Abstract
Abscisic acid (ABA) modifies the hydraulic properties of roots by increasing root water flux (Jv). The role of reactive oxygen species (ROS) in this ABA-induced process was evaluated. At the same time, some antioxidant enzyme activities in root tissues were measured. Phaseolus vulgaris plants were grown hydroponically, and different concentrations of ABA in combination with catalase enzyme or ascorbate were added to the nutrient solution. Catalase treatment had no effect by itself (no ABA) and had little or only a small stimulatory effect at ABA concentrations of 1, 50, and 100 μM, but it partially inhibited the ABA effect at 5 μM. Ascorbate by itself doubled Jv and root hydraulic conductance over the control value. In the presence of ABA, ascorbate partially or, at 100 μM, completely inhibited that ABA stimulation of Jv. These results are discussed in relationship to the possibility that ABA signaling in the roots involves ROS.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Abscisic acid (ABA) is a plant hormone involved in several biotic and abiotic stress responses, including drought, low temperature, flooding, and high salinity (Chinnusany and others 2004). One of the most common effects of ABA on plants at the leaf level is the induction of stomatal closing, and this action of ABA has been studied extensively (see Desikan and others 2004). Thus, it is known that ABA induces the production of reactive oxygen species (ROS) such as superoxide anion (O −2 ) and hydrogen peroxide (H2O2), and that these species are responsible in part for stomatal closing (Desikan and others 2004). However, other signaling pathways are also involved (see Köhler and others 2003).
At the root level, ABA enhances root water transport by increasing root hydraulic conductance (Collins and Morgan 1980; Ludewig and others 1988; Bassiri and Radin 1992; Hose and others 2000), but in some cases ABA has the opposite effect (Markhart and others 1979; Aroca and others 2003) or no effect (Wan and Zwiazek 2001). Recently, Schraut and others (2005) have found a strong correlation between endogenous ABA levels and water flux in maize roots. But the mechanisms involved in the ABA promotion of root water transport remain barely explored. It is known that ABA promotes H2O2 accumulation in root tissues (Lin and Kao 2001; Tsai and Kao 2004). At the same time, H2O2 exogenously applied to the root medium caused a modification of root hydraulic properties. In some cases H2O2 inhibits root hydraulic conductance (Ktitorova and others 2002; Lee and others 2004; Aroca and others 2005), but in other cases an increasing tendency was observed or even no effect at all was observed (Lee and others 2004; Aroca and others 2005). Such discrepancies may be explained by the use of different plant species, H2O2 concentrations, and times of exposure.
The aim of the present research was to evaluate the involvement of ROS on ABA-modified root hydraulic properties. Phaseolus vulgaris plants were grown hydroponically, and different concentrations of ABA were added to the nutrient solution. At the same time, ascorbate (an efficient and broad-range antioxidant; Noctor and Foyer 1998), and catalase (an enzyme that removes H2O2; Van Breusegem and others 2001) were added to the nutrient solution. Root hydraulic properties, root electrolyte leakage, and some root antioxidant enzyme activities were measured.
MATERIALS AND METHODS
Plant Material and Experimental Design
Phaseolus vulgaris seeds were germinated on wet Perlite at 25°C. Seven days after sowing, plants were placed in hydroponic solution containing 5 mM KNO3, 5 mM Ca(NO3)2, 2 mM MgSO4, 1 mM K2HPO4, 50 μM KCl, 25 μM H3BO3, 2 μM MnSO4, 2 μM ZnSO4, 0.5 μM CuSO4, 0.5 μM Na2MoO4, and 20 μM EDTA-Fe. Four days later ABA was added to the nutrient solution at final concentrations of 1, 5, 50, or 100 μM, 2 h after the lights came on. These ABA concentrations were chosen based on previous studies (Markhat and others 1979; Collins and Morgan 1980; Ludewig and others 1988; BassiriRad and Radin 1992; Zhang and others 1995; Wan and Zwiazek 2001). At the same time, catalase or calcium ascorbate at final concentrations of 100 units ml−1 or 5 mM, respectively, were added. Measurements were taken 24 h after additions. Growth conditions were 25°C, 16:8 h (light:dark) photoperiod, 200 μmol m−2 s−1 of photosynthetic photon flux density, and 60% relative humidity.
Root Hydraulic Properties
Root hydraulic conductance was measured as previously described by Aroca and others (2005). Stems of the plants were cut below the cotyledons, and a plastic pipette was attached to the stem with a short, flexible, silicone tube. The exuded sap from the first 15 min was discarded to avoid phloem contamination. Exuded sap from the following 1 h period was collected and weighed, and the osmolality was measured with a cryoscopic osmometer (Osmomat 030, Gonotec GmbH, Berlin, Germany). Osmolality of the nutrient solution was also measured. Root hydraulic conductance (L) was calculated by the following equation: L = Jv / ΔΨ, where Jv is the exuded sap flow rate, and ΔΨ is the osmotic potential difference between the exuded sap and the nutrient solution (BassiriRad and Radin 1992; Fernández-García and others 2002; Vysotskaya and others 2004; Aroca and others 2005).
Root Electrolyte Leakage
Root electrolyte leakage was measured as described before by Aroca and others (2005). Entire root systems from 15 plants were placed individually in a tube containing 20 ml of distilled water. After 3 h of incubation at room temperature, the conductivity of the solution was measured (Conductivity Meter, Wescan Instruments) and was referred to as C0. Then the tubes were placed at −80°C for 1 h and incubated again for 2 h at room temperature. The conductivity of the solution at this time was referred to as CT. The conductivity of distilled water before the root immersion was also measured and referred to as CW. The percentage of electrolyte leakage was calculated as follows: [(C0 – Cw) / (CT – Cw)] × 100.
Antioxidant Enzymes Activities
Enzyme extraction was done as described before by Aroca and others (2001). Briefly, 250 mg of root fresh weight were homogenized in a cold mortar with 5 ml of 100 mM phosphate buffer (pH 7.0) containing 0.1 mM DTPA (diethylenetriamine pentaacetic acid; a metal chelating agent) and 50 mg PVPP (polyvinylpolypyrrolidone), which removes phenolics and alkaloids from plant extracts, avoiding interference with spectrophotometric measurements and enhancing enzyme stability. The homogenate was filtered and centrifuged at 38,000 × g for 10 min. The supernatant was used to determine antioxidant enzyme activities. Ascorbate peroxidase (APX; EC1.11.1.11), glutathione reductase (GR; EC1.6.4.2), and superoxide dismutase (SOD; EC1.15.1.1) activities were measured as described previously by Aroca and others (2001). Catalase (EC1.11.1.6) activity was measured as described by Aebi (1984). Consumption of H2O2 (extinction coefficient of 39.6 mM−1 cm−1) at 240 nm for 1 min was monitored. The reaction mixture consisted of 50 mM phosphate buffer (pH 7.0) containing 10 mM H2O2 and 100 μl of enzyme extract in a 2 ml volume.
Statistical Analysis
Means of all treatments of each parameter were compared using ANOVA and Fisher LSD tests.
RESULTS
Root Hydraulic Properties
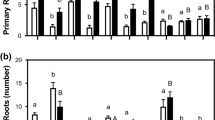
A preliminary experiment was conducted to find the time delay between the addition of ABA to the nutrient medium and its effect on root hydraulic properties. Having observed a 24-h delay, this time of exposure was chosen for subsequent experiments. Because the ABA effect on root hydraulic conductance may depend on its concentration (Hose and others 2000), ABA was applied to the nutrient solution at four different final concentrations (1, 5, 50, and 100 μM). The three lower concentrations of ABA (1, 5, and 50 μM) increased the free exuded sap rate of detopped roots (Jv) fourfold (Figure 1A). A sixfold increase on Jv was caused by 100 μM ABA. A similar response to ABA was observed for root hydraulic conductance (L). Abscisic acid from 1 to 50 μM increased L eightfold, and 100 μM ABA increased L 15-fold (Figure 1C). Abscisic acid treatments diminished the osmotic gradient between the nutrient solution and the exuded sap (ΔΨs); the gradient became shallower as the ABA concentration increased (Figure 1B). Thus, ABA caused a rise on Jv and L independent of the concentration until 50 μM, but at 100 μM ABA the increase was higher.
Root free exuded sap flow rate (Jv; A), osmotic gradient between exuded sap and nutrient solution (ΔΨs; B), and root hydraulic conductance (L; C) of Phaseolus vulgaris plants exposed during 24 h to 0, 1, 5, 50, or 100 μM of ABA alone (white columns) or in combination with 100 units ml−1 of catalase (black columns) or 5 mM calcium ascorbate (gray columns). Different letters indicate significant differences (p < 0.05) among treatments at each ABA concentration (a, b, c) or among ABA concentrations at each treatment: ABA-alone–treated plants (A, B, C, D), catalase-treated plants (I, J, K, L), and ascorbate-treated plants (V, W, X, Y). Columns represent mean ± SE (n = 49).
The effect of adding catalase to the nutrient solution was tested next. First, 100 U ml−1 of catalase alone did not modify any of the root hydraulic properties (Figure 1). However, roots treated with catalase plus 5 μM ABA had lower Jv than plants treated only with 5 μM ABA (Figure 1A). In contrast, plants treated with catalase and 50 μM ABA had higher Jv than plants treated only with 50 μM ABA (Figure 1A). No other significant effects of catalase on Jv were observed in ABA-treated plants. At the same time, catalase treatment had no significant effects on the other parameters of root hydraulic properties (L and ΔΨs) when compared to ABA treatment alone (Figure 1B, C). However, plants treated with 50 μM ABA plus catalase had a higher L than plants treated with 1 or 5 μM ABA plus catalase, contrary to what happened with ABA-only–treated plants (Figure 1C). At the same time, roots treated with 5 μM ABA plus catalase had lower L than 1 μM ABA-plus-catalase–treated plants, also opposite to what happened with plants treated with ABA only (Figure 1C).
Treatment with 5 mM calcium ascorbate alone increased Jv and L and decreased ΔΨs (Figure 1). However, ascorbate treatment inhibited the increase of Jv by ABA, because no more increases in Jv by ABA in ascorbate-treated plants were observed (Figure 1A). Also, ABA-plus-ascorbate–treated plants always had less Jv than ABA-only–treated plants (Figure 1A). By contrast, since ascorbate caused a dramatic decrease of ΔΨs, no significant differences between ABA alone and ABA plus ascorbate treated plants were observed on L, except at 50 μM ABA where ascorbate-treated plants had higher values (Figure 1C).
Therefore, the effects of catalase and ascorbate on modifying ABA effects on root hydraulic properties were more evident on Jv than on L, and were also dependent on ABA concentration. To confirm the effects on L would require studies on root hydraulic properties using other techniques such as cell or root pressure probes (Tomos and Leigh 1999). However, the results obtained with the technique used here were comparable with results obtained with other techniques, including cell and root pressure probes in other reports (Henzler and others 1999; Fernández-García and others 2002; Vysotskaya and others 2004).
Root Electrolyte Leakage (EL)
Because ABA can promote electrolyte leakage (EL) of cell membranes (Fan and Blake 1994; Jiang and Zhang 2001), and because an increase in EL can diminish root hydraulic properties (Aroca and others 2005), the effects of the used treatments on the EL of the roots were analyzed.
Abscisic acid applied at 5 and 100 μM diminished EL compared to nontreated roots; no other changes in EL caused by ABA were observed (Figure 2). Catalase treatment decreased EL compared to ABA-only–treated roots when it was added together with 1 μM ABA, but no other changes due to catalase were observed (Figure 2). Finally, ascorbate treatment increased EL compared to the other two treatments, even without application of ABA, and this increase was smallest in the presence of 5 μM ABA and highest in the presence of 100 μM ABA (Figure 2).
Electrolyte leakage of Phaseolus vulgaris plants exposed over 24 h to 0, 1, 5, 50, or 100 μM ABA alone (white columns) or in combination with 100 units ml−1 of catalase (black columns) or 5 mM calcium ascorbate (gray columns) (n = 15). Otherwise as for Figure 1.
Root Antioxidant Enzymes Activities
Oxidative stress increases EL (Aroca and others 2005). At the same time, as mentioned above, ABA can modify the activities of antioxidant enzymes, and promote ROS generation (Jiang and Zhang 2001). Therefore, the effects of the different treatments on the activity of some antioxidant enzymes were analyzed to establish correlations with the behavior of EL described above.
Abscisic acid treatment did not cause any significant change (p > 0.05) at any concentration in catalase or ascorbate peroxidase (APX) activities (Figure 3A, D). However, ABA increased superoxide dismutase (SOD) and glutathione reductase (GR) activities at 100 μM and 5 μM, respectively, but it decreased GR activity at 1 μM (Figure 3B, C).
Ascorbate peroxidase (APX, A), glutathione reductase (GR, B), superoxide dismutase (SOD, C), and catalase (D) enzyme activities of Phaseolus vulgaris plants exposed during 24 h to 0, 1, 5, 50, or 100 μM ABA alone (white columns) or in combination with 100 units ml−1 of catalase (black columns) or 5 mM calcium ascorbate (gray columns) (n = 4). Otherwise as for Figure 1.
Obviously, roots treated with exogenous catalase had an increase in catalase activity between 9 and 17 times (depending on ABA concentration), although no differences among the catalase treatments were observed (Figure 3D). At the same time, catalase-treated roots had lower activities of APX, GR, and SOD— from 5 to 100 μM ABA, at 5 and 50 μM ABA, and at 5 μM ABA— than ABA-alone–treated roots, respectively (Figure 3). On the contrary, catalase alone increased SOD activity significantly (p < 0.05) (Figure 3C).
Finally, ascorbate-treated roots had lower activities of APX, GR, and SOD enzymes than ABA-only–treated roots in the presence of 5 and 50 μM (Figure 3). At the same time, ascorbate treated roots decreased catalase activity in the presence of 50 μM ABA (Figure 3D), and increased SOD activity in the absence of ABA (Figure 3C).
DISCUSSION
During the past few years evidence has accumulated indicating that ABA action is in part mediated by ROS (Desikan and others 2004; Mori and Schroeder 2004). Here catalase and ascorbate were used as scavengers of ROS (Noctor and Foyer 1998; Van Breusegem and others 2001), in combination with different concentrations of ABA to ascertain the role of ROS in ABA-modified root hydraulic properties.
Like most of the previous reports on the effect of ABA on root hydraulic properties, in the present experimental conditions ABA increased both Jv and L (Figure 1; Collins and Morgan 1980; Ludewig and others 1988; BassiriRad and Radin 1992; Hose and others 2000; Schraut and others 2005). However, the increase in Jv in the presence of 5 μM ABA was lower with catalase than without it (Figure 1A). Therefore, at this ABA concentration, the promotion of Jv by ABA may depend in part on H2O2. The ABA-induced closure of stomata in leaves has also been shown to be partially inhibited by calalase (Zhang and others 2001). In contrast to the situation at 5 μM ABA, in the presence of 50 μM ABA, catalase-treated plants had higher values of Jv than untreated plants (Figure 1A). In addition, 50 μM-ABA–treated roots also had more EL than plants treated with 5 μM (Figure 2). Thus, because an increase in EL can reduce root hydraulic properties (Aroca and others 2005), it is possible that the mechanisms increasing Jv in the presence of 50 μM of ABA are being restricted by the EL of the roots.
Reactive oxygen species generation by ABA is positively correlated with ABA concentration (Jiang and Zhang 2001, 2002; Lin and Kao 2001). Obviously, catalase-treated plants had a greater capacity to remove H2O2 produced by the action of ABA exposure than untreated plants. Moreover, because plants treated with 50 μM ABA alone had equal or lower activities of antioxidant enzymes than 5 μM-ABA–treated plants, it is not surprising that 50-μM-ABA–treated plants had higher EL and therefore did not increase their Jv and L as did catalase-treated plants in the presence of 50 μM ABA.
Thus, it seems that the effects of catalase treatment on ABA-treated plants depend on the ABA concentration. At low concentrations (5 μM) of ABA, catalase acts as an inhibitor of the increase in Jv; at an intermediate concentration (50 μM) of ABA, it acts as an enhancer of Jv; and at a higher concentration (100 μM) of ABA, it had no effect. Nevertheless, because at 1 μM ABA no differences were found between catalase-treated plants and untreated plants on Jv or L, it is reasonable to think that other mechanisms independent of H2O2 generation are responsible for the rise of root hydraulic properties induced by ABA. A similar conclusion has been proposed before for the action of ABA in stomata closure or in the activation of antioxidant enzymes (Köhler and others 2003; Tsai and Kao 2004).
Surprisingly, ascorbate treatment alone increased Jv and L (Figure 1). In a recent study, Henzler and others (2004) found that hydroxyl radicals (•OH) have the capacity to close aquaporins (proteinaceous pores that facilitate the passing of water across membrane cells; see Luu and Maurel [2005] for review), and as a consequence they reduce the hydraulic conductivity of Chara corallina internodes. At the same time, several studies have shown that exogenous H2O2 can decrease root hydraulic conductance (Ktitorova and others 2002; Lee and others 2004; Aroca and others 2005). It is assumed that .OH and H2O2 are generated continuously in plant cells (Noctor and Foyer 1998), and because ascorbate can eliminate both molecules directly (Noctor and Foyer 1998), it is not surprising that ascorbate treatment enhanced Jv and L (Figure 1). However, this effect was not caused by catalase treatment which also removes H2O2 (Van Breusegem and others 2001). Catalase cannot cross membranes, but ascorbate can be transported into the cell (Pignocchi and Foyer 2003). Thus, it is possible that the two molecules caused different effects on the redox state of the cell and therefore different root responses (Foyer and Noctor 2005).
Plants treated with ABA and ascorbate together always had a lower Jv than plants treated with ABA alone (Figure 1A). Thus, ascorbate inhibited the promotion of Jv caused by ABA. The rise in EL in ascorbate-treated plants did not seem to be harmful because these plants had a higher Jv and L than untreated or catalase-treated plants (Figure 1A, C). However, such an increase in EL could be enough to avoid a further increase in Jv, repressing the enhancer mechanisms induced by ABA. Moreover, in the presence of the highest ABA concentration used (100 μM), the EL of ascorbate-treated roots was higher than in the presence of lower concentrations (Figure 2). At the same time, L was lower in ascorbate-treated plants at 100 μM ABA than at 50 μM ABA, contrary to what was obsrved in the other two treatments (Figure 1C). Thus, at 100 μM ABA, where ROS generation should be higher, it is possible that ascorbate was enhancing membrane oxidative damage. In fact, Cross and others (2003) found that H2O2 was most effective in killing Bacillus spores in the presence of ascorbate, by enhancing •OH generation.
Both catalase and ascorbate treatments alone increased SOD activity (Figure 3C). Because the product of SOD is H2O2, and because both treatments should remove H2O2 efficiently, it was expected that SOD activity increased to keep basal levels of H2O2 needed for normal growth (Mittler and others 2004). In the presence of ABA, however, catalase- and ascorbate-treated plants had APX, GR, and SOD activities equal to or lower than those in plants treated with ABA alone (Figure 3A–C). In the presence of ABA, H2O2 and other ROS generation should increase (Jiang and Zhang 2001; Lin and Kao 2001; Tsai and Kao 2004), but catalase and ascorbate added exogenously would remove H2O2 and ROS; therefore roots would need lower antioxidant enzyme levels to keep ROS at basal levels. As was proposed by Mittler and others (2004), antioxidant mechanisms in plants cells are fine tuned to keep ROS low so they can be used as signals.
In summary, evidence is presented for the first time that catalase and ascorbate added exogenously can modify the response of root hydraulic properties to ABA. Thus, at a low concentration of ABA (5 μM), H2O2 could function in part as the signal that mediated the ABA induced rise of Jv. However, at a higher concentration of ABA (50 μM), catalase alleviated the oxidative damage caused by ABA and enhanced Jv. Here, it is also reported for the first time that ascorbate increases root hydraulic properties. Moreover, it seems that the combination of ABA and ascorbate could cause deleterious effects on root hydraulic properties as the ABA concentration rises.
References
Aebi H. 1984. Catalase in vitro. Methods Enzymol 105:121–126
Aroca R, Amodeo G, Fernández-Illescas S, Herman EM, Chaumont F, et al. 2005. The role of aquaporins and membrane damage in chilling and hydrogen peroxide induce changes in the hydraulic conductance of maize roots. Plant Physiol 137:341–353
Aroca R, Irigoyen JJ, Sánchez-Díaz M. 2001. Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Sci 161:719–726
Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Díaz M, Tognoni F, et al. 2003. Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Sci 165:671–679
BassiriRad H, Radin JW. 1992. Temperature-dependent water and ion transport properties of barley and sorghum roots. Plant Physiol 99:34–37
Chinnusany V, Schumaker K, Zhu J-K. 2004. Molecular genetics perspectives on crosstalk and specificity in abiotic stress signalling in plants. J Exp Bot 55:225–236
Collins JC, Morgan M. 1980. The influence of temperature on the abscisic acid stimulated water flow from excised maize roots. New Phytol 84:19–26
Cross JB, Currier RP, Torraco DJ, Vanderberg LA, Wagner GL, et al. 2003. Killing of Bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl Environ Microbiol 69:2245–2252
Desikan R, Cheung M-K, Bright J, Henson D, Hancock JT, et al. 2004. ABA, hydrogen peroxide and nitric oxide signalling in stomatal guard cells. J Exp Bot 55:205–212
Fan S, Blake TJ. 1994. Abscisic acid induced electrolyte leakage in woody species with contrasting ecological requirements. Physiol Plant 90:414–419
Fernández-García N, Martínez V, Cerdá A, Carvajal M. 2002. Water and nutrient uptake of grafted tomato plants grown under saline conditions. J Plant Physiol 159:899–905
Foyer CH, Noctor G. 2005. Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in physiological context. Plant Cell Environ 28:1056–1071
Henzler T, Waterhouse RN, Smith AJ, Carvajal M, Cooke DT, et al. 1999. Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 210:50–60
Henzler T, Ye Q, Steudle E. 2004. Oxidative gating of water channels (aquaporins) in Chara by hydroxyl radicals. Plant Cell Environ 27:1184–1195
Hose E, Steudle E, Hartung W. 2000. Abscisic acid and hydraulic conductivity of maize roots: a study using cell- and root-pressure probes. Planta 211:874–882
Jiang M, Zhang J. 2001. Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Jiang M, Zhang J. 2002. Involvement of plasma-membrane NADPH oxidase in abscisic acid- and water stress-induced antioxidant defense in leaves of maize seedlings. Planta 215:1022–1030
Köhler B, Hills A, Blatt MR. 2003. Control of guard cell ion channels by hydrogen peroxide and abscisic acid indicates their action through alternate signaling pathways. Plant Physiol 131:385–388
Ktitorova IN, Skobeleva OV, Sharova EI, Ermakov EL. 2002. Hydrogen peroxide appears to mediate a decrease in hydraulic conductivity in wheat roots under salt stress [in Russian]. J Plant Physiol 49:369–380
Lee SH, Singh AP, Chung GC. 2004. Rapid accumulation of hydrogen peroxide in cucumber roots due to exposure to low temperature appears to mediate decrease in water transport. J Exp Bot 55:1733–1741
Lin CC, Kao CH. 2001. Abscisic acid induced changes in cell wall peroxidase activity and hydrogen peroxide level in roots of rice seedlings. Plant Sci 160:323–329
Ludewig M, Dörffling K, Seifert H. 1988. Abscisic acid and water transport in sunflowers. Planta 175:325–333
Luu DT, Maurel C. 2005. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ 28:85–96
Markhart AH III, Fiscus EL, Naylor AW, Kramer PJ. 1979. Effect of abscisic acid on root hydraulic conductivity. Plant Physiol 64:611–614
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends Plant Sci 9:490–498
Mori IC, Schroeder JI. 2004. Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetical mechanotransduction. Plant Physiol 135:702–708
Noctor G, Foyer CH. 1998. Ascorbate and glutathione: keeping active oxygen species under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Pignocchi C, Foyer CH. 2003. Apoplastic ascorbate metabolism and its role in the regulation of cell signalling. Curr Opin Plant Biol 6:379–389
Schraut D, Heilmeier H, Hartung W. 2005. Radial transport of water and abscisic acid (ABA) in roots of Zea mays under conditions of nutrient deficiency. J Exp Bot 56:879–886
Tomos AD, Leigh RA. 1999. The pressure probe: a versatile tool in plant cell physiology. Annu Rev Plant Physiol Plant Mol Biol 50:447–472
Tsai Y-C, Kao CH. 2004. The involvement of hydrogen peroxide in abscisic acid-induced activities of ascorbate peroxidase and glutathione reductase in rice roots. J Plant Growth Regul 43:207–212
Van Breusegem F, Vranová E, Dat JF, Inzé D. 2001. The role of active oxygen species in plant signal transduction. Plant Sci 161:405–414
Vysotskaya LB, Arkhipova TN, Timergalina LN, Dedov AV, Veselov SY, et al. 2004. Effect of partial root excision on transpiration, root hydraulic conductance and leaf growth in wheat seedlings. Plant Physiol Biochem 42:251–255
Wan X, Zwiazek JJ. 2001. Root water flow and leaf stomatal conductance in aspen (Populus tremuloides) seedlings treated with abscisic acid. Planta 213:741–747
Zhang J, Zhang X, Liang J. 1995. Exudation rate and hydraulic conductivity of maize roots are enhanced by soil drying and abscisic acid treatment. New Phytol 131:329–336
Zhang X, Zhang L, Dong F, Gao J, Galbraith DW, et al. 2001. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol 126:1438–1448
Acknowledgments
The author thanks Prof. M. J. Chrispeels (University of California San Diego) for making his laboratory facilities available for these experiments, as well as for stimulating discussions and for editing the manuscript. The author was supported by a postdoctoral fellowship from the Ministerio de Educación y Ciencia (Spain). The author is also grateful to Dr. M. J. Martín for help during the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aroca, R. Exogenous Catalase and Ascorbate Modify the Effects of Abscisic Acid (ABA) on Root Hydraulic Properties in Phaseolus vulgaris L. Plants. J Plant Growth Regul 25, 10–17 (2006). https://doi.org/10.1007/s00344-005-0075-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-005-0075-1