin gene could be involved in the increase of L caused by exogenous H2O2, a complete set of PIP aquaporins genes would be needed. At the same time, the use of specific antibodies against each PIP gene, or at least some of them, will also be required. These two research tools are already available for maize (Chaumont et al. 2001; Hachez et al. 2006), and the experiments described here could be confirmed in such plant species in future researches.
In conclusion, we reported here for the first time, a significant enhancement of L by exogenous application of H2O2. At the same time, a good correlation between PIP protein amount and their root localization and L has been found in hydroponic experiments. This report provides new insights to study how different environmental factors or endogenous substances modulate L in different ways, possibly linked to the different production of ROS. These results fit with the recent discovery by Dynowski et al. (2008), proving the capacity to transport H2O2 by some plant PIPs. At the same time, the more pronounced response of L to H2O2 in non AM roots than in AM ones, and the different behavior of PIP aquaporins expression and abundance between both kinds of roots, could be caused by the greater capacity of the AM roots to remove ROS. These different behaviors could explain, in part, the different response of L and PIP aquaporins to abiotic stresses found extensively between both kinds of roots (Ouziad et al. 2006; Porcel et al. 2006; Aroca et al. 2007).
References
Aroca R (2006) Exogenous catalase and ascorbate modify the effects of abscisic acid (ABA) on root hydraulic properties in Phaseolus vulgaris L. plants. J Plant Growth Regul 25:10–17. doi:10.1007/s00344-005-0075-1
Aroca R, Tognoni F, Irigoyen JJ, Sánchez-Díaz M, Pardossi A (2001) Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol Biochem 39:1067–1073. doi:10.1016/S0981-9428(01)01335-3
Aroca R, Irigoyen JJ, Sánchez-Díaz M (2003) Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol Plant 117:540–549. doi:10.1034/j.1399-3054.2003.00065.x
Aroca R, Amodeo A, Fernández-Illescas S, Herman EM, Chaumont F, Chrispeels MJ (2005) The role of aquaporins and membrane damage in chilling and hydrogen peroxide induced changes in the hydraulic conductance of maize roots. Plant Physiol 137:341–353. doi:10.1104/pp.104.051045
Aroca R, Ferrante A, Vernieri P, Chrispeels MJ (2006) Drought, abscisic acid and transpiration rate effects on the regulation of PIP aquaporin gene expression and abundance in Phaseolus vulgaris plants. Ann Bot (Lond) 98:1301–1310. doi:10.1093/aob/mcl219
Aroca R, Porcel R, Ruiz-Lozano JM (2007) How does arbuscular mycorrhizal symbiosis regulate root hydraulic properties and plasma membrane aquaporins in Phaseolus vulgaris under drought, cold or salinity stresses? New Phytol 173:808–816. doi:10.1111/j.1469-8137.2006.01961.x
Benabdellah K, Merlos M-A, Azcón-Aguilar C, Ferrol N (2009) GintGRX1, the first characterized glomeromycotan glutaredoxin, is a multifunctional enzyme that responds to oxidative stress. Fungal Genet Biol 46:94–103. doi:10.1016/j.fgb.2008.09.013
Boursiac Y, Chen S, Luu D-T, Sorieul M, van den Dries N, Maurel C (2005) Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol 139:790–805. doi:10.1104/pp.105.065029
Boursiac Y, Boudet J, Postaire O, Luu D-T, Tournaire-Roux C, Maurel C (2008) Stimulus-induced downregulation of root water transport involves reactive oxygen species-activated cell signalling and plasma membrane intrinsic protein internalization. Plant J 56:207–218. doi:10.1111/j.1365-313X.2008.03594.x
Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122:1025–1034. doi:10.1104/pp.122.4.1025
Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125:1206–1215. doi:10.1104/pp.125.3.1206
Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106:1325–1333. doi:10.1104/pp.106.4.1325
Desikan R, Mackerness SAH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127:159–172. doi:10.1104/pp.127.1.159
Dringen R, Hamprecht B (1997) Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res 759:67–75. doi:10.1016/S0006-8993(97)00233-3
Dynowski M, Schaaf G, Loque D, Moran O, Ludewig U (2008) Plant plasma membrane water channels conduct the signalling molecule H2O2. Biochem J 414:53–61. doi:10.1042/BJ20080287
Gai JP, Christie P, Feng G, Li XL (2006) Twenty years of research on community composition and species distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 16:229–239. doi:10.1007/s00572-005-0023-8
Giovannetti M, Mosse B (1980) An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol 84:489–500. doi:10.1111/j.1469-8137.1980.tb04556.x
Hachez C, Moshelion M, Zelazny E, Cavez D, Chaumont F (2006) Localization and quantification of plasma membrane aquaporin expression in maize primary root: a clue to understanding their role as cellular plumbers. Plant Mol Biol 62:305–323. doi:10.1007/s11103-006-9022-1
Hachez C, Heinen RB, Draye X, Chaumont F (2008) The expression pattern of plasma membrane aquaporins in maize leaf highlights their role in hydraulic regulation. Plant Mol Biol 68:337–353. doi:10.1007/s11103-008-9373-x
Harrison MJ (2005) Signaling in the arbuscular mycorrhizal symbiosis. Annu Rev Microbiol 59:19–42. doi:10.1146/annurev.micro.58.030603.123749
Hukin D, Doering-Saad C, Thomas CR, Pritchard J (2002) Sensitivity of cell hydraulic conductivity to mercury is coincident with symplasmic isolation and expression of plasmalemma aquaporin genes in growing maize roots. Planta 215:1047–1056. doi:10.1007/s00425-002-0841-2
Hung SH, Yu CW, Lin CH (2005) Hydrogen peroxide functions as a stress signal in plants. Bot Bull Acad Sin 46:1–10
Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Güçlü J, Vinh J, Heyes J, Franck KI, Schäffner AR, Bouchez D, Maurel C (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15:509–522. doi:10.1105/tpc.008888
Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126:1358–1369. doi:10.1104/pp.126.4.1358
Kaldenhoff R, Grote K, Zhu J-J, Zimmermann U (1998) Significance of plasmalemma aquaporins for water-transport in Arabidopsis thaliana. Plant J 14:121–128. doi:10.1046/j.1365-313X.1998.00111.x
Katsuhara M, Hanba YT, Shiratake K, Maeshima M (2008) Review: expanding roles of plant aquaporins in plasma membranes and cell organelles. Funct Plant Biol 35:1–14. doi:10.1071/FP07130
Kay R, Chan A, Daly M, McPherson J (1987) Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science 236:1299–1302. doi:10.1126/science.236.4806.1299
Ktitorova IN, Skobeleva OV, Sharova EI, Ermakov EI (2002) Hydrogen peroxide appears to mediate a decrease in hydraulic conductivity in wheat roots under salt stress. Russ J Plant Physiol 49:369–380. doi:10.1023/A:1015549220405
Lee SH, Singh AP, Chung GC (2004) Rapid accumulation of hydrogen peroxide in cucumber roots due to exposure to low temperature appears to mediate decreases in water transport. J Exp Bot 55:1733–1741. doi:10.1093/jxb/erh189
Liu JY, Maldonado-Mendoza I, Lopez-Meyer M, Cheung F, Town CD, Harrison MJ (2007) Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J 50:529–544. doi:10.1111/j.1365-313X.2007.03069.x
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔ c t method. Methods 25:402–408. doi:10.1006/meth.2001.1262
Martínez-Ballesta MC, Silva C, López-Berenguer C, Cabañero FJ, Carvajal M (2006) Plant aquaporins: new perspectives on water and nutrient uptake in saline environment. Plant Biol 8:535–546. doi:10.1055/s-2006-924172
Marulanda A, Azcón R, Ruiz-Lozano JM (2003) Contribution of six arbuscular mycorrhizal fungal isolates to water uptake by Lactuca sativa plants under drought stress. Physiol Plant 119:526–533. doi:10.1046/j.1399-3054.2003.00196.x
Maurel C (2007) Plant aquaporins: novel functions and regulation properties. FEBS Lett 581:2227–2236. doi:10.1016/j.febslet.2007.03.021
Maurel C, Verdoucq L, Luu D-T, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59:595–624. doi:10.1146/annurev.arplant.59.032607.092734
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489. doi:10.1111/j.1399-3054.2008.01090.x
Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104:20996–21001. doi:10.1073/pnas.0708586104
Moskova I, Todorova D, Alexieva V, Ivanov S, Sergiev I (2009) Effect of exogenous hydrogen peroxide on enzymatic and nonenzymatic antioxidants in leaves of young pea plants treated with paraquat. Plant Growth Regul 57:193–202. doi:10.1007/s10725-008-9336-x
Nonami H (1998) Plant water relations and control of cell elongation at low water potentials. J Plant Res 111:373–382. doi:10.1007/BF02507801
Ouziad F, Wilde P, Schmelzer E, Hildebrandt U, Bothe H (2006) Analysis of expression of aquaporins and Na+/H+ transporters in tomato colonized by arbuscular mycorrhizal fungi and affected by salt stress. Environ Exp Bot 57:177–186. doi:10.1016/j.envexpbot.2005.05.011
Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plant extracts using titanium (IV). Anal Biochem 139:487–492. doi:10.1016/0003-2697(84)90039-3
Pei Z-M, Kuchitsu K (2005) Early ABA signaling events in guard cells. J Plant Growth Regul 24:296–307. doi:10.1007/s00344-005-0095-x
Philips JM, Hayman DS (1970) Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Brit Mycol Soc 55:158–161
Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM (2006) PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol Biol 60:389–404. doi:10.1007/s11103-005-4210-y
Rhee JY, Lee SH, Singh AP, Chung GC, Ahn SJ (2007) Detoxification of hydrogen peroxide maintains the water transport activity in figleaf gourd (Cucurbita ficifolia) root system exposed to low temperature. Physiol Plant 130:177–184. doi:10.1111/j.1399-3054.2007.00895.x
Ruggiero C, De Pascale S, Angelino G, Maggio A (2003) Developmental changes in plant resistance to water flow in Pisum sativum (L.). Plant Soil 250:121–128. doi:10.1023/A:1022894731467
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317. doi:10.1007/s00572-003-0237-6
Sairam RK, Kumutha D, Ezhilmathi K, Deshmukh PS, Srivastava GC (2008) Physiology and biochemistry of waterlogging tolerance in plants. Biol Plant 52:401–412. doi:10.1007/s10535-008-0084-6
Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol 46:1568–1577. doi:10.1093/pcp/pci172
Santoni V, Vinh J, Pflieger D, Sommerer N, Maurel C (2003) A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochem J 372:289–296. doi:10.1042/BJ20030159
Schachtman DP, Goodger JQD (2008) Chemical root to shoot signaling under drought. Trends Plant Sci 13:281–287. doi:10.1016/j.tplants.2008.04.003
Schützendübel A, Polle A (2002) Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365. doi:10.1093/jexbot/53.372.1351
Steudle E, Peterson CA (1998) How does water get through roots? J Exp Bot 49:755–788. doi:10.1093/jexbot/49.322.775
Tattini M, Traversi ML (2008) Responses to changes in Ca2+ supply in two Mediterranean evergreens, Phillyrea latifolia and Pistacia lentiscus, during salinity stress and subsequent relief. Ann Bot (Lond) 102:609–622. doi:10.1093/aob/mcn134
Van Breusegem F, Bailey-Serres J, Mittler R (2008) Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol 147:978–984. doi:10.1104/pp.108.122325
Vernieri P, Lenzi A, Figaro M, Tognoni F, Pardossi A (2001) How the roots contribute to the ability of Phaseolus vulgaris L. to cope with chilling-induced water stress. J Exp Bot 52:2199–2206
Wahid A, Gelani S, Ashraf M, Foolad MR (2007) Heat tolerance in plants: an overview. Environ Exp Bot 61:199–223. doi:10.1016/j.envexpbot.2007.05.011
Wan X-Y, Liu J-Y (2008) Comparative proteomics analysis reveals an intimate protein network provoked by hydrogen peroxide stress in rice seedling leaves. Mol Cell Proteomics 7:1469–1488. doi:10.1074/mcp.M700488-MCP200
Wang PT, Song C-P (2008) Guard-cell signalling for hydrogen peroxide and abscisic acid. New Phytol 178:703–718. doi:10.1111/j.1469-8137.2008.02431.x
Wen K, St. Segin P, St.-Arnaud M, Jabaji-Hare S (2005) Real-time quantitative RT-PCR of defense-associated gene transcripts of Rhizoctonia solani-infected bean seedlings in response to inoculation with a nonpathogenic binucleate Rhizoctonia isolate. Phytopathology 95:345–353. doi:10.1094/PHYTO-95-0345
Wu Q-S, Xia R-X, Zou Y-N (2006) Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 163:1101–1110. doi:10.1016/j.jplph.2005.09.001
Yang H-M, Zhang J-H, Zhang X-Y (2005) Regulation mechanisms of stomatal oscillation. J Integr Plant Biol 47:1159–1172. doi:10.1111/j.1744-7909.2005.00146.x
Ye Q, Steudle E (2006) Oxidative gating of water channels (aquaporins) in corn roots. Plant Cell Environ 29:459–470. doi:10.1111/j.1365-3040.2005.01423.x
Zelazny E, Borst JW, Muylaert M, Batoko H, Hemminga MA, Chaumont F (2007) FRET imaging in living maize cells reveals that plasma membrane aquaporins interact to regulate their subcellular localization. Proc Natl Acad Sci USA 104:12359–12364. doi:10.1073/pnas.0701180104
Zhou Y, Setz N, Niemitz C, Qu H, Offler CE, Tyerman SD, Patrick JW (2007) Aquaporins and unloading of phloem-imported water in coats of developing bean seeds. Plant Cell Environ 30:1566–1577. doi:10.1111/j.1365-3040.2007.01732.x
Zimmermann HM, Steudle E (1998) Apoplastic transport across young maize roots: effect of the exodermis. Planta 206:7–19. doi:10.1007/s004250050368
Acknowledgments
This research was funded by a grant from the Ministerio de Ciencia e Inovación (AGL-2005-01237). K. Benabdellah was supported by the I3P Program from Consejo Superior de Investigaciones Científicas. The authors also thank C. Maurel and E. M. Herman for the generous gifts of the antibodies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benabdellah, K., Ruiz-Lozano, J.M. & Aroca, R. Hydrogen peroxide effects on root hydraulic properties and plasma membrane aquaporin regulation in Phaseolus vulgaris . Plant Mol Biol 70, 647–661 (2009). https://doi.org/10.1007/s11103-009-9497-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-009-9497-7