Abstract
Purpose
Several surgical strategies have been proposed to treat MRI-negative Cushing’s Disease. These include tumor removal, if identified, and if a tumor is not identified, resection of varying degrees of the pituitary gland, often guided by inferior petrosal sinus sampling (IPSS). The relative risks and benefits of each strategy have never been compared.
Methods
This systematic review of the literature included only studies on the results of surgery for MRI-negative patients with Cushing’s Disease in which the surgical strategy was clearly described and associated remission and/or hypopituitarism rates detailed for each strategy.
Results
We identified 12 studies that met inclusion criteria for remission rates and 5 studies for hypopituitarism rates. We divided cases into 6 resection strategies. Remission and hypopituitarism rates for each strategy were: (1) tumor identified, resect tumor only (68%, 0%); (2) resect tumor and surrounding capsule (85%, 0%); and if the tumor was not identified (3) resect inferior 1/3 of gland (78%, no data); (4) resect 30–50% of gland based on IPSS (68%, 13%); (5) resect > 50% but < 100% of gland (65%, 9%); (6) resect entire gland (66%, 67%). Strategy 3 only contained 9 patients.
Conclusion
Remission rates for MRI-negative Cushing’s Disease support surgery as a reasonable approach. Results are best if a tumor is found. If a tumor is not identified, one can either remove one-third of the gland guided by IPSS lateralization, or remove both lateral portions along with the inferior portion leaving sufficient central gland to preserve function. Our recommendations are limited by the lack of rigorous and objective data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
ACTH-secreting pituitary microadenoma, also called Cushing’s Disease (CD) is the most common cause of Cushing’s Syndrome, characterized by elevated levels of cortisol in the bloodstream. Untreated CS, especially in severe cases, has a poor prognosis and high mortality, with patient survival rates of only 50% after 5 years [1]. The treatmemt of choice for CD is surgical removal of the pituitary tumor, with cure rates reported in the range of 70–98% [2,3,4,5,6]. The goal of surgery is to remove the ACTH producing cells while leaving enough of the residual gland and stalk behind to presreve normal pituitary function. In cases where the tumor is visible and all hormone studies are concordant regarding the central source for ACTH production, the surgical strategy is more straightforward, generally involving removal of the entire tumor and its pseudocapsule, as well the adjacent dura or medial cavernous sinus wall if the tumor is in direct apposition [7].
Frustratingly, MRI-scans may be fail to disclose the location of the tumor in up to 40% of patients, leaving the surgeon without a specific target [8]. In cases of MRI-negative CD, several different surgical strategies have been proposed for gland exploration and partial gland extirpation with little agreement as to best practices, and no data comparing rates of remission and hypopituitarism with each strategy [4, 9]. If the tumor is not found, resection strategies include: inferior 1/3 gland removal [10], lateral 1/3 gland removal based on IPSS [11], 30–50% removal based on IPSS [12], 2/3 resection [4] and complete hypophysectomy [13].
This paper will compare rates of remission and iatrogenic hypopituitarism between these various resection strategies and try to create a practical treatment algorithm that can be employed in this situation.
Methods
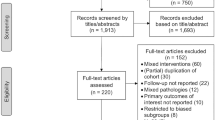
Studies were gathered through the online database of Weill Cornell Medicine’s Samuel J. Wood Library. The search date was any publication date in the past 20 years, and the date last searched was March 1st, 2022. No contact was made with study authors to identify additional studies. The terms “Cushing Disease”, “MRI Negative”, and Transsphenoidal surgery” were used. No other filters were applied. Subsequent studies were also identified through references from the original papers. This manuscript was prepared in accordance with PRISMA guidelines (Fig. 1) [14].
Study selection and data gathering
Patient groups were extracted from each study if the following criteria were met. (1) MRI-negative, (2) clear description of a resection strategy, (3) clear indication of remission rate with definition of remission criteria, (4) clear indication of rates of hypopituitarism. For the sake of this study, cases were divided into anterior dysfunction, posterior dysfunction of complete hypopituitarism when they were available. Data was not obtained or confirmed from the investigators. Average hypopituitarism and remission rates were calculated for each resection strategy for each study.
Six different strategies were identified from the literature (Table 1). If the tumor was found during surgery, the strategies were either to (1) resect the tumor or (2) resect the tumor, along with either a rim of surrounding pituitary tissue or the pseudocapsule. If the tumor was not found during surgery, the strategies were (3) resect the inferior 1/3 of the pituitary gland, (4) resect 30–50% of the pituitary gland based on IPSS data (we grouped lateral 1/3 resection with 50% resection based on the fact that it would be unlikely that the surgeon could accurately differentiate between 30% of 50% gland removal), (5) resect > 50% but not entire gland (this includes a bilateral lateral gland resection plus inferior resection), or (6) resect the entire pituitary gland i.e. complete hypophysectomy (Table 1). We did not include the strategy of blind vertical cuts to induce apoplexy, being unable to find results for this strategy. These strategies are numbered 1–6 for easier reference in the Figures and Tables (Table 2).
Hypopituitarism was defined as a loss of any hormone other than cortisol, requiring replacement and was separated into anterior, posterior or total hypopituitarism. Although some studies did not specify whether hypopituitarism was newly resulting or already existing, since we selected only MRI-negative cases, we presumed that that any post-operative hypopitutiarism was a result of the surgical intervention.
Determining remission was much more challenging since there is no single accepted method to establish remission for CD. Several different criteria have been used including serum cortisol, 24-h urinary free cortisol, overnight dexamethasone suppression, late-night salivary cortisol and low dose dexamethasone suppression [15]. Likewise, determination of recurrence is equally ambiguous and has been defined using a variety of different tests. Based on this lack of consensus, we decided to use the authors’ own definitions and just describe them to let the reader decide if they are sufficiently stringent. With regards to duration of remission, there was too much variability, and the follow-up was too heterogeneous to only include one criteria for long-term remission. As a result, the timeframe of remission was not considered and the average follow-up was presented, if clearly indicated. These limitations will be explored in more detail in the discussion.
Results
Twelve studies were included in this analysis, encompassing 27 study-resection strategy pairs, as some studies included multiple resection strategies. The remission rate and sample size for each study are shown in Fig. 2. Data on hypopituitarism, in which rates of hypopituitarism were clearly delineated based on resection strategy, were only available from only five studies. These five studies, encompassing ten study-resection strategy pairs, along with Cebula, et al., which did not separate out its hypopituitarism data by resection strategy, are shown in Fig. 3 [10]. Most of the studies were small, including roughly 20 patients, while a few were larger [10, 16, 17].
Diagnostic strategy and definition of remission
Each of the 12 studies examined outcomes of transsphenoidal surgery (TSS) for MR-negative Cushing’s Disease in a single medical center. The diagnosis of CD involved a combination of 24-h UFC, low- and high-dose dexamethasone suppression test, ACTH sampling, and/or histology (Table 3). Postoperative remission across all the studies was variable and established by a combination of postoperative serum cortisol, 24-h UFC, and suppression in response to low- and high-dose dexamethasone administration (Table 3).
Rates of remission and hypopituitarism stratified by study
The study with the highest remission rate (100%) was Semple, et al. [18]. Their study included 18 patients and scans were done from 1992 to 1997. However, two patients were lost to follow-up and in five patients, post-op serum cortisol on the day after surgery was in the normal range, which was considered a remission, a classification which would not be universally agreed upon today. If we only include patients with early post-op cortisol < 3, the remission was, at best, 13/18 patients or 72%. Also of note, in 15 patients, a tumor was identified at the time of surgery and removed. Two patients had total hypophysectomies and one had a hemihypophysectomy, the latter who died of SIADH. The study with the next highest remission rate of 92% was Watson et al. that employed a strategy of searching for a tumor based on IPSS. In this study, all 61 studied patients had an adenoma that was indentified during surgery, and all patients had the adenoma removed along with a pseudocapsule [19].
The study with the lowest remission rate (44%) was by Yamada et al. [20]. Their resection strategy was first to explore the gland with three vertical cuts on the side of IPSS laterality. If no tumor was identified a single cut was made on the contralateral side as well as a horizontal cut. If no tumor was found, the medial wall of the cavernous sinsues and diaphragma sella were inspected. If a tumor was found, adenomectomy plus pseudcapsular resection was performed. If no tumor was found, hemihypophysectomy was performed on the side of the IPSS laterality. If there was no laterality, then either anterior complete hypophysectomy or hemihypophysectomy combined with additional contralateral and medial partial resection was performed. Of note, if the tumor was identified during surgery, the remission rate was 100% compared with cases where no tumor was identified, where the remission rate was only 9%.
Resection strategy
It is important to differentiate Strategies 1 and 2, in which a tumor was identified intraoperatively, from Strategies 3–6, in which no tumor was identified. The former would be expected to have higher remisison rates compared with the latter, with the exception of Strategy 6, in which a total hypophysectomy was performed. Across all the studies, Strategy 4 (30–50% resection of one side of the pituitary gland guided by IPSS) was by far the most common resection strategy with 217 patients (Fig. 4). Following that, Strategy 2 was the next most frequent, with 131 patients, followed by Stretegies 1, 6, and 5, with 73, 62, and 57 patients respectively. Strategy 3 (resection of the inferior 1/3 of the gland) was the least common with 9 patients. Four of the studies employed a single resection strategy across all of their patients, while the remaining seven employed multiple strategies, guided by the intraoperative findings and IPSS results.
Remission rate by strategy
Remission rate was reported for a total of 549 patients (Fig. 4). Remission rates for each strategy were (1) resect tumor only (68%); (2) resect tumor and surrounding capsule (85%); and if the tumor was not identified, (3) resect inferior 1/3 of gland (78%); (4) resect 30–50% of gland based on IPSS (68%); (5) resect > 50% but < 100% of gland (65%); (6) resect entire gland (66%). Remission was lowest for Strategy 5, i.e. tumor not identified. resect > 50% but < 100% of the gland, and highest for Strategy 2, i.e. tumor identified, resect tumor plus a rim of surrounding tissue/capsule. Remission rates for the remaining strategies were comparable, including even complete hypophysectomy, which was not associated with increased remission.
Hypopituitarism rate by strategy
Hypopituitarism data was not reported as frequently, with a total of 184 patients in whom either anterior or posterior hypopituitarism were mentioned (Fig. 5). Hypopituitarism rates for each strategy were (1) resect tumor only (0%); (2) resect tumor and surrounding capsule (0%); and if the tumor was not identified, (3) resect inferior 1/3 of gland (no data); (4) resect 30–50% of gland based on IPSS (13%); (5) resect > 50% but < 100% of gland (9%); (6) resect entire gland (67%). The hypopituitarism rate was highest for Strategy 6, i.e. total hypophysectomy, and lowest for Strategies 1 and 2, i.e. resect tumor ± surrounding pseudocapsule. The combiniation of remission rate and hypopituitarism rate by resection strategy are presented together in Fig. 6.
Discussion
This paper provides the first overview of the various resection strategies, and their results, for the situation in which a surgeon must operate on a patient with biochemical Cushing’s Disease and a normal MRI scan. The literature indicates that cure rates can range from 40 to 90%, a range so broad it renders patient counseling and surgical decision-making quite confusing [18, 20]. Our data reveals several important points. (1) If a tumor can be found, surgical results are better than if a tumor cannot be found, and the best strategy is to remove the tumor and its pseudocapsule, which in some series is also described as removal of a small portion of surrounding normal pituitary gland. In this situation, remission rates approach 85% and hypopituitarism may be as low as 0%. (2) If no tumor can be found, although there is a report that removal of the inferior 1/3 of the gland may have the highest remission rate, this group contains only 9 patients and rates of hypopituitarism are not reported, so whether this strategy is optimal is unknown. (3) For this reason, if no tumor is found, the next best results occur when the surgeon removes the lateral 1/3 of the gland based on IPSS. While some surgeons report removing 50% of the gland on the side of IPSS lateralization, such an aggressive surgery risks disconnecting the stalk from the gland and causing hypopituitarism. Moreover, IPSS is fallable, and if only 1/3 is removed on the lateralized side, if the patient is not cured, the surgeon can go back and remove additional tissue on the other side, as well inferiorly, with an acceptable risk of hypopituitarism.
If IPSS is not clearly lateralizing, the best strategy appears to be bilateral removal of the lateral aspect (~ 25%) of the gland, as well as a small portion of the inferior part of the gland, leaving the stalk in communication with central part of the gland. Hypopituitarism rates following this strategy were actually lower than if only the lateral 30–50% were removed based on IPSS, which can be explained by the fact that some of the IPSS-guided studies included a strategy where the lateral 50% of the gland is removed, not just the lateral 1/3, which may have encroached on the stalk’s connection to the central gland.
This summary of our findings supports the following suggested treatment algorithm presented in Fig. 7.
The diagnosis of CD and definition of remission
One of the major weaknesses of this study lies in the ambiguity in assuring the diagnosis in CD and definitively confirming remission after treatment. Several other diseases can cause Cushing’s Syndrome, such as adrenal tumors, ectopic ACTH production, ectopic CRH-production and pseudo-Cushing’s [21]. We cannot rule out the possibility that in some cases included in this series, patient’s may not have had CD, which would artificially lower the success of surgery.
Likewise, the diagnosis of CD, as well as the determination of remission after surgery, were different in each study. The CD diagnosis depends on some combination of 24-h urinary free cortisol, low-dose dexamethasone suppression, CRH–low-dose dexamethasone suppression, salivary cortisol, high-dose dexamethasone suppression, and dynamic testing and inferior petrosal sinus sampling [21, 22]. Remission rates are determined based on early post-operative cortisol reduction below a certain threshold, normalization of urinary free cortisol, serum cortisol and/or dexamethasone suppressibility [4, 23,24,25] or even normalization of clinical features in the absence of any postoperative biochemistry [26]. Finally, remissions are not always durable and recurrence may reach 25% after 5– 10 years Thus, the success of any given strategy may be lower than is reported [27].
Another weakness in this study is that there is no objective measure of how much of the pituitary gland was actually removed by each surgeon, other than their intraoperative estimate. Surgeons are notoriously inaccurate at making these determinations. However, no studies used post-operative MRI scans to examine the volume of normal gland remaining, which may be an area for future study. Likewise, the rate of post-operative hypopituitarism may be related less to the volume of removed gland but rather the precise region removed, since cell-types are not distributed stochastically throughout the gland. Finally, hypopituitarism can also result from vascular injury, separate from direct partial gland removal.
These uncertainties in the definition and durability of CD remission and hypopituitarism makes meaningful comparison of data between centers difficult. For this reason, we chose to present in tabular form, the definitions used in each study, to render the data more transparent.
Variability in MR-negativity and IPSS
Several explanations exist for lack of tumor appearance on MRI. The first is based on the nature of small tumors, in that when they are less than 3 mm, there is not enough outward pressure to create a pseudocapsule, so the island of ACTH-producing cells lacks the density to appear on MRI [28]. These cases may be more difficult to cure with a focal surgery and might require a “regional” resection for success. Lack of appearance on MRI may also depend on the sensitivity of MRI and the specific sequence applied. The introduction of spoiled gradient recall acquisition, spin-echo sequences, 3 T magnets and intraoperative ultrasonography have all been shown to increase the sensitivity of MRI [19, 29,29,31]. These techniques were not used consistently in the studies included in this manuscript, and so some of the patients may have included tumors which might have been considered MRI-positive in a different study. Another possibility is the co-existance of empty sella syndrome, which is more commonly found in MRI-negative cases, with the pressure of the CSF potentially compressing and obscuring a tumor which might otherwise be apparent [32]. Empty sella was not noted in the studies included in this paper, and the impact of empty sella on surgical outcome is unknown.
Likewise, the results of IPSS vary from enter to center depending on technique. Variables include the exact position of the catheters, which can be placed in different location within the inferior petrosal sinues as well as the cavernous sinus [33]. The sensitivity is also highly dependent on the unique the anatomy of the patient, some having asymmetric drainage of the sellar region. Moreover, the ratio of central to peripheral cortisol felt to be diagnostic, and the use of concomittent prolactin sampling to maximize specificity also varies between centers, making the absolute sensitivity and specificity of the test unknown [34].
Additional strategies
This paper has focused specifically on surgical strategies that can employed by the neurosurgeon in the operating room. However, other treatment options exist besides medical therapy should surgery fail to induce remission. These include complete hypophysectomy, bilateral adrenalectomy and stereotactic radiosurgery (SRS).
Complete hypophysectomy is typically performed when no adenoma is visualized during surgery or previous partial hypophysectomy fails to result in remission [35]. Previous case series have found that complete hypophysectomy does not result in increased rates of remission compared to the less aggressive surgical options of selective adenomectomy and partial hypophysectomy[13, 36, 37]. Unique to complete hypophysectomy, however, are elevated rates of hypopituitarism, which have been reported in 79–85% of patients [37, 38].
Bilateral adrenalectomy is indicated in patients for whom other treatments have been unsuccessful or immediate reduction of cortisol levels is needed [39]. The procedure can now be done with a minimally invasive laporoscopic approach, decreasing the risk of complications and shortening the hospital stay [39]. The procedure has proven to be effective in reducing symptoms such as proximal myopathy, hirsutism, glucose tolerance, and weight loss [40]. Downsides of bilateral adrenalectomy include the lifelong need for mineralocorticoid and glucocorticoid replacement and the risk of Nelson Syndrome, a sequela of Cushing’s disease in which unresected pituitary tumor continues to grow and secrete ACTH [39]. Incidence of Nelson Syndrome following bilateral adrenalectomy has been reported to be between 8 and 38% [41], with higher rates for younger patients [42].
SRS is the primary method of radiation for pituitary tumors and is typically done in conjunction with medical therapy due to the delay between radiation initiation and reduction of hypercortisolemia [39]. Compared to other forms of radiotherapy, SRS leads to faster resolution of elevated pituitary hormone levels while having a lower risk of hypopituitarism and radiation-induced neoplasms [43]. Despite this however, remission rates following SRS vary widely, from 17 to 83% in case series with greater than 10 patients and a median follow-up of 2 years [43]. Jagannathan, et al. reported a 22% hypopituitarism rate in a series of 90 patients, with hypothyroidism and growth hormone deficiency being the two most common deficits [21].
Conclusions
Remission rates are fairly high for MRI-negative Cushing’s Disease, supporting surgery as a reasonable approach for this challenging situation. The best results are achieved if a tumor is found following gland exploration, in which case both the tumor and the surrouding pseudocapsulre should be removed. If a tumor is not identified, one can either remove one-third of the gland on the side of the IPSS lateralization, or remove both lateral portions, as well as the inferior portion, leaving enough of the central wedge (roughly 50%) in communication with the stalk to prevent hypopituitarism, since the results for these two approaches appear comparable. These recommendations must be viewed with circumspect as the existing data in the literature are rife with inconsistencies.
References
Plotz CM, Knowlton AI, Ragan C (1952) The natural history of Cushing’s syndrome. Am J Med 13(5):597–614. https://doi.org/10.1016/0002-9343(52)90027-2
Aranda G et al (2015) Long-term remission and recurrence rate in a cohort of Cushing’s disease: the need for long-term follow-up. Pituitary 18(1):142–149. https://doi.org/10.1007/s11102-014-0567-8
Barbetta L, Dall’Asta C, Tomei G, Locatelli M, Giovanelli M, Ambrosi B (2001) Assessment of cure and recurrence after pituitary surgery for Cushing’s disease. Acta Neurochir 143(5):477–481; discussion 481–482. https://doi.org/10.1007/s007010170077.
Carr SB, Kleinschmidt-DeMasters BK, Kerr JM, Kiseljak-Vassiliades K, Wierman ME, Lillehei KO (2018) Negative surgical exploration in patients with Cushing’s disease: benefit of two-thirds gland resection on remission rate and a review of the literature. J Neurosurg 129(5):1260–1267. https://doi.org/10.3171/2017.5.JNS162901
Chee GH, Mathias DB, James RA, Kendall-Taylor P (2001) Transsphenoidal pituitary surgery in Cushing’s disease: can we predict outcome? Clin Endocrinol 54(5):617–626. https://doi.org/10.1046/j.1365-2265.2001.01261.x
Lonser RR, Wind JJ, Nieman LK, Weil RJ, DeVroom HL, Oldfield EH (2013) Outcome of surgical treatment of 200 CHILDREN with Cushing’s disease. J Clin Endocrinol Metab 98(3):892–901. https://doi.org/10.1210/jc.2012-3604
Oldfield EH, Vortmeyer AO (2006) Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg 104(1):7–19. https://doi.org/10.3171/jns.2006.104.1.7
Salenave S et al (2004) Pituitary magnetic resonance imaging findings do not influence surgical outcome in adrenocorticotropin-secreting microadenomas. J Clin Endocrinol Metab 89(7):3371–3376. https://doi.org/10.1210/jc.2003-031908
Tritos NA, Biller BMK, Swearingen B (2011) Management of Cushing disease. Nat Rev Endocrinol 7(5):279–289. https://doi.org/10.1038/nrendo.2011.12
Cebula H et al (2017) Efficacy of endoscopic endonasal transsphenoidal surgery for Cushing’s disease in 230 patients with positive and negative MRI. Acta Neurochir 159(7):1227–1236. https://doi.org/10.1007/s00701-017-3140-1
Andereggen L et al (2021) Lateral one-third gland resection in Cushing patients with failed adenoma identification leads to low remission rates: long-term observations from a small, single-center cohort. Acta Neurochir 163(11):3161–3169. https://doi.org/10.1007/s00701-021-04830-2
Sun Y et al (2012) Diagnosis and therapy for Cushing’s disease with negative dynamic MRI finding: a single-centre experience. Clin Endocrinol 76(6):868–876. https://doi.org/10.1111/j.1365-2265.2011.04279.x
Hammer GD et al (2004) Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J Clin Endocrinol Metab 89(12):6348–6357. https://doi.org/10.1210/jc.2003-032180
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Hinojosa-Amaya JM, Varlamov EV, McCartney S, Fleseriu M (2019) Hypercortisolemia recurrence in Cushing’s Disease; a diagnostic challenge. Front Endocrinol 10:740. https://doi.org/10.3389/fendo.2019.00740
Dai C et al (2020) Outcomes of transsphenoidal surgery in cushing disease patients with negative pituitary magnetic resonance imaging findings: a single-center experience. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 26(11):1320–1330. https://doi.org/10.4158/EP-2020-0177
Pouratian N, Prevedello DM, Jagannathan J, Lopes MB, Vance ML, Laws ER (2007) Outcomes and management of patients with Cushing’s disease without pathological confirmation of tumor resection after transsphenoidal surgery. J Clin Endocrinol Metab 92(9):3383–3388. https://doi.org/10.1210/jc.2007-0208
Semple PL, Vance ML, Findling J, Laws ER (2000) Transsphenoidal surgery for Cushing’s disease: outcome in patients with a normal magnetic resonance imaging scan. Neurosurgery 46(3): 553–558; discussion 558–559, https://doi.org/10.1097/00006123-200003000-00005
Watson JC, Shawker TH, Nieman LK, DeVroom HL, Doppman JL, Oldfield EH (1998) Localization of pituitary adenomas by using intraoperative ultrasound in patients with Cushing’s disease and no demonstrable pituitary tumor on magnetic resonance imaging. J Neurosurg 89(6):927–932. https://doi.org/10.3171/jns.1998.89.6.0927
Yamada S et al (2012) Surgical management and outcomes in patients with Cushing disease with negative pituitary magnetic resonance imaging. World Neurosurg 77(3–4):525–532. https://doi.org/10.1016/j.wneu.2011.06.033
Jagannathan J, Sheehan JP, Jane JA (2007) Evaluation and management of Cushing syndrome in cases of negative sellar magnetic resonance imaging. Neurosurg Focus 23(3):E3. https://doi.org/10.3171/foc.2007.23.3.4
Hinojosa-Amaya JM, Cuevas-Ramos D (2021) The definition of remission and recurrence of Cushing’s disease. Best Pract Res Clin Endocrinol Metab 35(1):101485. https://doi.org/10.1016/j.beem.2021.101485
Invitti C, Pecori Giraldi F, de Martin M, Cavagnini F (1999) Diagnosis and management of Cushing’s syndrome: results of an Italian multicentre study Study Group of the Italian Society of Endocrinology on the Pathophysiology of the Hypothalamic-Pituitary-Adrenal Axis. J Clin Endocrinol Metab 84(2):440–448. https://doi.org/10.1210/jcem.84.2.5465
Mampalam TJ, Tyrrell JB, Wilson CB (1988) Transsphenoidal microsurgery for Cushing disease. A report of 216 cases. Ann Intern Med 109(6):487–493. https://doi.org/10.7326/0003-4819-109-6-487
Sonino N, Zielezny M, Fava GA, Fallo F, Boscaro M (1996) Risk factors and long-term outcome in pituitary-dependent Cushing’s disease. J Clin Endocrinol Metab 81(7):2647–2652. https://doi.org/10.1210/jcem.81.7.8675592
Bigos ST, Somma M, Rasio E, Eastman RC, Lanthier A, Johnston HH, Hardy J (1980) Cushing's disease: management by transsphenoidal pituitary microsurgery. J Clin Endocrinol Metab 50(2):348–354. https://doi.org/10.1210/jcem-50-2-348
Patil CG et al (2008) Late recurrences of Cushing’s disease after initial successful transsphenoidal surgery. J Clin Endocrinol Metab 93(2):358–362. https://doi.org/10.1210/jc.2007-2013
Grober Y, Grober H, Wintermark M, Jane JA, Oldfield EH (2018) Comparison of MRI techniques for detecting microadenomas in Cushing’s disease. J Neurosurg 128(4):1051–1057. https://doi.org/10.3171/2017.3.JNS163122
Batista D et al (2005) Detection of adrenocorticotropin-secreting pituitary adenomas by magnetic resonance imaging in children and adolescents with cushing disease. J Clin Endocrinol Metab 90(9):5134–5140. https://doi.org/10.1210/jc.2004-1778
Erickson D et al (2010) 3 Tesla magnetic resonance imaging with and without corticotropin releasing hormone stimulation for the detection of microadenomas in Cushing’s syndrome. Clin Endocrinol 72(6):793–799. https://doi.org/10.1111/j.1365-2265.2009.03723.x
Patronas N et al (2003) Spoiled gradient recalled acquisition in the steady state technique is superior to conventional postcontrast spin echo technique for magnetic resonance imaging detection of adrenocorticotropin-secreting pituitary tumors. J Clin Endocrinol Metab 88(4):1565–1569. https://doi.org/10.1210/jc.2002-021438
Himes BT, Bhargav AG, Brown DA, Kaufmann TJ, Bancos I, Gompel JJV (2020) Does pituitary compression/empty sella syndrome contribute to MRI-negative Cushing’s disease? A single-institution experience. Neurosurg Focus 48(6):E3. https://doi.org/10.3171/2020.3.FOCUS2084
Lefournier V et al (2003) Accuracy of bilateral inferior petrosal or cavernous sinuses sampling in predicting the lateralization of Cushing’s disease pituitary microadenoma: influence of catheter position and anatomy of venous drainage. J Clin Endocrinol Metab 88(1):196–203. https://doi.org/10.1210/jc.2002-020374
Perlman JE et al (2021) Pitfalls in performing and interpreting inferior petrosal sinus sampling: personal experience and literature review. J Clin Endocrinol Metab 106(5):e1953–e1967. https://doi.org/10.1210/clinem/dgab012
Kelly DF (2007) Transsphenoidal surgery for Cushing’s disease: a review of success rates, remission predictors, management of failed surgery, and Nelson’s Syndrome. Neurosurg Focus 23(3):1–6. https://doi.org/10.3171/foc.2007.23.3.7
Bochicchio D, Losa M, Buchfelder M (1995) Factors influencing the immediate and late outcome of Cushing’s disease treated by transsphenoidal surgery: a retrospective study by the European Cushing’s Disease Survey Group. J Clin Endocrinol Metab 80(11):3114–3120. https://doi.org/10.1210/jcem.80.11.7593411
Ciric I, et al (2012) Transsphenoidal surgery for Cushing disease: experience with 136 patients. Neurosurgery. 70(1):70–80; discussion 80–81. https://doi.org/10.1227/NEU.0b013e31822dda2c.
Brand IR, Dalton GA, Fletcher RF (1985) Long-term follow up of trans-sphenoidal hypophysectomy for Cushing’s disease. J R Soc Med 78(4):291–293. https://doi.org/10.1177/014107688507800404
Liu J, Fleseriu M, Delashaw J, Ciric I, Couldwell W (2007) Treatment options for cushing disease after unsuccessful surgery. Medscape Neurosurg. Focus. [Online]. http://www.medscape.com/viewarticle/566309. Accessed 27 March 2022
Vella A, Thompson GB, Grant CS, van Heerden JA, Farley DR, Young WF (2001) Laparoscopic adrenalectomy for adrenocorticotropin-dependent Cushing’s syndrome. J Clin Endocrinol Metab 86(4):1596–1599. https://doi.org/10.1210/jcem.86.4.7399
Assié G et al (2007) Corticotroph tumor progression after adrenalectomy in Cushing’s disease: a reappraisal of Nelson’s Syndrome. J Clin Endocrinol Metab 92(1):172–179. https://doi.org/10.1210/jc.2006-1328
Kemink L, Pieters G, Hermus A, Smals A, Kloppenborg P (1994) Patient’s age is a simple predictive factor for the development of Nelson’s syndrome after total adrenalectomy for Cushing’s disease. J Clin Endocrinol Metab 79(3):887–889. https://doi.org/10.1210/jcem.79.3.8077377
Sheehan JP et al (2005) Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg 102(4):678–691. https://doi.org/10.3171/jns.2005.102.4.0678
Zhang K et al (2020) Surgical outcomes and multidisciplinary management strategy of Cushing’s disease: a single-center experience in China. Neurosurg Focus 48(6):E7. https://doi.org/10.3171/2020.3.FOCUS2067
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AY and TS wrote the main manuscript text, AY conducted the literature search and data analysis, and TS and FH provided guidance in the study design and data analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All patient data in this retrospective review was used in compliance with national ethical guidelines of the United States.
Consent to participate
No human subjects were involved in the conducting of this literature review.
Consent to publish
No human subjects were involved in the conducting of this literature review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, A.B., Henderson, F. & Schwartz, T.H. Surgical strategies in the treatment of MR-negative Cushing’s Disease: a systematic review and treatment algorithm. Pituitary 25, 551–562 (2022). https://doi.org/10.1007/s11102-022-01239-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-022-01239-7