Abstract
Plants have developed sophisticated gravity sensing mechanisms to interpret environmental signals that are vital for optimum plant growth. Loss of SHOOT GRAVITROPISM 5 (SGR5) gene function has been shown to affect the gravitropic response of Arabidopsis inflorescence stems. SGR5 is a member of the INDETERMINATE DOMAIN (IDD) zinc finger protein family of putative transcription factors. As part of an ongoing functional analysis of Arabidopsis IDD genes (AtIDD) we have extended the characterisation of SGR5, and show that gravity sensing amyloplasts in the shoot endodermis of sgr5 mutants sediment more slowly than wild type, suggesting a defect in gravity perception. This is correlated with lower amyloplast starch levels, which may account for the reduced gravitropic sensitivity in sgr5. Further, we find that sgr5 mutants have a severely attenuated stem circumnutation movement typified by a reduced amplitude and an decreased periodicity. adg1-1 and sex1-1 mutants, which contain no starch or increased starch, respectively, also show alterations in the amplitude and period of circumnutation. Together these results suggest that plant growth movement may depend on starch levels and/or gravity sensing. Overall, we propose that loss of SGR5 regulatory activity affects starch accumulation in Arabidopsis shoot tissues and causes decreased sensitivity to gravity and diminished circumnutational movements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability of plants to adjust their growth habit in response to environmental stimuli is key to their survival and reproductive success. For example, plants must sense and respond to gravity such that roots generally grow downward (positive gravitropism) and shoots generally grow upward (negative gravitropism). The process of gravitropism can be divided into 3 sequential steps: (1) gravity perception, (2) signal transduction and (3) differential growth response (Fukaki and Tasaka 1999; Haswell 2003).
In roots, the site of gravity perception is the columella cells of the root cap (Blancaflor et al. 1998). However, genetic evidence suggests that the endodermis is the site of gravity perception in the shoot (Fukaki et al. 1998). The Arabidopsis mutants, scarecrow (scr) and shortroot (shr), which lack a normal endodermal cell layer, are defective in shoot gravitropism but not root gravitropism (Benfey et al. 1993; Scheres et al. 1995; DiLaurenzio et al. 1996). This implies that the endodermis is essential for shoot gravitropism and that it is most likely the site of gravity sensing in the shoot.
The most widely accepted model for gravity perception is the starch-statolith theory (reviewed in Sack 1997). It states that gravity-sensing cells (statocytes) contain starch filled amyloplasts (statoliths) that sediment in the direction of gravity. Indeed, sedimenting amyloplasts have been observed in the shoot endodermis and columella root cap cells (Olsen et al. 1984; Caspar and Pickard 1989; Fukaki et al. 1998; Weise and Kiss 1999). Amyloplasts in the endodermis of inflorescence stems of a starchless mutant of Arabidopsis do not sediment with gravity (Weise and Kiss 1999). This mutant shows reduced gravitropism in roots, hypocotyls and stems, providing support for the starch-statolith model (Kiss et al. 1996; Kiss et al. 1997; Weise and Kiss 1999).
Following perception of the gravity stimulus the signal must be transduced to elongating cells in order to elicit a differential growth response. The Cholodny-Went hypothesis proposes that tropic growth stimulated by gravity or light is brought about by an uneven lateral distribution of auxin across a plant organ. This causes differential rates of cell elongation on opposite sides of the organ resulting in organ bending (reviewed in Yamamoto 2003). Work with radiolabelled auxin and auxin-responsive reporters corroborates this long-standing theory (Young et al. 1990; Friml et al. 2002; Ottenschlager et al. 2003). The asymmetric distribution of auxin is most likely achieved through the targeting of one or more members of the PIN family of auxin efflux carriers to the plasma membrane on one side of the cell (Friml et al. 2002). Indeed, Arabidopsis PIN3 localises to the inner lateral membrane of the shoot endodermis making it an ideal candidate for transporting auxin laterally.
The study of Arabidopsis mutants has greatly aided our understanding of the mechanisms underlying shoot gravitropism (Fukaki et al. 1996; Yamauchi et al. 1997; Weise and Kiss 1999; Yamamoto et al. 2002). Three mutant alleles at the SHOOT GRAVITROPISM 5 (SGR5) locus have been described previously (Yamauchi et al. 1997; Morita et al. 2006). The sgr5 mutants show a reduced gravity response in inflorescence stems, whereas root and hypocotyl gravitropism are not altered. The angle of lateral shoot outgrowth is also affected, presumably because lateral branches possess a defect in gravitropism similar to the primary stem (Yamauchi et al. 1997). Mutations in sgr5 do not alter phototropism, suggesting that the primary defect is not in auxin transport or downstream differential growth responses.
The SGR5 locus corresponds to AtIDD15, a member of the plant specific INDETERMINATE DOMAIN (IDD) gene family (Colasanti et al. 2006; Morita et al. 2006). The IDD family is highly conserved across divergent plant species and contains 16 members in Arabidopsis (Colasanti et al. 2006). IDD proteins are characterised by the INDETERMINATE (ID) domain, a sequence specific DNA binding domain containing four zinc finger motifs (Kozaki et al. 2004; Colasanti et al. 2006). The inner two zinc fingers of the ID domain (ZF2 and ZF3) are necessary for binding an 11 bp consensus sequence in vitro whereas the remaining two (ZF1 and ZF4) do not appear to bind DNA and may have some other function (Kozaki et al. 2004; Welch et al. 2007). Interestingly, sgr5-1 has a mutation that disrupts ZF3 of AtIDD15 demonstrating that DNA binding is necessary for wild-type function (Morita et al. 2006). All IDD proteins also contain an N-terminal nuclear localization signal which, together with their capacity for sequence specific DNA binding, strongly suggests that they function as transcription factors (Kozaki et al. 2004; Colasanti et al. 2006; Morita et al. 2006; Wong and Colasanti 2007).
SGR5/AtIDD15 mRNA is expressed in all major organs of the plant although the highest levels of expression of an SGR5 promoter-GUS reporter were detected in the shoot endodermis (Morita et al. 2006). Consistent with SGR5 functioning in the endodermis, the shoot gravitropism defect of sgr5-1 was rescued by expressing SGR5 under the control of the endodermal-specific SCR promoter. This suggests that SGR5 is involved in an early stage of shoot gravitropism, either during gravity perception or in the early signalling events downstream of perception in the endodermis. However, most amyloplasts in sgr5-1 endodermal cells sediment in the direction of gravity, leading Morita et al. (2006) to propose that SGR5/AtIDD15 acts downstream of amyloplast sedimentation.
Here we describe sgr5-4, a new partial loss of function mutant allele at the SGR5/AtIDD15 locus. Inflorescence stems of sgr5-4 mutants show reduced gravitropism similar to previously characterised sgr5 alleles. Circumnutatory movement is also diminished in sgr5 mutants. In contrast to former speculation, we show that whilst amyloplasts in the endodermis of sgr5-3 and sgr5-4 stems do sediment towards gravity, they move more slowly than wild type. We propose that SGR5/AtIDD15 regulates the transcription of one or more genes that are ultimately required for starch metabolism in shoots and that this affects gravity perception by altering the rate at which amyloplasts sediment.
Materials and methods
Plant materials and growth conditions
Col-0 and Ler-0 were used as wild-type controls. The sgr5-3 allele in the Col ecotype was obtained from the Arabidopsis Biological Resource Center (SALK_087766) and has been described previously (Morita et al. 2006). The sgr5-4 allele in the Ler ecotype was obtained from the John Innes Centre Gene Trap collection and was donated by the European Arabidopsis Stock Centre (NASC ID N172127). sgr5-3 and sgr5-4 were backcrossed four times to wild type (Col) or three times to wild type (Ler) respectively. In each case, homozygous mutants from the final backcross retained the shoot gravitropism phenotype. In all experiments presented here sgr5-3 homozygotes from the second backcross were used, whereas homozygotes from the original sgr5-4 Ler line were used. adg1-1 (Col) seeds were kindly donated by Sylva Donaldson, and sex1-1 (Col) seeds were obtained from the Arabidopsis Biological Resource Center (Stock # CS212). Seeds were imbibed in water at 4°C for 2–6 days before sowing onto Sunshine Mix #4 Aggregate Plus (SunGro) treated with 1 g/l 20-20-20 fertiliser, in square pots. Plants were grown under fluorescent and incandescent white light (150 μmol/m2/sec) on 16 h light/8 h dark cycles, at 20–23°C.
Determination of the sgr5-4 genomic insertion site
Plant genomic DNA was purified by a rapid extraction protocol. Briefly, leaf tissue was ground in liquid nitrogen and mixed with 750 μl extraction buffer (100 mM Tris-HCl, 50 mM EDTA, 500 mM NaCl, pH 8.0). After adding 50 μl 10% SDS, samples were incubated at 65°C for 5 min and plant debris was removed by centrifugation. Next, 250 μl 5M potassium acetate was added and the DNA was precipitated with isopropanol. The 3′ end of the Ds element and genomic sequence flanking it were amplified by PCR from sgr5-4 genomic DNA using primers DS3’-1: 5′-CGATTACCGTATTTATCCCGTTCG-3′ (Parinov, 1999) and 15RP: 5′-CTGACGACATGTAACTCAC-3′. The PCR product was purified according to the QIAquick Spin PCR purification protocol (Qiagen) and DNA sequencing was performed with primers DS3’-1 and 15RP using a BigDye Terminator v3.1 and ABI3730 DNA Analyzer.
Semi-quantitative RT-PCR expression analysis
Total RNA was extracted from 100 mg frozen leaf tissue using TRIzol® reagent (Invitrogen) and then purified according to the RNeasy method (Qiagen) as per manufacturer’s protocol. First strand cDNA synthesis was performed with 1 μg total RNA template, using oligo(dT)18 primers and RevertAid™ H Minus M-MuLV Reverse Transcriptase (Fermentas). PCR was performed on serial two-fold dilutions of cDNA template to determine the range in which amplification occurs exponentially. PCR reactions using undiluted cDNA template also amplified SGR5 from wild-type plants but not sgr5-4 (data not shown). SGR5 primers: 15LP, 5′-CCGGCATGCTCTTCTAGAACC-3′ and 15RP, 5′- CTGACGACATGTAACTCAC -3′ anneal downstream of the Ds insertion in exon 3. GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE (GAPDH) primers: GAPDHLP, 5′-CACTTGAAGGGTGGTGCCAAG-3′ and GAPDHRP, 5′-CCTGTTGTCGCCAACGAAGTC-3′ were used as a control for expression.
Gravitropism assay
Plants were grown in pots until the primary inflorescence stems were 4–9 cm tall (approximately 3.5 weeks) and gravistimulated by turning pots horizontally onto their sides and placing them in darkness. At each point during the time course a subset of plants were removed from the dark and photographed from the side. The angle relative to the vertical of each primary shoot apex was measured from photographs using ImageJ image analysis software.
Time-lapse photography and circumnutation assay
Plants were grown as described until the primary inflorescence stems were 4–9 cm tall. For time-lapse photography plants were illuminated by overhead white fluorescent light for 3 h before images were taken. A Canon EOS Digital Rebel camera connected to an iMac G4 computer was used for imaging with Remote Capture software (Canon, Inc). Images were recorded every 5 min over a minimum 415 min time period under continuous light. Collected images were collated as an image sequence at 6 frames per second with QuickTime software (Apple Computer, Inc.) to create each movie (Supplementary Figs. S1 and S2). Each experiment was performed at least 10 times to confirm reproducibility. For circumnutation analyses the angles of the shoot apices relative to the vertical were measured from each set of photographs using ImageJ analysis software and plotted graphically to track the bending movement of inflorescence stems two-dimensionally.
Amyloplast sedimentation analysis
Plants were grown until the primary stems were 4 to 9 cm tall, then gravistimulated for 0, 20 or 60 min, by turning them upside down. Following gravistimulation, a 5 mm stem segment was excised from between 3 and 4 cm below the shoot apex. Tissue segments were embedded in Shandon Cryomatrix™ (Thermo Scientific), then cryofixed by freezing in liquid nitrogen. Longitudinal tissue sections 14 μm thick were cut using a Shandon Cryotome® SME cryostat (Thermo Electron Corporation) and mounted onto Superfrost Plus slides (Fisher Scientific). Sections were fixed in 4% paraformaldehyde, 50 mM potassium phosphate (pH 7.0) then washed in 50 mM potassium phosphate buffer (pH 7.0) followed by deionised water and stained with 0.1 % toluidine blue. Coverslips were mounted in deionised water and samples were visualised using a Leica DMLS2 microscope. For each population, the average position of amyloplasts in 10 endodermal cells from each of 2 tissue sections were scored from each of 5 plants (i.e. n = 100). This was done by visually dividing each endodermal cell into 4 equal sectors along its length, numbered 0–3, where 0 was the apical-most sector and 3 was the basal-most sector (Fig. 5b). For each endodermal cell a score of 0, 1, 2 or 3 was given, depending on the average position of amyloplasts.
Starch staining
Plants were grown until inflorescence stems were 4.5–8 cm tall. Segments were excised from inflorescence stems, 3–4 cm below the apex and fixed overnight in 3% (v/v) glutaraldehyde, 1.25% (v/v) formaldehyde, 50 mM sodium phosphate buffer (pH 7.0). Next, the tissue was dehydrated in an ethanol series, transferred through a series to Citrisolv (Fisher Scientific) then infiltrated and embedded in Paraplast Plus (Fisher Scientific). Transverse sections 12 μm thick were cut with a Leica microtome and mounted onto Superfrost Plus slides. Sections were dewaxed in Citrisolv, rehydrated through an ethanol series and stained with 0.2% w/v iodine, 2% w/v potassium iodide (IKI). Coverslips were mounted in IKI stain and sealed with nail polish.
Starch extraction and quantification
Plants were grown until the stems had bolted 3 to 9 cm and tissue was harvested at the end of the 16 h light period. For stem tissue, segments were excised from 2 to 4 cm below the shoot apex and pooled into samples from at least 7 plants each. For leaves, each sample contained 2 expanded rosettes leaves from a single plant. Starch was extracted with 0.7 M perchloric acid and the insoluble fraction was cleared with 80% (v/v) ethanol then resuspended in water as described (Delatte et al. 2005). Samples were boiled for 15 min then starch was measured using the Total Starch assay kit (Megazyme) according to the manufacturer’s instructions, except that volumes were reduced to accommodate the lower starch levels in these tissues.
Results
Identification of sgr5 loss of function alleles
The Arabidopsis IDD gene family is comprised of 16 closely related members encoding proteins that share a zinc finger-containing DNA binding domain first described for the maize INDETERMINATE1 (ID1) protein (Colasanti et al. 2006). In an effort to characterise the specific functions of individual IDD genes, we sought to isolate insertion mutants in members of the IDD family from Arabidopsis (M. Tanimoto, R. Tremblay and J. Colasanti, unpublished). We obtained one insertion in the SGR5/AtIDD15 gene from the SALK T-DNA collection (Alonso 2003) and another allele from the John Innes Centre Gene Trap collection (Parinov et al. 1999). The SALK line is in the Col ecotype and contains a T-DNA insertion in intron 1 (Fig. 1a). DNA sequencing of the genomic region flanking one side the T-DNA showed that this allele is identical to sgr5-3, which has been described previously (Morita et al. 2006). In the second allele, in the Ler background, the SGR5/AtIDD15 gene is disrupted by a Ds transposable element inserted into the large third exon (Fig. 1a). We designated this new allele sgr5-4.
sgr5 mutant alleles. (a) IDD15/SGR5 gene structure. Open and closed boxes represent the untranslated and coding regions of the exons respectively. Black lines indicate the introns. The locations of the T-DNA and Ds insertions in sgr5-3 and sgr5-4 respectively, are shown as triangles above the gene. (b) Semiquantitative RT-PCR expression analysis of SGR5/AtIDD15 in sgr5-4. RNA was prepared from leaf tissue from wild type (Ler) (lane 1), and sgr5-4 (lane 2). GAPDH primers were used as a control
Sequence analysis of the sgr5-3 transcript predicted that the region downstream of the T-DNA insertion is not translated in the correct reading frame, and thus sgr5-3 is believed to be a null allele (Morita et al. 2006). Similarly, the Ds element in sgr5-4 most likely disrupts SGR5 gene function. This was borne out by semi-quantitative RT-PCR expression analysis using primers downstream of the Ds insertion in sgr5-4, which showed that no transcript is detected (Fig. 1b).
Mutations in sgr5 alter the angles of shoot lateral organs
Prior to bolting, no morphological differences between sgr5-4 and wild-type plants were observed (Fig. 2a). However, later in development it was apparent that sgr5-4 axillary shoots initially grow out at a wider angle from the primary stem compared with wild type, although they eventually reorient their growth in an upward direction (Fig. 2b). A similar phenotype has been reported in previously characterised sgr5 alleles and also in other shoot gravitropism (sgr) mutants (Fukaki et al. 1996; Yamauchi et al. 1997; Morita et al. 2006). At nodes higher up the stem, the angles formed between the siliques and the primary stem are also less acute in sgr5-4 than wild type (Fig. 2c). This phenotype does not appear to be dependent on the Ler background since it also occurs in sgr5-3 in the Col ecotype (Fig. 2d). Therefore, SGR5 appears to have a role in regulating the angle of lateral organ outgrowth in shoots. No other differences in shoot development or morphology were observed.
Morphological phenotypes of sgr5 mutants. (a) Rosette stage plants. Wild type (Ler) left, sgr5-4 right. (b) Mature plants (4.5 weeks old) showing axillary branch angles. Wild type (Ler) left, sgr5-4 right. (c and d) Silique angles. (c) Wild type (Ler) left, sgr5-4 right (d) Wild type (Col) left, sgr5-3 right
sgr5-4 is defective in shoot gravitropism
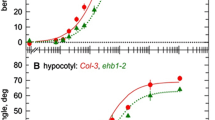
The lateral branch defect described above is often reported to be associated with a reduced response to gravity most likely because a wild-type gravity response is necessary for correct organ positioning. To test the gravitropic response of sgr5-4 inflorescence stems we gravistimulated actively growing plants by turning them onto their sides (by 90°) and measured the kinetics of stem reorientation in darkness. Wild-type (Ler) stems adjusted their growth toward the vertical within 8 h (Fig. 3a, b, d) whereas sgr5-4 stems responded to gravistimulation at a slower rate than wild type, failing to reach a vertical position even after 24 h (Fig. 3a, c, e). This response was similar to that of sgr5-3 (Col) under our conditions, although wild-type controls in the Col background responded to gravity at a faster rate than wild type (Ler) (Fig. 3a). Thus, sgr5-4 shows a less severe phenotype compared to its wild-type control than sgr5-3, suggesting that it may be a weaker mutant allele. Comparison of the sgr5-4 (Ler) gravity response with that of sgr5-3 introgressed into the Ler ecotype supports this theory (data not shown). Therefore, in agreement with Morita et al. (2006) it is clear that sgr5 mutants are compromised in their ability to reorient vertically in response to gravistimulation.
Gravitropic response of sgr5 inflorescence stems. (a) Time course for gravitropism. Plants were gravistimulated by placing them horizontally in darkness. The angles of the primary shoot apices with respect to the vertical were measured at each point during the time course. Open circles, wild type (Ler); closed circles, sgr5-4; open triangles, wild type (Col); closed triangles, sgr5-3 (n ≥ 7). Error bars represent standard error of the mean. (b, d) Wild type, Ler and (C,E) sgr5-4 inflorescence stems after (b, c) 0 h and (d, e) 8 h of horizontal gravistimulation. The arrow indicates the direction of gravity
sgr5 alters shoot circumnutation
Circumnutation is the rhythmic, oscillatory movement of a plant organ that occurs during growth (Brown 1993; Johnsson 1997). Some mutants defective in gravitropism also show altered circumnutatory movement, although the dependence of circumnutation on gravity responsiveness is still somewhat controversial (reviewed in Kiss 2006). To determine whether SGR5 is involved in the regulation of circumnutation in inflorescence stems, we performed time-lapse photography of sgr5 mutant shoots in both Col and Ler ecotype backgrounds, and mapped the angle of the shoot tip over a 415 min time course. In all cases plants had not attained their final height prior to filming and therefore were capable of growth movements (Supplementary Fig. S1). The experiment was repeated at least 10 times, with each replicate yielding qualitatively identical results. Wild-type stems in the Ler ecotype rotated around a central axis with a period of approximately 230 min, reaching a maximum of approximately 15–18° from vertical (Figs. 4 and S1). By contrast, wild-type Col stem movement showed a much more dramatic circumnutation, with a maximum movement ranging from 45 to 77 degrees from vertical, thus showing a bending amplitude of about four-fold higher than Ler (Figs. 4 and S1). Wild-type Col plants also showed a slightly shorter period than Ler, averaging about 190 min for the growth stages examined here. Therefore, circumnutation differences vary between the two ecotypes tested here, with Col shoot movement having a greater amplitude and shorter periodicity.
Circumnutation in sgr5 inflorescence stems. Angles of the primary shoot apices were measured with respect to the vertical axis. Data for a single representative plant from each genotype are shown. Open circles, wild type (Ler); closed circles, sgr5-4; open triangles, wild type (Col) and closed triangles sgr5-3
Mutations in the SGR5 gene had a striking effect on circumnutation in both ecotypes (Figs. 4 and S1). In sgr5-3 and sgr5-4 mutant stems, the amplitude of circumnutatory movement was decreased compared with wild-type controls, although sgr5-3 (Col) appeared to have a stronger effect than sgr5-4 (Ler). Interestingly, loss of SGR5 function seems to alter the period of shoot circumnutation as well. Again, this was most apparent in the sgr5-3 allele, which showed a periodicity of about 70 min, compared to 190 min for wild-type (Col) plants. In the sgr5-4 mutant it was difficult to determine the period of movement above background noise levels since the maximum amplitude of bending measured was only about 3 degrees. Nonetheless, this movement is still perceptible by time-lapse visualization (Fig. S1). For both mutant and wild-type plants circumnutation ceased once growth of the shoot was complete (data not shown).
SGR5 influences amyloplast sedimentation rates
The site of gravity perception in the Arabidopsis shoot resides within the cells of the endodermis (Fukaki et al. 1998). Morita et al. (2006) showed that SGR5/AtIDD15 functions in the endodermis but that most gravity sensing amyloplasts in vertically growing sgr5 stems are located at the base of endodermal cells. However, we reasoned that sgr5 amyloplasts might re-sediment more slowly than wild type following a change in the direction of gravity. To test this hypothesis, we gravistimulated plants by turning them upside down, cryofixed the gravisensitive region of the stems, and cut longitudinal tissue sections using a cryotome. The average position of amyloplasts along the length of the endodermal cells was recorded at discrete time points. Prior to gravistimulation, in wild-type plants of the Col and Ler ecotypes, amyloplasts were situated close to the basal end of endodermal cells (Fig. 5a–c). In contrast, sgr5-3 and sgr5-4 amyloplasts were located at slightly more apical positions within the endodermal cells. Most wild-type Col amyloplasts sedimented to the apical end of the cell within 20 min of gravistimulation, while wild-type Ler statoliths moved more slowly, reaching only the middle of the cell on average within the same time period (Fig. 5a, c). Nonetheless, most Ler amyloplasts sedimented to the apical end of the cell within 60 min after reorientation (Fig. 5c). In sgr5-3 and sgr5-4 mutant stems, amyloplast movement was significantly retarded compared to wild-type controls. The rate of sedimentation was slowest in sgr5-4, with no observable movement occurring during the first 20 min of gravistimulation (Fig. 5a, c). Neither sgr5-3 nor sgr5-4 amyloplasts sedimented fully within 60 min of gravistimulation (Fig. 5c). Therefore, SGR5 appears to affect the rate at which gravity sensing amyloplasts in the shoot endodermis sediment in response to gravity.
Amyloplast sedimentation in sgr5 endodermal cells. Plants were gravistimulated by turning them upside down for 0, 20 or 60 min, then longitudinal cryosections were prepared from the elongation zone of the stems. (a) In each panel a single endodermal cell is shown, with the cortex positioned to the left and the vasculature to the right. The average position of amyloplasts along the apical-basal axis of the cell is indicated with a horizontal arrow. Images in all panels are shown at the same magnification. Bar = 20 μm. (b) Schematic illustrating the scoring system used to determine the position of amyloplasts within the endodermal cells. Each cell was visually divided into four equal segments along its length, numbered 0–3, where 0 was the apical-most segment and 3 was the basal-most segment. (The apical end of the cell appears at the bottom of the image since plants were inverted). The position of amyloplasts along the apical-basal axis of the cell was then scored based on which segment they were located in on average. The direction of gravity is shown with a vertical arrow. (c) Average position of amyloplasts in gravistimulated sgr5 and wild-type endodermal cells, using the scoring system described in b (n = 100). Error bars represent standard error of the mean
SGR5 affects starch accumulation in plastids
According to the starch-statolith model, starch may provide amyloplasts with the relatively high density required for them to act as gravity sensing statoliths (Sack 1997). Consistent with this, starchless and low starch mutants show reduced gravity responses similar to sgr5, and amyloplasts in the shoot endodermis of a starchless mutant are unable to sediment to the bottom of the cell (Kiss et al. 1996; Kiss et al. 1997; Weise and Kiss 1999). To determine whether the defects in gravity response and amyloplast sedimentation observed in sgr5 mutants could be due to lower amounts of starch, we tested whether sgr5 mutants contain altered levels of amyloplast starch. Transverse sections from the elongation zone of growing inflorescence stems were prepared from wax-embedded tissue and stained for starch with iodine/potassium iodide solution (IKI). In the Arabidopsis shoot the outer tissues of wild-type inflorescence stems are arranged radially in distinct cell layers. The outermost layer, the epidermis, surrounds 3 or 4 cortical cell layers, followed by a single layer of endodermal cells (Fig. 6a, c). The vascular bundles and pith make up the central tissues. Amyloplasts in the endodermis of wild-type stems stained intensely with IKI demonstrating the presence of starch (Fig. 6a, c, e, g). However, wild-type plants in the Col ecotype stained more intensely compared with Ler. We observed no obvious morphological differences in the tissue structure of sgr5 mutants compared with wild type and no apparent differences in the number of amyloplasts (Fig. 6 b, d). However, sgr5-3 and sgr5-4 amyloplasts showed lower levels of IKI staining than their respective wild-type counterparts, indicating that they contain less starch (Fig. 6 b, d, f, h). This suggests that SGR5 promotes starch accumulation in amyloplasts.
Starch staining of sgr5 plastids. Transverse sections through the elongation zone of inflorescence stems, stained with IKI. All sections were stained together so that the levels of staining were directly comparable to one another. (a) Wild type (Ler), (b) sgr5-4, (c) wild type (Col), (d) sgr5-3. ep, epidermis; co, cortex; en, endodermis; pi, pith. Bar = 20 μm. A single magnified endodermal cell is shown in (e) wild type (Ler), (f) sgr5-4, (g) wild type (Col), (h) sgr5-3. Amyloplasts are indicated by arrowheads. Bar = 10 μm
Wild-type chloroplasts in the cortical cell layers also reacted weakly with IKI (Fig. 6a, c). In sgr5-3 and sgr5-4, starch staining in the chloroplasts was lower than wild-type controls, similar to our observations for amyloplasts (Fig. 6b, d). Therefore SGR5 regulates plastid starch levels in the cortex and endodermis of inflorescence stems. To verify that sgr5 stems contain less starch than wild type, we performed quantitative assays of total starch levels from stem tissue. sgr5-4 (Ler) stems contained approximately 28% less total starch than wild type (Ler), whereas sgr5-3 (Col) contained 8% less starch than wild type (Col) (Fig. 7a, b). We also measured total leaf starch in order to test the tissue specificity of SGR5 function. sgr5-4 rosette leaves contained 31% less starch than wild type (Ler) whereas sgr5-3 leaf starch was found not to be significantly lower than wild type (Col) (Fig. 7c, d). Together these results show that SGR5 controls starch levels in leaves as well as inflorescence stems.
Quantitative measurements of sgr5 starch levels. Starch concentration (mg/g fresh weight) from (a) stems, Ler background (P = 5.69 × 10−10), (b) stems, Col background (P = 9.73 × 10−4), (c) rosette leaves, Ler background (P = 5.01 × 10−4), and (d) rosette leaves, Col background (P = 0.222) (n = 8). Error bars represent standard error of the mean
Starch mutants show altered circumnutation
Our study of sgr5 alleles suggests that altered stem circumnutation may be associated with reduced starch levels. To assess whether starch might have a general effect on circumnutation, we performed time-lapse experiments to track stem movement in mutants at loci that directly regulate starch metabolism. The adg1-1 mutation causes loss of function of the gene coding for the small subunit of ADP-GLUCOSE PYROPHOSPHORYLASE, and lacks starch in stems, leaves and roots (Lin et al. 1988; Wang et al. 1998). SEX1 (STARCH EXCESS 1) controls starch degradation, with loss of function at this locus causing a starch excess phenotype (Yu et al. 2001). Both adg1-1 and sex1-1 stems showed a greater periodicity in their movement compared with wild type (Col) (Figs. 8 and S1). These results were qualitatively identical in 9 out of 11 experiments for adg1-1 and all of 9 experiments for sex1-1 (data not shown). The relative angle of bending in adg1-1 compared to wild type was inconsistent across our 11 experiments (data not shown). However, overall, the angle of bending was reduced in adg1-1 compared to wild type (Figs. 8 and S1). In contrast, sex1-1 stems bent at a consistently larger angle than wild type in all experiments (Figs. 8 and S1). Therefore, mutants with altered starch levels appear to show differences in the amplitude and period of stem circumnutation.
Discussion
In this study we have further characterised mutant alleles of SGR5/AtIDD15 in two different Arabidopsis ecotypes and show that the attenuated gravitropic response and circumnutation phenotypes are associated with reduced amyloplast sedimentation kinetics in shoot endodermal cells. In both Col and Ler, a reduction in SGR5 activity also results in decreased starch levels in amyloplasts as well as overall lower starch levels in stems. In addition we observed less acute cauline branch and pedicel angles in sgr5 mutants in both ecotypes. This trait is most likely directly related to the gravitropic phenotype since altered lateral organ angles are reported to be common for mutants of all shoot gravitropism (sgr) loci (Yamauchi et al. 1997).
SGR5 facilitates gravity perception in Arabidopsis
Previous work has demonstrated that SGR5 functions in the shoot endodermis, the site of gravity perception (Morita et al. 2006). Genetic dissection of the gravitropic response highlights key elements of gravity perception defined by mutant phenotypes such as (1) improper development of endodermal tissues, (2) defects in vacuolar biogenesis or function and (3) abnormal amyloplast formation and/or starch levels. The sgr mutants have been shown to act at the first two of these stages. For example, the SGR1 and SGR7 loci are allelic to the transcription factor genes SCARECROW (SCR) and SHORT ROOT (SHR), respectively (Fukaki et al. 1998). The sgr1 and sgr7 mutants lack normal endodermal layers, which results in loss of gravitropic sensing and agravitropic shoots (Fukaki et al. 1996; Fukaki et al. 1998).
The sgr mutants that affect vacuole formation or function include sgr2, sgr3, sgr4 and sgr8 (Morita and Tasaka 2004). SGR2 encodes a phospholipase A1-like protein that localises to the vacuole (Kato et al. 2002) whereas the SGR3 and SGR4 genes encode a pair of interacting SNARE proteins that may be involved in vesicular transport to the vacuole (Yano et al. 2003). Similarly, the SGR8/GRV2 gene product may play a role in endocytosis and vacuole formation (Silady et al. 2004). Although the underlying molecular mechanisms need to be confirmed, loss of function for any of these genes results in either an agravitropic or reduced gravitropic response caused by the inability of amyloplasts to sediment normally within the cell.
We have shown that amyloplast sedimentation is also affected in sgr5 mutants and propose that this is due to lower starch levels in shoot endodermal amyloplasts. Numerous reports suggest that lowered starch levels in amyloplasts cause reduced gravitropic response in shoots (Caspar and Pickard 1989; Kiss et al. 1996; Kiss et al. 1997; Weise and Kiss 1999; Vitha et al. 2007). In particular, mutants at the PHOSPHOGLUCOMUTASE (PGM) locus show reduced gravitropism of inflorescence stems, and amyloplasts in the shoot endodermis do not sediment towards gravity (Weise and Kiss 1999). PGM encodes an enzyme involved in starch synthesis in plastids (Caspar and Pickard 1989) which suggests that defects in gravitropism can be a direct consequence of having no starch. A recent analysis of the Arabidopsis starch excess mutant, sex1, finds an enhanced gravitropic response, providing further evidence that the degree of gravitropic movement is directly correlated with starch content (Vitha et al. 2007).
We also found that the speed at which amyloplasts sediment is correlated with amyloplast starch levels. Of the lines we tested, wild-type Col plants contained the highest concentrations of amyloplast starch as revealed by IKI staining, whilst wild-type plants in the Ler ecotype had less starch. Consistent with our hypothesis, Col amyloplasts sedimented at a faster rate than Ler and showed a stronger gravitropic response. Therefore the difference in gravitropic sensitivity observed between Col and Ler plants may be attributed, at least partially, to differences in amyloplast starch concentration. sgr5-3 mutants contained less amyloplast starch than either of the wild types and sgr5-4 plants showed even lower levels. Indeed amyloplast sedimentation occurred more slowly in sgr5-4 than in sgr5-3. These results support the starch-statolith model of gravity perception, highlighting the importance of amyloplast sedimentation for sensing gravity (Kiss et al. 1996; Kiss et al. 1997; Weise and Kiss 1999). Therefore we suggest that the simplest explanation is that SGR5 has a role in regulating the accumulation or deposition of starch within endodermal cells of plant shoots, which results in changes in the sedimentation of stem endodermal statoliths, and culminates in altered gravitropism and circumnutation.
Starch mutants show altered circumnutation movements
In addition to the attenuated gravitropic response reported here and by Morita et al. (2006), we also find that loss of SGR5 activity dampens circumnutational movements in both Col and Ler ecotypes. The causes of helical growth movements in plants have long been debated, but it is now acknowledged that there is a connection to gravitropic movements (Haswell 2003; Kitazawa et al. 2005). More importantly, gravity perception in the shoot endodermis appears to be a key regulator of shoot circumnutation. Loss of endodermal cell specification or amyloplast sedimentation in sgr1/scr, sgr2, sgr4, sgr7/shr, sgr8/grv2 or pgm mutants results in reduced circumnutational movement similar to sgr5 (Hatakeda et al. 2003; Silady et al. 2004; Kitazawa et al. 2005). These results further support our hypothesis that SGR5 acts at the level of gravity perception.
Circumnutation is also altered in the starch mutants adg1-1 and sex1-1, suggesting that starch may have a general role in circumnutation. adg1-1, which lacks starch, shows a smaller angle of bending than wild type, whereas sex1-1, which contains elevated starch levels, bends at a greater angle than wild type. Therefore the angle of bending appears to be directly proportional to starch content. Both increased starch and the absence of starch appear to increase the period of circumnutation. However, in sgr5-3, which has reduced starch levels, the period is shorter than wild type. Thus, while it seems that starch influences the period of circumnutation movements, there does not appear to be a general trend in relation to starch levels. Perhaps the angle of bending is regulated by gravity sensing and hence amyloplast starch, whereas starch could influence the period of movements by a separate mechanism. The connection between shoot starch levels and circumnutation is further supported by a report that circumnutational movements eventually cease in Arabidopsis plants kept in the dark for 48 h or more (Someya et al. 2006), possibly because of starch depletion in gravity sensing amyloplasts. However, it is interesting to note that starch does not appear to be absolutely required for circumnutation since adg1-1 stems, which contain no detectable starch (Lin et al. 1988), are still capable of movement.
The SGR5/AtIDD15 protein has a distinct role in regulating plant behaviour
The IDD gene family is a conserved group of plant specific zinc finger protein encoding genes based on the founding member from maize, INDETERMINATE1 (ID1) (Colasanti et al. 2006). Loss of ID1 gene function causes an extremely late flowering phenotype in maize (Colasanti et al. 1998). Mutations in AtIDD10/JACKDAW affect tissue patterning in the root of Arabidopsis (Welch et al. 2007). SGR5, or AtIDD15, is only the third member of the IDD gene family in any plant species reported to have a loss-of-function phenotype. Similarity of transcription factor proteins does not necessarily predict function, and members of a family are often found to control diverse, unrelated functions. In this case loss of SGR5 function in both the Col and Ler ecotypes shows a reduced response to gravity and dampened circumnutation, but, unlike maize id1 or Arabidopsis idd10 mutants, there is no effect on flowering time or root development (S. Chatfield, R. Tremblay and J. Colasanti, unpublished).
The Arabidopsis genome contains 16 AtIDD genes that share from 63% to 85% identity to each other in the ID-domain, a region of approximately 200 amino acids surrounding four distinct zinc finger motifs that define the IDD family in higher plants. Phylogenetic analysis shows that AtIDD15 and two other genes, AtIDD14 and AtIDD16, form a highly similar subgroup, group ‘A’, which is most distant from other members of this gene family (Colasanti et al. 2006). No loss of function mutants have yet been described for AtIDD14 or AtIDD16, so it is not known whether they function redundantly and have roles similar to AtIDD15 in regulating starch accumulation in amyloplasts or whether they have completely novel functions. However, since SGR5/AtIDD15 is expressed in roots (Morita et al. 2006) and sgr5 mutants show no root phenotype, this implies that SGR5 may function redundantly with other genes in this tissue. Both rice and maize have similar group ‘A’ clusters of three IDD genes that share similarities with their Arabidopsis counterparts. Therefore it would be interesting to obtain mutants in the corresponding SGR5/AtIDD15 orthologues in these plant species to determine whether there are similar effects on starch deposition and, by extension, gravitropic effects.
SGR5 can alter Arabidopsis response to an environmental stimulus
Both the transition to flowering and gravitropic movement illustrate the importance of environmental stimuli on plant growth and development. For example, in the case of flowering, many plants depend on defined photoperiods to signal the optimal time for reproductive success. The timing of floral transition ultimately is a major determinant of final plant form. Similarly, gravity directs plant growth movement in directions that are optimal for survival; e.g. positive gravitropism facilitates root growth down toward water/nutrients and negative gravitropism is required for shoots to grow upward. Here we show that elimination of SGR5 function alters plant response to an environmental stimulus in a way that modifies plant movement. This is interesting in light of speculation that transcription factor genes are key targets for plant evolution (reviewed in Doebley and Lukens 1998). Kitazawa et al. (2005) discovered that a mutant variant of the Pharbitis nil SCR/SGR1 gene, PnSCR, is responsible for reduced gravitropic sensitivity and attenuated circumnutation of the weeping variety of morning glory. Therefore it is conceivable that alterations in transcriptional regulator activity due to natural variation could be responsible for the broad spectrum of gravitational sensitivities and circumnutational movements that exist in plants.
Abbreviations
- ADG1:
-
ADP-GLUCOSE PYROPHOSPHORYLASE 1
- bp:
-
Base pair
- Col:
-
Columbia
- Ds:
-
Dissociation element
- EDTA:
-
Ethylene diamine tetraacetic acid
- GAPDH:
-
GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE
- ID1:
-
INDETERMINATE1
- IDD:
-
INDETERMINATE Domain
- GUS:
-
β-Glucuronidase
- IKI:
-
Iodine/potassium iodide
- kb:
-
Kilobase
- Ler :
-
Landsberg erecta
- PCR:
-
Polymerase chain reaction
- PGM:
-
Phosphoglucomutase
- RT-PCR:
-
Reverse transcriptase polymerase chain reaction
- SCR:
-
SCARECROW
- SDS:
-
Sodium dodecyl sulphate
- SEX1:
-
STARCH EXCESS 1
- SGR:
-
SHOOT GRAVITROPISM
- SHR:
-
SHORT ROOT
- T-DNA:
-
Transfer DNA
- w/v:
-
Weight per volume
- ZF:
-
Zinc finger
References
Alonso JM (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301:1849–1849
Benfey PN, Linstead PJ, Roberts K, Schiefelbein JW, Hauser MT, Aeschbacher RA (1993) Root Development in Arabidopsis—four mutants with dramatically altered root morphogenesis. Development 119:57–70
Blancaflor EB, Fasano JM, Gilroy S (1998) Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol 116:213–222
Brown AH (1993) Circumnutations—From Darwin to Space-flights. Plant Physiol 101:345–348
Caspar T, Pickard BG (1989) Gravitropism in a starchless mutant in Arabidopsis—Implications for the starch-statolith theory of gravity sensing. Planta 177:185–197
Colasanti J, Tremblay R, Wong AYM, Coneva V, Kozaki A, Mable BK (2006) The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genomics 7:158
Colasanti J, Yuan Z, Sundaresan V (1998) The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell 93:593–603
Delatte T, Trevisan M, Parker ML, Zeeman SC (2005) Arabidopsis mutants Atisa1 and Atisa2 have identical phenotypes and lack the same multimeric isoamylase, which influences the branch point distribution of amylopectin during starch synthesis. Plant J 41:815–830
DiLaurenzio L, WysockaDiller J, Malamy JE, Pysh L, Helariutta Y, Freshour G, Hahn MG, Feldmann KA, Benfey PN (1996) The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86:423–433
Doebley J, Lukens L (1998) Transcriptional regulators and the evolution of plant form. Plant Cell 10:1075–1082
Friml J, Wisniewska J, Benkova E, Mendgen K, Palme K (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415:806–809
Fukaki H, Fujisawa H, Tasaka M (1996) SGR-1, SGR2, and SGR3: Novel genetic loci involved in shoot gravitropism in Arabidopsis thaliana. Plant Physiol 110:945–955
Fukaki H, Tasaka M (1999) Gravity perception, gravitropic response of inflorescence stems in Arabidopsis thaliana, Life Sciences: Microgravity Research Ii, pp 763–770
Fukaki H, Wysocka-Diller J, Kato T, Fujisawa H, Benfey PN, Tasaka M (1998) Genetic evidence that the endodermis is essential for shoot gravitropism in Arabidopsis thaliana. Plant J 14:425–430
Haswell ES (2003) Gravity perception: How plants stand up for themselves. Curr Biol 13:R761–R763
Hatakeda Y, Kamada M, Goto N, Fukaki H, Tasaka M, Suge H, Takahashi H (2003) Gravitropic response plays an important role in the nutational movements of the shoots of Pharbitis nil and Arabidopsis thaliana. Physiol Plant 118:464–473
Johnsson A (1997) Circumnutations: Results from recent experiments on Earth and in space. Planta 203:S147–S158
Kato T, Morita MT, Fukaki H, Yamauchi Y, Uehara M, Niihama M, Tasaka M (2002) SGR2, a phospholipase-like protein, and ZIG/SGR4, a SNARE, are involved in the shoot gravitropism of Arabidopsis. Plant Cell 14:33–46
Kiss JZ (2006) Up, down, and all around: how plants sense and respond to environmental stimuli. Proc Natl Acad Sci USA 103:829–830
Kiss JZ, Guisinger MM, Miller AJ, Stackhouse KS (1997) Reduced gravitropism in hypocotyls of starch-deficient mutants of Arabidopsis. Plant Cell Physiol 38:518–525
Kiss JZ, Wright JB, Caspar T (1996) Gravitropism in roots of intermediate-starch mutants of Arabidopsis. Physiol Plant 97:237–244
Kitazawa D, Hatakeda Y, Kamada M, Fujii N, Miyazawa Y, Hoshino A, Iida S, Fukaki H, Morita MT, Tasaka M, Suge H, Takahashi H (2005) Shoot circumnutation and winding movements require gravisensing cells. Proc Natl Acad Sci USA 102:18742–18747
Kozaki A, Hake S, Colasanti J (2004) The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res 32:1710–1720
Lin TP, Caspar T, Somerville C, Preiss J (1988) Isolation and Characterization of a starchless mutant of Arabidopsis thaliana (L) Heynh lacking ADP glucose pyrophosphorylase activity. Plant Physiol 86:1131–1135
Morita MT, Sakaguchi K, Kiyose SI, Taira K, Kato T, Nakamura M, Tasaka M (2006) A C2H2-type zinc finger protein, SGR5, is involved in early events of gravitropism in Arabidopsis inflorescence stems. Plant J 47:619–628
Morita MT, Tasaka M (2004) Gravity sensing and signaling. Curr Opin Plant Biol 7:712–718
Olsen GM, Mirza JI, Maher EP, Iversen TH (1984) Ultrastructure and movements of cell organelles in the root cap of agravitropic and normal seedlings of Arabidopsis thaliana. Physiol Plant 60:523–531
Ottenschlager I, Wolff P, Wolverton C, Bhalerao RP, Sandberg G, Ishikawa H, Evans M, Palme K (2003) Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA 100:2987–2991
Parinov S, Sevugan M, Ye D, Yang WC, Kumaran M, Sundaresan V (1999) Analysis of flanking sequences from Dissociation insertion lines: a database for reverse genetics in Arabidopsis. Plant Cell 11:2263–2270
Sack FD (1997) Plastids and gravitropic sensing. Planta 203:S63–S68
Scheres B, Dilaurenzio L, Willemsen V, Hauser MT, Janmaat K, Weisbeek P, Benfey PN (1995) Mutations affecting the radial organization of the Arabidopsis root display specific defects throughout the embryonic axis. Development 121:53–62
Silady RA, Kato T, Lukowitz W, Sieber P, Tasaka M, Somerville CR (2004) The gravitropism defective 2 mutants of Arabidopsis are deficient in a protein implicated in endocytosis in Caenorhabditis elegans. Plant Physiol 136:3095–3103
Someya N, Niinuma K, Kimura M, Yamaguchi I, Hamamoto H (2006) Circumnutation of Arabidopsis thaliana inflorescence stems. Biol Plant 50:287–290
Vitha S, Ming Y, Sack FD, Kiss JZ (2007) Gravitropism in the starch excess mutant of Arabidopsis thaliana. Am J Bot 94:590–598
Wang SM, Lue WL, Yu TS, Long JH, Wang CN, Eimert K, Chen J (1998) Characterization of ADG1, an Arabidopsis locus encoding for ADPG pyrophosphorylase small subunit, demonstrates that the presence of the small subunit is required for large subunit stability. Plant J 13:63–70
Weise SE, Kiss JZ (1999) Gravitropism of inflorescence stems in starch-deficient mutants of Arabidopsis. Int J Plant Sci 160:521–527
Welch D, Hassan H, Blilou I, Immink R, Heidstra R, Scheres B (2007) Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Genes Dev 21:2196–2204
Wong AYM, Colasanti J (2007) Maize floral regulator protein INDETERMINATE1 is localized to developing leaves and is not altered by light or the sink/source transition. J Exp Bot 58:403–414
Yamamoto K, Pyke KA, Kiss JZ (2002) Reduced gravitropism in inflorescence stems and hypocotyls, but not roots, of Arabidopsis mutants with large plastids. Physiol Plant 114:627–636
Yamamoto KT (2003) Happy end in sight after 70 years of controversy. Trends Plant Sci 8:359–360
Yamauchi Y, Fukaki H, Fujisawa H, Tasaka M (1997) Mutations in the SGR4, SGR5 and SGR6 loci of Arabidopsis thaliana alter the shoot gravitropism. Plant Cell Physiol 38:530–535
Yano D, Sato M, Saito C, Sato MH, Morita MT, Tasaka M (2003) A SNARE complex containing SGR3/AtVAM3 and ZIG/VTI11 in gravity-sensing cells is important for Arabidopsis shoot gravitropism. Proc Natl Acad Sci USA 100:8589–8594
Young LM, Evans ML, Hertel R (1990) Correlations between gravitropic curvature and auxin movement across gravistimulated roots of Zea mays. Plant Physiol 92:792–796
Yu TS, Kofler H, Hausler RE, Hille D, Flugge UI, Zeeman SC, Smith AM, Kossmann J, Lloyd J, Ritte G, Steup M, Lue WL, Chen JC, Weber A (2001) The Arabidopsis sex1 mutant is defective in the R1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell 13:1907–1918
Acknowledgements
We thank Michael Mucci for expert plant care and Elizabeth Holmes for performing DNA sequencing. Thanks also to Steven Chatfield, Steven Rothstein and Thierry Delatte for useful discussions, and Viktoriya Coneva for comments on the manuscript. MT was funded by the Ontario Ministry of Agriculture Challenge Fund and RT was a recipient of an Ontario Graduate Scholarship. Research in JC’s lab is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery grant), the Canadian Foundation for Innovation, the Ontario Ministry of Agriculture and Food and the Ontario Innovation Trust.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Supplementary Fig. S1: Time-lapse movie of sgr5 circumnutation. Time-lapse movie of sgr5 mutant alleles in Col and Ler ecotypes. Images of plants growing under white fluorescent light were recorded every 5 min for 415 min and played back at 6 frames/s. Order of plants from left to right is sgr5-3, wild type (Col), sgr5-4 and wild type (Ler) (MOV 4500 kb)
Supplementary Fig. S2: Time-lapse movie of circumnutation in starch mutants. Images of plants growing under fluorescent white light were captured every 5 min for 500 min and played back at 6 frames/s. From left to right plants are adg1-1, wild type (Col) and sex1-1 (MOV 3416 kb)
Rights and permissions
About this article
Cite this article
Tanimoto, M., Tremblay, R. & Colasanti, J. Altered gravitropic response, amyloplast sedimentation and circumnutation in the Arabidopsis shoot gravitropism 5 mutant are associated with reduced starch levels. Plant Mol Biol 67, 57–69 (2008). https://doi.org/10.1007/s11103-008-9301-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-008-9301-0