Abstract
Rice plants (Oryza sativa L.) take up iron using iron-chelating compounds known as mugineic acid family phytosiderophores (MAs). In the biosynthetic pathway of MAs, nicotianamine aminotransferase (NAAT) catalyses the key step from nicotianamine to the 3′′-keto form. In the present study, we identified six rice NAAT genes (OsNAAT1–6) by screening a cDNA library made from Fe-deficient rice roots and by searching databases. Among the NAAT homologues, OsNAAT1 belongs to a subgroup containing barley functional NAAT (HvNAAT-A and HvNAAT-B) as well as a maize homologue cloned by cDNA library screening (ZmNAAT1). Northern blot and RT-PCR analysis showed that OsNAAT1, but not OsNAAT2–6, was strongly up-regulated by Fe deficiency, both in roots and shoots. The OsNAAT1 protein had NAAT enzyme activity in vitro, confirming that the OsNAAT1 gene encodes functional NAAT. Promoter–GUS analysis revealed that OsNAAT1 was expressed in companion and pericycle cells adjacent to the protoxylem of Fe-sufficient roots. In addition, expression was induced in all cells of Fe-deficient roots, with particularly strong GUS activity evident in the companion and pericycle cells. OsNAAT1 expression was also observed in the companion cells of Fe-sufficient shoots, and was clearly induced in all the cells of Fe-deficient leaves. These expression patterns highly resemble those of OsNAS1, OsNAS2 and OsDMAS1, the genes responsible for MAs biosynthesis for Fe acquisition. These findings strongly suggest that rice synthesises MAs in whole Fe-deficient roots to acquire Fe from the rhizosphere, and also in phloem cells to maintain metal homeostasis facilitated by MAs-mediated long-distance transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Graminaceous plants take up Fe using a unique mechanism known as Strategy II, secreting Fe-chelating compounds into the rhizosphere when the plants sense Fe deficiency (Takagi 1976; Römheld and Marschner 1986). The chelating compounds, which have six coordination sites (three –COOH, two –NH and one –OH) that bind to Fe, are termed mugineic acid family phytosiderophores (MAs). Although Fe is mainly present as oxidised Fe(III) compounds, poorly soluble in neutral to alkaline soil, chelation of MAs to Fe(III) dramatically increases the solubility of Fe(III) in the rhizosphere, making graminaceous plants capable of taking up Fe as Fe(III)–MAs complexes. The biosynthesis and secretion of MAs markedly increase in roots in response to Fe deficiency (Takagi 1976; Takagi et al. 1984). The tolerance to Fe deficiency among graminaceous plants is thought to be dependent on the amount and kinds of MAs that they secrete. Rice (Oryza sativa L.), sorghum (Sorghum bicolor L.) and maize (Zea mays L.) secrete only small amounts of 2′-deoxymugineic acid (DMA) among the possible MAs, and thus are susceptible to low-Fe availability. In contrast, barley (Hordeum vulgare L.) secretes large amounts of MAs, including mugineic acid and 3-epihydroxymugineic acid, in addition to DMA, under Fe deficiency; therefore, it is more tolerant to Fe deficiency than other graminaceous plants (Mori and Nishizawa 1987; Lytle and Jolley 1991; Nakanishi et al. 1993; Kanazawa et al. 1994; Higuchi et al. 1996; Ma et al. 1999).
The biosynthetic pathway of MAs in graminaceous plants has been identified through extensive biochemical and physiological studies (Mori and Nishizawa 1987; Kawai et al. 1988, Shojima et al. 1990; Ma and Nomoto 1993; Ma et al. 1999). Methionine is the precursor of MAs (Mori and Nishizawa 1987) and is adenosylated by S-adenosylmethionine (SAM) synthetase (Takizawa et al. 1996). Nicotianamine synthase (NAS) catalyses the trimerisation of SAM to nicotianamine (NA) (Higuchi et al. 1994). Nicotianamine aminotransferase (NAAT) catalyses the amino transfer of NA to produce the 3′′-keto intermediate (Shojima et al. 1990; Kanazawa et al. 1995) and is a key enzyme in the biosynthetic pathway of MAs; this is the first step specific to graminaceous plants. Deoxymugineic acid synthase (DMAS) reduces the 3′′-keto form to DMA (Bashir et al. 2006). All the MAs share their biosynthetic pathway from methionine to DMA, which is then hydroxylated to form other MAs in barley by IDS2 and IDS3 dioxygenases (Nakanishi et al. 1993, 2000; Kobayashi et al. 2001).
The genes involved in MAs biosynthesis have been cloned from graminaceous plants. NAS genes were first isolated from barley (HvNAS1–7) through enzyme purification from Fe-deficient barley roots (Higuchi et al. 1999). Subsequently, NAS genes were again isolated from barley (NASHOR1 and NASHOR2; Herbik et al. 1999), as well as from rice (OsNAS1–3; Higuchi et al. 2001) and maize (ZmNAS1–3; Mizuno et al. 2003). Two barley NAAT genes, HvNAAT-A and HvNAAT-B, were also cloned through enzyme purification from Fe-deficient barley roots (Takahashi et al. 1999). Recently, DMAS genes were also cloned from rice (OsDMAS1), barley (HvDMAS1), wheat (TaDMAS1) and maize (ZmDMAS1), through identification of an Fe deficiency-inducible aldo–keto reductase gene and establishment of an enzyme activity assay (Bashir et al. 2006).
Graminaceous plants take up Fe(III)–MAs complexes through specific transporters. The gene encoding the transporter was first cloned from the maize yellow stripe1 (ys1) mutant (Curie et al. 2001), which is defective in Fe(III)–MAs uptake (von Wirén et al. 1994). YS1 transports not only metal–MAs complexes but also metal–NA complexes (Schaaf et al. 2004). In rice, among 18 putative YS1 homologues (OsYSLs), OsYSL2 is strongly expressed in Fe-deficient leaves, and its encoding protein transports Fe(II)–NA and Mn(II)–NA complexes (Koike et al. 2004). In barley, HvYS1 is also up-regulated in root epidermal cells in response to Fe deficiency and transports the Fe(III)–MAs complex (Murata et al. 2006). In non-graminaceous plants, YSL transporters are considered to play important roles in metal homeostasis by transporting metal–NA complexes because non-graminaceous plants synthesise NA but not MAs (Colangelo and Guerinot 2006).
Rice is one of the most important crop species in the world, and its low tolerance to Fe deficiency in calcareous and marginal soils restricts the world food supply. Thus, molecular characterisation of rice genes involved in the MAs-based Fe uptake system is of special importance. In the present report, six rice NAAT genes, OsNAAT1–6, were identified to determine the uncharacterised key step in DMA biosynthesis in rice. Among the rice NAAT genes, OsNAAT1 was found to be strongly induced under Fe deficiency. By using in vitro enzyme activity assay system, we demonstrated that OsNAAT1 encodes a functional protein with NAAT activity. OsNAAT1 promoter–GUS analysis strongly suggested that DMA is synthesised in all root cells for Fe acquisition from the rhizosphere, and also plays a role in long-distance transport of Fe in rice. The cloning and characterisation of the OsNAAT1 gene should serve as an important step in uncovering the role of DMA in rice Fe nutrition.
Materials and methods
Plant materials
Wild-type and transgenic rice seeds were germinated on Murashige and Skoog (MS) medium and transferred into a nutrient solution in a glass house with 30°C light/25°C dark periods under natural light conditions. The composition of the nutrient solution was 2 mM Ca(NO3)2, 0.5 mM MgSO4, 0.7 mM K2SO4, 0.1 mM KCl, 0.1 mM KH2PO4, 10 μM H3BO3, 0.1 mM Fe(III)–EDTA, 0.5 μM MnSO4, 0.5 μM ZnSO4, 0.2 μM CuSO4 and 0.01 μM (NH4)6Mo7O24. The pH of the culture solution was adjusted daily to 5.3 with 1 N HCl. When the fifth leaves appeared, plants were cultured without Fe. Control plants were cultured continuously in the standard culture solution. Shoots and roots were harvested 2 weeks after transplanting for Northern blot and histochemical analyses, or 1 week after transplanting for quantitative real-time PCR (RT-PCR) analysis.
Cloning OsNAAT and ZmNAAT genes
To clone rice NAAT homologues, a cDNA library was synthesised using poly(A) + RNA extracted from Fe-deficient rice roots (Higuchi et al. 2001). In 2002, putative homologues of HvNAAT-A and HvNAAT-B genes were searched in rice databases, predicting the presence of four rice NAAT genes, which we designated as OsNAAT1–4. Specific primers were designed: OsNAAT1-F: 5′-TAAGAGGATAATTGATTTGCTTAC-3′, OsNAAT1-R: 5′-CTGATCATTCCAATCCTAGTACAAT-3′, OsNAAT2-F: 5′-CAACAAAATTCTCGATCAATTAAG-3′, OsNAAT2-R: 5′-ATGAAATATCTCAACACCTTTGTGC-3′, OsNAAT3-F: 5′-CACCAATGCCCTTGGGGTGGTGAA-3′, OsNAAT3-R: 5′-CTGAAAGCCTGAAACTATTCACGAG-3′, OsNAAT4-F: 5′-CCGAGCTATTGCAGAGTACCTATC-3′, OsNAAT4-R: 5′-GGAGTGCTTCCATAAACAAGGTGA-3′. These primers were used to successfully amplify probes of the corresponding genes from the cDNA library. Approximately 400,000 colonies of the cDNA library were screened using colony hybridisation. Isolated cDNA clones were sequenced using a Thermo Sequenase Cycle Sequencing kit (Shimadzu, Kyoto, Japan) and a DNA sequencer (DSQ-2000L; Shimadzu). To clone maize NAAT homologues, a cDNA library was synthesised using poly(A) + RNA extracted from Fe-deficient maize roots (Mizuno et al. 2003). Based on HvNAAT-A sequence, a primer pair was designed: forward, 5′-GCCGTAGCAGAGCACTTGTCACAG and reverse, 5′-GATGACCATCGCGGTGGTGTTCTT. An amplified fragment using these primers and the maize cDNA library as a template was used for colony hybridisation.
Northern blot analysis
Total RNA was isolated from rice shoots and roots and was subjected to Northern blot analysis, as described by Higuchi et al. (1999), using the same probes as those used in colony hybridisation. No obvious cross-hybridisation was observed, confirming the specific nature of the probes.
Quantitative RT-PCR analysis
Total RNA was extracted from rice leaves and roots using a RNeasy Plant Kit (QIAGEN, Tokyo, Japan), and was treated with RNase-free DNase I (TaKaRa, Japan) to remove contaminating genomic DNA. First-strand cDNA was synthesised using ReverTra Ace reverce transcriptase (TOYOBO, Japan) by priming with oligo-d(T)17. The NAAT fragments were amplified by PCR in a SmartCycler (TaKaRa) with SYBR Green I and ExTaq™ Real-Time-PCR version (TaKaRa). Gene-specific primers used for PCR were as follows: OsNAAT1 forward, 5′-TAAGAGGATAATTGATTTGCTTAC, OsNAAT1 reverse, 5′-CTGATCATTCCAATCCTAGTACAAT, OsNAAT2 forward, 5′-CAACAAAATTCTCGATCAATTAAG, OsNAAT2 reverse, 5′-ATGAAATATCTCAACACCTTTGTGC, OsNAAT3 forward, 5′-CACCAATGCCCTTGGGGTGGTGAA, OsNAAT3 reverse, 5′-CACGAGCTAGCTGGCTTCCTTGA, OsNAAT4 forward, 5′-CCGAGCTATTGCAGAGTACCTATC, OsNAAT4 reverse, 5′-GGAGTGCTTCCATAAACAAGGTGA, OsNAAT5 forward, 5′-GAAACTGGATCTGTCCTGCC, OsNAAT5 reverse, 5′-TTCGGCTTGCTATGTCGCGA, OsNAAT6 forward, 5′-CACTTCTGTTCGGTGTTGAA, and OsNAAT6 reverse, 5′-TAGCTTTACTTGAACTGCTC. The primers used for internal control in RT-PCR were Actin forward, 5′-CGCCAYACNGGTGYTATGGTTGG, and Actin reverse, 5′-ACACGGAGCTCATTGTAGAA.
Protein expression and purification
To subclone OsNAAT1 into pMAL-c2 (New England Biolabs, Ipswich, MA, USA), the ORF sequence was amplified using primers 5′-gagagaagatctATGGCACCGACGACGGCGGCGGCGG-3′ and 5′-gagagatctagaCTAGATATAATTTAAAGGGTTTTTC-3′, which contain EcoRI and BamHI restriction sites, respectively. The amplified fragments were cloned into pBluescript II SK+ (Stratagene, La Jolla, CA, USA). The verified fragment was excised with EcoRI and BamHI, and was subcloned into pMAL-c2. The resultant fusion plasmid was introduced into Escherichia coli strain XL1-Blue to produce the OsNAAT1-MBP fusion protein. The protein was purified as described by Higuchi et al. (1999).
NAAT enzyme assay
The NAAT enzyme assay was carried out according to the methods of Ohata et al. (1993) and Kanazawa et al. (1994). Five micrograms of OsNAAT1 fusion protein was centrifuged in an Amicon Ultrafree-MC 30-kDa cutoff filter unit (Millipore, Billerica, MA, USA) at 6,200 × g and 4°C for 15 min. The flow-through was discarded, and 50 μl of N-[Tris(hydroxymethyl)methyl]-3-aminopropanesulfonic acid buffer (50 mM TAPS, 5 mM KCl, 5 mM MgCl2, 10 mM 2-oxoglutaric acid, 10 μM pyridoxal 5′-phosphate (PLP), 150 μM nicotianamine (Hasegawakoryo)) were added to the filter unit. The solution was mixed several times by pipetting and incubated at 26°C for 30 min. The filter unit was then placed in a new tube and centrifuged at 6,200 × g and 4°C for 15 min. The flow-through was collected and 4 μl of 0.25 M NaBH4 were added to allow the reduction for 1 min at room temperature. Then, 50 μl of each sample were analysed by HPLC using DMA as a standard (Mori and Nishizawa 1987; Takahashi et al. 1999). All reactions were performed in duplicate.
Rice transformation and histochemical analysis
The 1.7-kb 5′-upstream region of the OsNAAT1 gene was amplified by PCR using genomic DNA as a template. The primers used were the forward primer 5′-ctctctaagcttCTTAATGGCACAGAGGGAAAAACCT-3′ and the reverse primer 5′-ctctcttctagaGGCCGTGCTCTGTTTTTTGTTGGT-3′, which contain XbaI and HindIII restriction sites, respectively. The amplified and verified fragment was excised by XbaI and HindIII and was subcloned upstream of the uidA ORF, which encodes β-glucuronidase (GUS), in the pIG121Hm vector (Hiei et al. 1994). An Agrobacterium tumefaciens strain (C58) carrying the above construct was used to transform rice (Oryza sativa L. cv. Tsukinohikari) as described previously (Higuchi et al. 2001). T1 seeds were germinated and cultured as described above, and subjected to GUS expression analysis as described by Inoue et al. (2003).
Results
Cloning of OsNAAT1
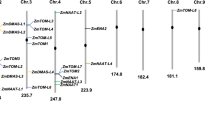
To clone OsNAAT genes, HvNAAT homologues in a cDNA library made from Fe-deficient rice roots were screened (Higuchi et al. 2001). Sequence analysis of the obtained clones revealed the presence of four distinct cDNA clones, designated as OsNAAT1 (Accession No. AB206814), OsNAAT2 (AK060537), OsNAAT3 (Os02g0302400) and OsNAAT4 (AK107186). A database search identified the presence of two more cDNA-encoding putative NAAT, designated as OsNAAT5 (Os11g0552000) and OsNAAT6 (AK060557). Among these, OsNAAT1 is the most homologous to HvNAAT-A and HvNAAT-B (Fig. 1). The OsNAAT1 is 1423 nucleotides in length, located on rice chromosome 3, and encodes a predicted 444 polypeptides. Searching for conserved domains revealed that OsNAAT1 has PLP-dependent aminotransferase class I and II domains (pfam00155.12). The OsNAAT1 gene contains an Fe deficiency-responsive element 1 (IDE1)-like sequence at 290–273 bases upstream from the putative translation start site (Kobayashi et al. 2005). We also screened a maize cDNA library made from Fe-deficient roots to isolate HvNAAT homologues. One positive clone putatively encoding a close homologue of HvNAAT was identified, which we designated as ZmNAAT1 (Fig. 1a).
Sequence characteristics of OsNAAT and other NAAT homologues. (a) The deduced amino acid sequence of OsNAAT1 is aligned with HvNAAT-A, HvNAAT-B and ZmNAAT1. The shaded areas represent identical residues in at least three of the four proteins. The asterisk and box indicate the lysine residue of putative pyridoxal phosphate binding site and the neighbouring consensus sequence, respectively. (b) Unrooted phylogenic tree for the deduced amino acid sequence of NAAT homologues. BT009504, Triticum aestivum clone wr1.pk0085.h9:fis, full insert mRNA sequence; AY014359, Zea mays PCO115235 mRNA sequence; CN130225, Sorghum bicolor cDNA clone RHOH1_40_C05_A002 3′, mRNA sequence; NM_124776, Arabidopsis thaliana aminotransferase, putative (At5g53970) mRNA; DQ006809, Medicago truncatula tyrosine aminotransferase mRNA; DQ003328, Glycine max tyrosine aminotransferase mRNA; BT012990, Lycopersicon esculentum clone 114210R, mRNA sequence; AJ458993, Coleus blumei mRNA for tyrosine aminotransferase (tat gene); NM_123007, Arabidopsis thaliana aminotransferase-related (At5g36160) mRNA; AY054204, Arabidopsis thaliana At2g20610/F23N11.7 mRNA; NM_201760, Arabidopsis thaliana SUR1 (SUPERROOT 1); transaminase (SUR1) mRNA; AK176613, Arabidopsis thaliana mRNA for tyrosine transaminase-like protein; NM_118983, Arabidopsis thaliana aminotransferase-related (At4g28410) mRNA; NM_128044; Arabidopsis thaliana TAT3 (TYROSINE AMINOTRANSFERASE 3); transaminase (TAT3) mRNA; NM_118490, Arabidopsis thaliana aminotransferase class I and II family protein (At4g23590) mRNA; NM_118491, Arabidopsis thaliana CORI3 (CORONATINE INDUCED 1, JASMONIC ACID RESPONSIVE 2); transaminase (CORI3) mRNA; AY187682, Brassica oleracea cystine lyase BOCL-3 mRNA
To compare the amino acid sequences of NAAT homologues, we used CLUSTAL W, which found 17 additional homologous proteins from graminaceous and non-graminaceous plants (Fig. 1b). Among the NAAT homologues, HvNAAT-A, HvNAAT-B, OsNAAT1 and ZmNAAT1 form a distinct subgroup. This subgroup also contains putative NAAT from wheat (BT009504), maize (AY014359) and sorghum (CN130225) but contained no homologous sequences from non-graminaceous species. OsNAAT1 is 76% homologous to HvNAAT-A, 74% to HvNAAT-B, and 75% to ZmNAAT1. In contrast, OsNAAT1 displays lower homology to OsNAAT2 (61%), OsNAAT3 (56%), OsNAAT4 (61%), OsNAAT5 (63%), OsNAAT6 (46%) and an Arabidopsis putative tyrosine aminotransferase, NM_124776 (55%).
Expression pattern of OsNAATs
To further estimate possible candidates of OsNAAT genes related to MAs-based Fe uptake, expression pattern of OsNAAT genes in response to Fe deficiency was analysed. Northern blot analysis of OsNAAT1–4 genes was performed using rice plants under Fe-sufficient or Fe-deficient conditions (Fig 2a). OsNAAT1 was weakly expressed in roots of Fe-sufficient plants and was strongly induced in both roots and shoots of Fe-deficient plants. OsNAAT2 was constitutively expressed in both roots and shoots at low levels. OsNAAT3 was constitutively expressed in the shoots of both Fe-sufficient and Fe-deficient plants, and OsNAAT4 was expressed in the roots of both Fe-sufficient and Fe-deficient plants. Quantitative RT-PCR analysis was further conducted to detect the expression of OsNAAT1–6 genes (Tables 1 and 2). Under Fe deficiency, OsNAAT1 expression was induced to 18.2- and 8.0-fold in roots and leaves, respectively. In contrast, transcript abundance of the other OsNAAT genes (OsNAAT2, OsNAAT3, OsNAAT4, OsNAAT5 and OsNAAT6) showed no clear change in response to Fe deficiency both in roots and leaves (Table 1). Transcript abundance of OsNAAT1 reached up to more than 107 copies per 1 μg of total RNA in Fe-deficient roots and leaves (Table 2). Expression of OsNAAT1-6 was also monitored by microarray analyses, which confirmed the Northern and RT-PCR results and also suggested a dominance of OsNAAT1 transcripts among the OsNAAT1-6 in Fe-deficient roots (data not shown).
Northern blot analysis of OsNAAT. Rice plants were grown hydroponically under Fe sufficiency or deficiency for 2 weeks. (a) +Fe, Fe sufficiency; −Fe, Fe deficiency; S, shoots; R, roots. (b) CR, Fe-sufficient roots; FR, Fe-deficient roots; CS, Fe-sufficient shoots; FY, Fe-deficient chlorotic young leaves; FG, Fe-deficient green old leaves
A Northern blot analysis of OsNAAT1 was then performed using the green old leaves and yellow young leaves (Fig. 2b). Transcripts of OsNAAT1 were more abundant in the yellow young leaves than in green old leaves. These expression patterns of OsNAAT1 are similar to those of OsNAS1, OsNAS2 and OsDMAS1 (Inoue et al. 2003; Bashir et al. 2006). We also examined the expression of ZmNAAT1 in response to Fe deficiency by Northern blot analysis. ZmNAAT1 expression was strongly induced under Fe deficiency in roots, but not in leaves (data not shown), resembling the expression pattern of HvNAAT-A and HvNAAT-B (Takahashi et al. 1999).
OsNAAT1 has nicotianamine aminotransferase activity
From the sequence comparison and expression patterns of OsNAATs, OsNAAT1 was thought to play a dominant role in rice DMA biosynthesis for Fe acquisition. Therefore, the enzyme activity of OsNAAT1 was examined using a fusion protein to maltose-binding protein (MBP). Performance of the in vitro reaction with recombinant OsNAAT1–MBP was identified by DMA detection using HPLC (Fig. 3). The HPLC signal of the OsNAAT1–MBP reaction demonstrated the generation of DMA, showing identical retention time to that of standard DMA. In contrast, the signal of the MBP reaction showed no peak in the corresponding retention time. Therefore, the OsNAAT1 protein was confirmed to possess NAAT enzyme activity.
HPLC profile of enzymatic activity of the recombinant OsNAAT1 protein. Enzymatic activity was determined through detection of DMA by HPLC, followed by the chemical reduction of a 3′′-keto acid generated by NAAT. (a) OsNAAT1-MBP, (b) DMA standard, (c) free MBP. The peaks corresponding to DMA are indicated by arrowheads
Spatial pattern of OsNAAT1 expression
To gain a more detailed insight into the physiological roles of the OsNAAT1 gene, the localisation of its expression in both Fe-sufficient and Fe-deficient rice plants was investigated through histochemical localisation of the OsNAAT1 promoter–GUS transformants. GUS activity expressed by the OsNAAT1 promoter was histochemically detected by blue colour staining (Fig. 4).
Histochemical localisation of the OsNAAT1 promoter–GUS expression in transgenic rice plants grown under Fe sufficiency (a–c, g, i) or Fe deficiency (d–f, h, j, k). (a, d) Root transverse sections. (b, e) Enlarged part of the stele. (c, f) Enlarged part of the epidermis and exodermis. (g, h) Root longitudinal section. (i) Fe-sufficient leaf. (j) Chlorotic young leaf of Fe-deficient plants. (k) Green old leaf of Fe-deficient plants. Scale bars = 100 μm for (a, d); 50 μm for (b, e, i–k); 25 μm for (c, f); and 500 μm for (g, h)
In the roots of Fe-sufficient plants, GUS staining was mainly detected within the stele, and was also observed in some part of the epidermal and exodermal cells (Fig. 4a, c). At higher magnification, the staining was detected in part of the pericycle cells adjacent to the protoxylem and metaxylem, as well as in the protophloem cells along the vascular bundles in Fe-sufficient roots (Fig. 4b). Longitudinal sections of Fe-sufficient roots showed that staining was mainly detected in the stele (Fig. 4g). In roots of Fe-deficient plants, the promoter activity of OsNAAT1 was much stronger and was detected in all tissues, including the epidermis, exodermis, cortex and whole stele (Fig. 4d–f, h). Strong staining was obvious in pericycle cells adjacent to the protoxylem (Fig. 4e), and was also evident in Fe-sufficient roots. Under both Fe sufficiency and Fe deficiency, staining was detected in cells surrounding the metaxylem I, which is typically the region from which lateral roots emerge (data not shown).
In leaves, GUS staining under Fe sufficiency was slight and was only detected in phloem companion cells (Fig. 4i). In contrast, strong staining was detected in all vascular bundles and mesophyll cells in chlorotic young leaves of Fe-deficient plants (Fig. 4j). GUS staining was especially prominent in the vascular bundles, both in xylem and phloem cells. In older green leaves of Fe-deficient plants, GUS staining was detected in vascular bundles but not in mesophyll cells (Fig. 4k). These spatial patterns of OsNAAT1 expression were highly consistent with results from the Northern blot and RT-PCR analyses (Fig. 2; Tables 1 and 2).
Discussion
OsNAAT1 encodes a functional nicotianamine aminotransferase involved in the biosynthesis of DMA in rice
The rice OsNAAT1 gene, which encodes a key enzyme in the biosynthesis of MAs, was isolated for the first time. The OsNAAT1 protein possesses NAAT activity in vitro (Fig. 3), and the gene expression was strongly upregulated in Fe-deficient roots and shoots (Figs. 2 and 4; Tables 1 and 2). In contrast, the expression of OsNAAT2–6 was not induced in response to Fe deficiency (Fig. 2a; Table 1). Furthermore, OsNAAT1 belongs to a distinct subgroup containing HvNAAT-A and HvNAAT-B, the barley functional NAAT genes responsible for biosynthesis of MAs (Takahashi et al. 1999, 2001; Fig. 1). A close homologue of HvNAAT from maize, ZmNAAT1, was also cloned. ZmNAAT1 also belongs to the same subgroup with HvNAAT-A and HvNAAT-B (Fig. 1b) and exhibits Fe deficiency-induced expression. These results suggest that OsNAAT1 plays a major role in DMA biosynthesis for rice Strategy II Fe acquisition. Characterisation of transgenic rice lines with altered OsNAAT1 expression would further clarify the importance of OsNAAT1 in DMA biosynthesis.
DMA is suggested to be biosynthesised in all root cells under Fe-deficient conditions
To clarify the role of DMA biosynthesis and secretion in Fe homeostasis, expression patterns of the genes involved in DMA biosynthesis need to be spatially determined. To this end, promoter–GUS transgenic rice plants were previously produced and analysed to clarify localisation regarding the expression of the OsNAS1–3 and OsDMAS1 genes (Inoue et al. 2003; Bashir et al. 2006). Thus, the present finding on the expression pattern of OsNAAT1 in comparison to previous data on OsNAS and OsDMAS expression now enables estimation of the site of DMA production in rice plants. OsNAS1, OsNAS2 and OsDMAS1 are expressed in all root cells under Fe-deficient conditions (Inoue et al. 2003; Bashir et al. 2006). The localisation of OsNAAT1 expression closely resembled these previously identified patterns (Fig. 4), strongly suggesting that DMA is synthesised in all root cells under Fe-deficient conditions. To meet the increased demand for methionine required for the synthesis of DMA, the methionine cycle is highly active in Fe-deficient wheat roots (Ma et al. 1995). Microarray analysis and Northern blot analysis revealed that expression of the genes required for all the predicted steps in the methionine cycle are strongly induced in response to Fe deficiency in roots of barley and rice (Negishi et al. 2002; Kobayashi et al. 2005; Suzuki et al. 2006). Some of the genes participating in the methionine cycle and biosynthesis of MAs possess similar sequences to Fe deficiency-responsive cis-acting elements, IDE1 and IDE2, in their upstream regions (Kobayashi et al. 2003, 2005). Furthermore, IDE1 and IDE2 confer similar expression patterns to OsNAS1, OsNAS2, OsDMAS1 and OsNAAT1 promoters in rice (Kobayashi et al. 2004). More recently, the genes participating in DMA biosynthesis in rice, including OsNAS1, OsNAS2, OsDMAS1 and OsNAAT1, have been found to be under the regulation of an Fe deficiency-inducible bHLH transcription factor, OsIRO2 (Ogo et al. 2006, 2007). Thus, the genes involved in DMA biosynthesis are thought to be co-ordinately regulated in an Fe deficiency-inducible fashion.
These results raise the possibility that a series of the genes involved in the methionine cycle and DMA biosynthesis may be expressed in the same cells, suggesting that the biosynthesis from methionine to DMA takes place in all root cells.
In germinating seeds, OsNAAT1 is expressed in the vascular bundle of the scutellum, the coleoptile, the coleorhiza, and the base of the seminal root (Nozoye et al. 2007). This observation, along with the expression of other genes participating in the biosynthesis of DMA (OsNAS1–3 and OsDMAS1) and Fe transport (OsYSL2 and OsIRT1), suggest that DMA and NA are produced and involved in Fe transport during seed germination (Nozoye et al. 2007).
DMA is involved in long-distance transport of Fe via the phloem
Previously, we proposed that NA is involved in the long-distance transport of Fe in rice (Inoue et al. 2003; Koike et al. 2004). Three OsNAS genes and an Fe(II)–NA complex transporter gene, OsYSL2, are expressed in phloem companion cells in rice. In the present report, we propose that DMA, in addition to NA, is also involved in Fe transport via the phloem. OsNAAT1 is expressed in the phloem companion cells of Fe-deficient leaves (Fig. 4), where OsNAS1–3 and OsDMAS1 are also expressed (Inoue et al. 2003; Bashir et al. 2006). These results support the possibility that DMA is synthesised in the phloem companion cells, and may be involved in Fe transport. This is further supported by the findings that a large amount of DMA is detected in phloem sap from leaves of Fe-sufficient rice plants (Mori et al. 1991) and a large amount of DMA is detected in both Fe-sufficient and Fe-deficient rice leaves (Higuchi et al. 2001). Since a computer simulation predicts that the Fe(III)–DMA complex appears to be slightly less stable at a higher pH, near to that in phloem (pH 7.8–8.0) (von Wirén et al. 1999), unknown factors are likely to participate in Fe–DMA transport via the phloem.
We attempted to develop Fe-deficiency tolerance in rice by introducing genes for MAs biosynthesis to increase the secretion of MAs under Fe-deficient conditions (Takahashi et al. 2001). Other strategies, applying a reconstructed ferric chelate reductase, Refre1-372, or overexpression of a transcription factor, OsIRO2, also lead to enhanced tolerance to Fe deficiency (Ishimaru et al. 2007; Ogo et al. 2007). A future strategy to produce Fe-deficiency tolerant plants could be realised through facilitating Fe translocation to increase Fe availability by a combined manipulation of the genes involved in DMA biosynthesis and the YSL transporter genes.
Abbreviations
- DMA:
-
2′-Deoxymugineic acid
- GUS:
-
β-Glucuronidase
- MAs:
-
Mugineic acid family phytosiderophores
- NA:
-
Nicotianamine
References
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281:32395–32402
Colangelo EP, Guerinot ML (2006) Put the metal to petal: metal uptake and transport throughout plants. Curr Opin Plant Biol 9:322–330
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe 1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349
Herbik A, Koch G, Mock HP, Dushkov D, Czihal A, Thielmann J, Stephan UW, Baumlein H (1999) Isolation, characterization and cDNA cloning of nicotianamine synthase from barley. A key enzyme for iron homeostasis in plants. Eur J Biochem 265:231–239
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza Sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Higuchi K, Kanazawa K, Nishizawa NK, Mori S (1994) Purification and characterization of nicotianamine synthase from Fe deficient barley roots. Plant Soil 165:173–179
Higuchi K, Kanazawa K, Nishizawa NK, Mori S (1996) The role of nicotianamine synthase in response to Fe nutrition status in Gramineae. Plant Soil 178:171–177
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa NK, Mori S (1999) Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol 119:471–479
Higuchi K, Watanabe S, Takahashi M, Kawasaki S, Nakanishi H, Nishizawa NK, Mori S (2001) Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions. Plant J 25:159–167
Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2003) Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long-distance transport of iron and differentially regulated by iron. Plant J 36:366–381
Ishimaru Y, Kim S, Tsukamoto T, Oki H, Kobayashi T, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2007) Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc Natl Acad Sci USA 104:7373–7378
Kanazawa K, Higuchi K, Nishizawa NK, Fushiya S, Chino M, Mori S (1994) Nicotianamine aminotransferase activities are correlated to the phytosiderophore secretions under Fe-deficient conditions in Gramineae. J Exp Bot 45:1903–1906
Kanazawa K, Higuchi K, Nishizawa NK, Fushiya S, Mori S (1995) Detection of two distinct isozymes of nicotianamine aminotransferase in Fe-deficient barley roots. J Exp Bot 46:1241–1244
Kawai S, Itoh K, Takagi S, Nomoto K (1988) Studies on phytosiderophore: biosynthesis of mugineic acid and 2′-deoxymugineic acid in Hordeum vulgare L. var Minorimugi. Tetrahyd Lett 29:1053–1056
Kobayashi T, Nakanishi H, Takahashi M, Kawasaki S, Nishizawa NK, Mori S (2001) In vivo evidence that Ids3 from Hordeum vulgare encodes a dioxygenase that converts 2′-deoxymugineic acid to mugineic acid in transgenic rice. Planta 212:864–871
Kobayashi T, Nakayama Y, Itai RN, Nakanishi H, Yoshihara T, Mori S, Nishizawa NK (2003) Identification of novel cis-acting elements, IDE1 and IDE2, of the barley IDS2 gene promoter conferring iron-deficiency-inducible, root-specific expression in heterogeneous tobacco plants. Plant J 36:780–793
Kobayashi T, Nakayama Y, Takahashi M, Inoue H, Nakanishi H, Yoshihara T, Mori S, Nishizawa NK (2004) Construction of artificial promoters highly responsive to iron deficiency. Soil Sci Plant Nutr 50:1167–1175
Kobayashi T, Suzuki M, Inoue H, Itai RN, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2005) Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot 56:1305–1316
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J 39:415–424
Lytle CM, Jolley VD (1991) Iron deficiency stress response of various C-3 and C-4 grain crop genotypes: strategy II mechanism evaluated. J Plant Nutr 14:341–361
Ma JF, Nomoto K (1993) Two related biosynthetic pathways of mugineic acids in Gramineous plants. Plant Physiol 102:373–378
Ma JF, Shinada T, Matsuda C, Nomoto K (1995) Biosynthesis of phytosiderophores, mugineic acids, associated with methionine cycling. J Biol Chem 270:16549–16554
Ma JF, Taketa S, Chang YC, Iwashita T, Matsumoto H, Takeda K, Nomoto K (1999) Genes controlling hydroxylations of phytosiderophores are located on different chromosomes in barley (Hordeum vulgare L.). Planta 207:590–596
Mizuno D, Higuchi K, Sakamoto T, Nakanishi H, Mori S, Nishizawa NK (2003) Three nicotianamine synthase genes isolated from maize are differentially regulated by iron nutritional status. Plant Physiol 132:1989–1997
Mori S, Nishizawa N (1987) Methionine as a dominant precursor of phytosiderophores in Graminaceae plants. Plant Cell Physiol 28:1081–1092
Mori S, Nishizawa N, Hayashi H, Chino M, Yoshimura E, Ishihara J (1991) Why are young rice plants highly susceptible to iron deficiency? Plant Soil 130:143–156
Murata Y, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T (2006) A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J 46:563–572
Nakanishi H, Okumura N, Umehara Y, Nishizawa NK, Chino M, Mori S (1993) Expression of a gene specific for iron deficiency (Ids3) in the roots of Hordeum vulgare. Plant Cell Physiol 34:401–410
Nakanishi H, Yamaguchi H, Sasakuma T, Nishizawa NK, Mori S (2000) Two dioxygenase genes, Ids3 and Ids2, from Hordeum vulgare are involved in the biosynthesis of mugineic acid family phytosiderophores. Plant Mol Biol 44:199–207
Negishi T, Nakanishi H, Yazaki J, Kishimoto N, Fujii F, Shimbo K, Yamamoto K, Sakata K, Sasaki T, Kikuchi S, Mori S, Nishizawa NK (2002) cDNA microarray analysis of gene expression during Fe-deficiency stress in barley suggests that polar transport of vesicles is implicated in phytosiderophore secretion in Fe-deficient barley roots. Plant J 30:83–94
Nozoye T, Inoue H, Takahashi M, Ishimaru Y, Nakanishi H, Mori S, Nishizawa NK (2007) The expression of iron homeostasis-related genes during rice germination. Plant Mol Biol 64:35–47
Ogo Y, Itai RN, Nakanishi H, Inoue H, Kobayashi T, Suzuki M, Takahashi M, Mori S, Nishizawa NK (2006) Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J Exp Bot 57:2867–2878
Ogo Y, Itai RN, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK (2007) The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J 51:366–377
Ohata T, Kanazawa K, Mihashi S, Nishizawa NK, Fushiya S, Nozoe S, Chino M, Mori S (1993) Biosynthetic pathway of phytosiderophores in iron-deficient Graminaceous plants. Development of an assay system for the detection of nicotianamine aminotransferase activity. Soil Sci Plant Nutr 39:745–749
Römheld V, Marschner H (1986) Evidence for a specific uptake system for iron phytosiderophore in roots of grasses. Plant Physiol 80:175–180
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, von Wirén N (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279:9091–9096
Shojima S, Nishizawa NK, Fushiya S, Nozoe S, Irifune T, Mori S (1990) Biosynthesis of phytosiderophores. In-vitro biosynthesis of 2′-deoxymugineic acid from l-methionine and nicotianamine. Plant Physiol 93:1497–1503
Suzuki M, Takahashi M, Tsukamoto T, Watanabe S, Matsuhashi S, Yazaki J, Kishimoto N, Kikuchi S, Nakanishi H, Mori S, Nishizawa NK (2006) Biosynthesis and secretion of mugineic acid family phytosiderophores in zinc-deficient barley. Plant J 48:85–97
Takagi S (1976) Naturally occuring iron-chelating compounds in oat- and rice-root washing. I. Activity measurement and preliminary characterization. Soil Sci Plant Nutr 22:423–433
Takagi S, Nomoto K, Takemoto S (1984) Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J Plant Nutr 7:469–477
Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S (1999) Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol 121:947–956
Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S (2001) Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol 19:466–469
Takizawa R, Nishizawa NK, Nakanishi H, Mori S (1996) Effect of iron deficiency on S-adenosylmethionine synthetase in barley roots. J Plant Nutr 19:1189–1200
von Wirén N, Mori S, Marschner H, Römheld V (1994) Iron inefficiency in maize mutant ys1 (Zea mays L. cv yellow-stripe) is caused by a defect in uptake of iron phytosiderophores. Plant Physiol 106:71–77
von Wirén N, Klair S, Bansal S, Briat JF, Khodr H, Shioiri T, Leigh RA, Hider RC (1999) Nicotianamine chelates both Fe(III) and Fe(II). Implications for metal transport in plants. Plant Physiol 119:1107–1114
Acknowledgement
We thank Mr. Tatsuya Sakamoto for assistance with cloning of ZmNAAT.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Inoue, H., Takahashi, M., Kobayashi, T. et al. Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol Biol 66, 193–203 (2008). https://doi.org/10.1007/s11103-007-9262-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-007-9262-8