Abstract
Wolfram syndrome (WS) is mainly caused by mutations in the WFS1 gene and characterized by diabetes mellitus, optic atrophy, hearing loss, and central diabetes insipidus (CDI). WFS1 is an endoplasmic reticulum (ER)-resident transmembrane protein, and Wfs1 knockout (Wfs1−/−) mice, which have been used as a mouse model for WS, reportedly manifested impairment of glucose tolerance due to pancreatic β-cell loss. In the present study, we examined water balance, arginine vasopressin (AVP) secretion, and ER stress in AVP neurons of the hypothalamus in Wfs1−/− mice. There were no differences in urine volumes between Wfs1−/− and wild-type mice with free access to water. Conversely, when mice were subjected to intermittent water deprivation (WD) for 20 weeks, during which water was unavailable for 2 days a week, urine volumes were larger in Wfs1−/− mice, accompanied by lower urine AVP concentrations and urine osmolality, compared to wild-type mice. The mRNA expression of immunoglobulin heavy chain binding protein, a marker of ER stress, was significantly increased in the supraoptic nucleus and paraventricular nuclei in Wfs1−/− mice compared to wild-type mice after WD. Our results thus showed that Wfs1 knockout leads to a decrease in AVP secretion during dehydration, which could explain in part the mechanisms by which Wfs1 mutations cause CDI in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The endoplasmic reticulum (ER) is an essential cellular organelle and is responsible for the posttranslational modification and proper folding of membrane and secretory proteins. ER stress occurs when the demand for protein synthesis exceeds the protein folding capacity. Excessive ER stress results in cellular dysfunction or cell death, and it has been implicated in the pathogenesis of various endocrine or neurodegenerative disorders [1, 2]. The unfolded protein response (UPR) is an adaptive cellular response that ameliorates ER function and prevents ER stress-induced cell death. This is mediated by 3 transmembrane proteins: inositol requiring 1 (IRE1), double-stranded-RNA-dependent protein kinase-like ER kinase (PERK), and activating transcription factor 6 (ATF6). The immunoglobulin heavy chain binding protein (BiP) works as a chaperone and suppresses these signaling pathways under basal conditions; when ER stress occurs, these 3 signaling pathways are activated by dissociation of BiP. Among these signaling pathways, ATF6α is known to upregulate BiP mRNA expression, leading to enhanced protein folding capacity [3,4,5].
Arginine vasopressin (AVP) is an antidiuretic hormone that is mainly synthesized in the supraoptic nucleus (SON) and paraventricular nuclei in the hypothalamus (PVN) [6]. We previously showed that BiP mRNA was highly expressed in AVP neurons even under basal conditions [7], suggesting that AVP neurons are exposed to ER stress to meet the demand for AVP synthesis and secretion for the maintenance of water balance. Central diabetes insipidus (CDI) is a disorder resulting from a deficiency of AVP and characterized by polyuria and polydipsia. While most causes of CDI are acquired, there is a subclass called familial neurohypophysial diabetes insipidus (FNDI) in which polyuria manifests several months or years after birth [8, 9]. Previous studies including ours showed that excessive ER stress is a pathogenetic cause of the impairment of AVP secretion and cell death of AVP neurons in rodent models of FNDI [9,10,11,12,13].
Wolfram syndrome (WS) is a rare autosomal recessive disorder characterized by early-onset diabetes mellitus, progressive optic atrophy, deafness, and CDI [14, 15]. WS is mostly caused by mutations of Wfs1 gene, encoding the ER-resident transmembrane protein wolframin (WFS1) [14, 16], which has been shown to play a key role in maintaining ER homeostasis [17,18,19]. Diabetes mellitus and optic atrophy are common clinical manifestations in WS, and they usually occur in the first decade [20]. On the other hand, CDI in WS usually occurs around the second or third decade, and its incidence is reported to range from 30 to 87% [21,22,23]. Previous studies reported that Wfs1 knockout animals recaptured the phenotype of diabetes mellitus in patients with WS, and that ER stress induced by Wfs1 knockout caused the dysfunction of β-cells [24,25,26].
In the present study, we examined water balance, AVP secretion, and ER stress in AVP neurons of the hypothalamus in Wfs1−/− mice in order to clarify the underlying mechanisms by which CDI manifests in WS.
Materials and methods
Animals
Wfs1−/− mice were generated previously [24]. The strain was produced by intercrossing male and female heterozygotes (Wfs1+/−), and their wild-type (Wfs1+/+) littermates were used as control. Animal care and use were performed in accordance with the institutional guidelines. Animal experiments were performed according to procedures approved by the Animal Experimentation Committee of the Nagoya University Graduate School of Medicine. Mice were maintained in a room with controlled temperature on a 12-h/12-h light–dark cycle (23.0 ± 0.5 °C, lights on 09:00 to 21:00).
Measurement of urine volume, urine AVP concentration, and urine osmolality
Two-month-old male mice were divided into 2 groups; one group had free access to water and the other was subjected to intermittent water deprivation (WD), which consisted of repeated cycles of continuous WD for 48 h followed by 5 days with free access to water, as reported previously [10]. Mice were housed in metabolic cages, and the 24-h pooled urine was collected for 20 weeks. In the intermittent WD group, urine volume was measured on the first day during WD, or on days 4 and 5 after WD (Supplemental Fig. 1). Urine AVP concentrations were measured by radioimmunoassay (AVP kit Yamasa; Yamasa Corporation, Chiba, Japan). Urine osmolality was determined by the cryoscopic method (Oriental Yeast Co., Ltd., Tokyo, Japan).
Measurement of food intake, body weight, and blood glucose concentration
Food intake and body weight were measured every week for 28 weeks. 5 days after the 20th intermittent WD, blood samples were taken by tail bleeding at the beginning of the light cycle between 09:00 and 10:00 in 28-week-old mice, and blood glucose concentrations were measured using a glucometer (Medisafe Mini; Terumo, Tokyo, Japan).
Immunohistochemistry
Immunohistochemistry was performed as described previously [10, 27,28,29]. Briefly, mice were transcardially perfused, and the brains were fixed with 4% paraformaldehyde and cut into 50-μm coronal sections using a vibratome (VT1200 S; Leica Biosystems, Wetzlar, Germany). After the sections were washed and blocked, they were incubated with primary antibodies overnight at 4 °C. The slices were rinsed and then incubated with secondary antibodies for 2 h at RT. Images were captured by a fluorescence microscope (BZ-9000; Keyence, Osaka, Japan) and processed using Adobe Photoshop CS5 software (Adobe Systems, San Jose, CA, USA). Neurons were counted from the matched slices at 0.7 and 0.82 mm caudal from bregma for the SON and PVN, respectively [29].
Antibodies
The primary antibodies used in this study were as follows: rabbit anti-WFS1 (1:200) [24, 30], guinea pig anti-AVP (1:2000; T-5048; Peninsula, San Diego, CA, USA), and mouse anti-neurophysin I [OT-NP; 1:100; PS38; kindly provided by Dr. Harold Gainer, National Institutes of Health (NIH), Bethesda, MD, USA] [31, 32]. The secondary antibodies used in this study were as follows: Alexa Fluor 488-conjugated goat anti-mouse IgG (H + L) highly cross-adsorbed (1:1000; A11029; Invitrogen, San Diego, CA, USA), Cy3-conjugated affinipure donkey anti-guinea pig IgG (H + L) (1:500; 706-165-148; Jackson ImmunoResearch, West Grove, PA, USA), and Alexa Fluor 647-conjugated donkey anti-rabbit IgG (H + L) highly cross-adsorbed (1:1000; A31573; Invitrogen).
In situ hybridization
In situ hybridization were performed as reported previously [7, 10, 27,28,29]. Briefly, brains were cut into 16-μm coronal sections on a cryostat at − 20 °C, thaw-mounted on Superfrost Plus microscope slides (Matsunami Glass Ind., Osaka, Japan), and stored at − 80 °C. The matched slices at 0.70 mm (SON) and 0.82 mm (PVN) caudal from bregma from each mouse were examined. The probes for Avp and Bip mRNA were radiolabeled with [35S] UTP and [35S] CTP (PerkinElmer Life Sciences, Waltham, MA, USA). After the procedures of prehybridization, hybridization, and posthybridization, the sections were exposed to BioMax MR film (Carestream Health, Rochester, NY, USA) for 7 h (Avp mRNA) or 48 h (Bip mRNA). The expression levels of mRNA were quantified by measuring of the integrated optimal densities of the images, and expressed in arbitrary units (AU) using ImageJ software (NIH).
Statistical analysis
Statistical analysis was performed by unpaired t test, one-way ANOVA, or two-way ANOVA, with repeated measures followed by the Bonferroni test as appropriate. Data are shown as mean ± SE. Values of P < 0.05 were considered statistically significant.
Results
WFS1 is expressed in AVP neurons in the hypothalamus
Immunohistochemistry using specific anti-WFS1 antibody revealed that WFS1 proteins were expressed in the SON and PVN in wild-type (WT) mice, and colocalized with AVP and oxytocin (Fig. 1). The quantitative analyses revealed that 95.9% (658/686) AVP neurons and 89.1% (303/340) oxytocin neurons were WFS1-positive. Conversely, WFS1 proteins were not expressed in AVP neurons in the SON or PVN in Wfs1−/− mice (Supplemental Fig. 2).
WFS1 is expressed in AVP neurons in the hypothalamus. Immunofluorescence staining for WFS1 (green), AVP (red), and oxytocin (yellow) in the SON (a–e and k–p) and PVN (f–j) of 2-month-old WT mice. Lower magnification images staining for WFS1 (a and f), AVP (b and g), and oxytocin (c and h). d and i are merged images of staining for WFS1 and AVP. e and j are merged images of staining for WFS1 and oxytocin. Higher magnification images staining for WFS1 (k and n), AVP (l), and oxytocin (o). m and p are merged images of staining for WFS1 and AVP, WFS1 and oxytocin, respectively. Scale bars, 100 μm (a–j), 10 μm (k–p)
Increased urine volume and decreased AVP secretion in Wfs1−/− mice during WD
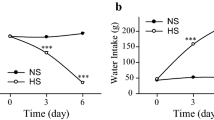
There were no significant differences in urine volume or water intake for 20 weeks between Wfs1−/− and WT mice with access to water ad libitum (Fig. 2a, b). There were also no significant differences in urine volume or water intake between genotypes for 20 weeks when measured on days 4 and 5 after 2 day-WD every week (Fig. 2c, d). However, urine volume was significantly greater in Wfs1−/− than in WT mice when measured during 20th WD, but not during 1st or 12th WD (Fig. 2e). The urine AVP concentration as well as urine osmolality was significantly lower in Wfs1−/− than in WT mice during 20th WD (Fig. 2f, g).
Increased urine volume and decreased AVP secretion in Wfs1−/− mice during WD. Urine volume (a) and water intake (b) in WT (○) and Wfs1−/− mice (●) with water access ad libitum. Mean urine volume (c) and water intake (d) measured 4 and 5 days after each WD in WT (○) and Wfs1−/− mice (●) subjected to intermittent WD. Urine volume (e), urine AVP concentration (f), and urine osmolality (g) in WT (white bars) and Wfs1−/− mice (black bars) during the 1st, 12th, and 20th WD. Results are expressed as mean ± S.E. n = 4 animals per group with water access ad libitum, 7–8 animals per group subjected to intermittent WD. *P < 0.05, compared to WT mice during WD. % BW percentage body weight
Knockout of Wfs1 increases the expression of BiP in response to WD
AVP mRNA expression levels in the SON and PVN were not significantly different under basal conditions between Wfs1−/− and WT mice (data not shown); they also did not significantly differ between genotypes after 1st (data not shown) or 20th WD (Fig. 3a–c). However, while there was no significant difference in BiP mRNA expression levels in the SON and PVN under basal conditions between genotypes (data not shown), the levels were significantly increased in Wfs1−/− than in WT mice after 1st (data not shown) and 20th WD (Fig. 3D-F).
Knockout of Wfs1 increases the expression of BiP in response to WD. Expression of AVP (a, b) and BiP (d, e) mRNA in the SON and PVN in WT (white bars) and Wfs1−/− mice (black bars) after intermittent WD for 20 weeks. Representative images of in situ hybridization for AVP (c) and BiP (f) mRNA. Mean expression levels of AVP and BiP mRNA in WT mice are expressed as 100. Results are expressed as mean ± SE. n = 6–8 animals per group. *P < 0.05, compared to WT mice
Food intake, body weight, and blood glucose concentration
There were no significant differences in food intake or body weight for 20 weeks between Wfs1−/− and WT mice with access to water ad libitum or subjected to intermittent WD (data not shown). There were no significant differences in blood glucose concentrations (Wfs1−/− 147.00 ± 7.86 mg/dL vs WT 157.63 ± 6.20 mg/dL) between genotypes in 28-week-old mice subjected to intermittent WD. These data suggest knockout of Wfs1 did not affect energy homeostasis or glucose metabolism in the current study.
Discussion
In the present study, we demonstrated that WFS1 is expressed in AVP neurons in the SON and PVN of WT mice, and that the urine volume was larger after repeated WD in Wfs1−/− mice, which was accompanied by a lower urine AVP concentration and lower urine osmolality, compared to WT mice. To the best of our knowledge, this is the first study to show the impairment of AVP secretion in WS model animals.
Previous studies showed that both Wfs1 mRNA and proteins were expressed in the SON and PVN [33,34,35]. In the current study, we not only confirmed but also extended these findings by showing that WFS1 is localized in both AVP and oxytocin neurons in the SON and PVN.
A previous report indicated that plasma insulin levels did not decrease until 36 weeks after birth in Wfs1−/− mice [24]. While hyperglycemia due to insulin deficiency could cause osmotic diuresis, it is not the case in the current study, as we observed urine volume and water intake in Wfs1−/− mice from 8 to 28 weeks after birth.
FNDI model mice manifest increases in urine volume even with ad libitum access to water [9, 10]. Compared to FNDI model mice, the phenotype of Wfs1−/− mice with respect to water balance was mild. This is consistent with the clinical manifestation: patients with FNDI develop progressive polyuria several months or years after birth [36], while those with WS develop CDI, which is sometimes partial, in the second or third decade of life [21].
WFS1 plays important roles in regulating ER function, cytoplasmic calcium homeostasis [19], mitochondrial function [19], and UPR activation [18]. It has been reported that Wfs1 knockout animals show increased BiP expression in the pancreas, retina, and brainstem [24, 25, 37, 38]. In this study, we showed that BiP mRNA expression was increased in AVP neurons of Wfs1−/− mice compared to WT mice after WD. Elevated BiP expression might reflect ER stress in AVP neurons, as BiP has been used as a marker of ER stress [39, 40]. Alternatively, BiP upregulation might result from activated ATF6α signaling due to knockout of WFS1, as a previous study using pancreatic β-cell lines showed that WFS1 downregulates ATF6α by the ubiquitin–proteasome pathway, and that knockdown of WFS1 induced an increase in the expression of ATF6α and BiP [18]. We previously reported that upregulation of BiP in the AVP neurons under dehydration was abolished in ATF6α knockout mice [28]. In any case, a relatively mild phenotype of Wfs1−/− mice in water balance might be due to the increased expression of BiP, given that BiP plays a protective role in AVP neurons [27]. It is also of note that, while urine AVP concentrations were lower after WD in Wfs1−/− mice than in WT mice, there were no differences in AVP mRNA in the SON and PVN between genotypes. These data suggest the possibility that knockout of Wfs1 affected AVP neurons at the posttranslational level.
There are a few limitations in this study. First, we chose male mice for analysis based on a previous study showing that the phenotype of diabetes mellitus in Wfs1 knockout mice was more evident in male than in female mice [24]. However, it is possible that the phenotypes could be prominent in females, as in the case of FNDI mice [9]. Second, although it is likely that knockout of Wfs1 induced ER stress and affected intracellular trafficking at the level of the ER, we have not examined the morphology of the ER in AVP neurons by electron microscopy.
In conclusion, we showed that Wfs1 knockout mice manifested decreases in AVP secretion during dehydration, which was accompanied by increased BiP expression in AVP neurons.
References
Sano R, Reed JC (2013) ER stress-induced cell death mechanisms. Biochim Biophys Acta Mol Cell Res 1833:3460–3470. https://doi.org/10.1016/j.bbamcr.2013.06.028
Zhang IX, Raghavan M, Satin LS (2020) The endoplasmic reticulum and calcium homeostasis in pancreatic beta cells. Endocrinol (United States) 161:1–14. https://doi.org/10.1210/endocr/bqz028
Haze K, Yoshida H, Yanagi H et al (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10:3787–3799. https://doi.org/10.1091/mbc.10.11.3787
Yoshida H, Okada T, Haze K et al (2000) ATF6 activated by proteolysis binds in the presence of NF-Y (CBF) directly to the cis-acting element responsible for the mammalian unfolded protein response. Mol Cell Biol 20:6755–6767. https://doi.org/10.1128/mcb.20.18.6755-6767.2000
Wang M, Wey S, Zhang Y et al (2009) Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxidants Redox Signal 11:2307–2316. https://doi.org/10.1089/ars.2009.2485
Bisset GW, Chowdrey HS (1988) Control of release of vasopressin by neuroendocrine reflexes. Q J Exp Physiol 73:811–872. https://doi.org/10.1113/expphysiol.1988.sp003223
Hagiwara D, Arima H, Morishita Y et al (2012) BiP mRNA expression is upregulated by dehydration in vasopressin neurons in the hypothalamus in mice. Peptides 33:346–350. https://doi.org/10.1016/j.peptides.2011.12.011
Arima H, Azuma Y, Morishita Y, Hagiwara D (2016) Central diabetes insipidus. Nagoya J Med Sci 78:349–357. https://doi.org/10.18999/nagjms.78.4.349
Hayashi M, Arima H, Ozaki N et al (2009) Progressive polyuria without vasopressin neuron loss in a mouse model for familial neurohypophysial diabetes insipidus. Am J Physiol Regul Integr Comp Physiol 296:1641–1649. https://doi.org/10.1152/ajpregu.00034.2009
Hagiwara D, Arima H, Morishita Y et al (2014) Arginine vasopressin neuronal loss results from autophagy-associated cell death in a mouse model for familial neurohypophysial diabetes insipidus. Cell Death Dis. https://doi.org/10.1038/cddis.2014.124
Hagiwara D, Grinevich V, Arima H (2019) A novel mechanism of autophagy-associated cell death of vasopressin neurons in familial neurohypophysial diabetes insipidus. Cell Tissue Res 375:259–266. https://doi.org/10.1007/s00441-018-2872-4
Davies J, Murphy D (2002) Autophagy in hypothalamic neurones of rats expressing a familial neurohypophysical diabetes insipidus transgene. J Neuroendocrinol 14:629–637. https://doi.org/10.1046/j.1365-2826.2002.00822.x
Russell TA, Ito M, Ito M et al (2003) A murine model of autosomal dominant neurohypophyseal diabetes insipidus reveals progressive loss of vasopressin-producing neurons. J Clin Invest 112:1697–1706. https://doi.org/10.1172/JCI200318616
Inoue H, Tanizawa Y, Wasson J et al (1998) A gene encoding a transmembrane protein is mutated in patients with diabetes mellitus and optic atrophy (Wolfram syndrome). Nat Genet 20:143–148. https://doi.org/10.1038/2441
Strom TM, Hörtnagel K, Hofmann S et al (1998) Diabetes insipidus, diabetes mellitus, optic atrophy and deafness (DIDMOAD) caused by mutations in a novel gene (wolframin) coding for a predicted transmembrane protein. Hum Mol Genet 7:2021–2028. https://doi.org/10.1093/hmg/7.13.2021
Takeda K, Inoue H, Tanizawa Y et al (2001) WFS1 (Wolfram syndrome 1) gene product: predominant subcellular localization to endoplasmic reticulum in cultured cells and neuronal expression in rat brain. Hum Mol Genet 10:477–484. https://doi.org/10.1093/hmg/10.5.477
Ueda K, Kawano J, Takeda K et al (2005) Endoplasmic reticulum stress induces Wfs1 gene expression in pancreatic β-cells via transcriptional activation. Eur J Endocrinol 153:167–176. https://doi.org/10.1530/eje.1.01945
Fonseca SG, Ishigaki S, Oslowski CM et al (2010) Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. J Clin Invest 120:744–755. https://doi.org/10.1172/JCI39678
Angebault C, Fauconnier J, Patergnani S et al (2018) ER-mitochondria cross-talk is regulated by the Ca2+ sensor NCS1 and is impaired in Wolfram syndrome. Sci Signal 11:eaaq1380. https://doi.org/10.1126/scisignal.aaq1380
Bundey SEMAFBT (1995) Neurodegeneration and diabetes: UK nationwide study of Wolfram (DIDMOAD) syndrome. Lancet 346:1458–1463. https://doi.org/10.1016/S0140-6736(95)92473-6
Smith CJA, Crock PA, King BR et al (2004) Phenotype-genotype correlations in a series of Wolfram syndrome families. Diabetes Care 27:2003–2009. https://doi.org/10.2337/diacare.27.8.2003
Matsunaga K, Tanabe K, Inoue H et al (2014) Wolfram syndrome in the japanese population; molecular analysis of wfs1 gene and characterization of clinical features. PLoS ONE. https://doi.org/10.1371/journal.pone.0106906
Medlej R, Wasson J, Baz P et al (2004) Diabetes mellitus and optic atrophy: a study of Wolfram syndrome in the Lebanese population. J Clin Endocrinol Metab 89:1656–1661. https://doi.org/10.1210/jc.2002-030015
Ishihara H, Takeda S, Tamura A et al (2004) Disruption of the WFS1 gene in mice causes progressive β-cell loss and impaired stimulus—secretion coupling in insulin secretion. Hum Mol Genet 13:1159–1170. https://doi.org/10.1093/hmg/ddh125
Plaas M, Seppa K, Reimets R et al (2017) Wfs1-deficient rats develop primary symptoms of Wolfram syndrome: insulin-dependent diabetes, optic nerve atrophy and medullary degeneration. Sci Rep. https://doi.org/10.1038/s41598-017-09392-x
Akiyama M, Hatanaka M, Ohta Y et al (2009) Increased insulin demand promotes while pioglitazone prevents pancreatic beta cell apoptosis in Wfs1 knockout mice. Diabetologia 52:653–663. https://doi.org/10.1007/s00125-009-1270-6
Kawaguchi Y, Hagiwara D, Miyata T et al (2020) Endoplasmic reticulum chaperone BiP/GRP78 knockdown leads to autophagy and cell death of arginine vasopressin neurons in mice. Sci Rep. https://doi.org/10.1038/s41598-020-76839-z
Azuma Y, Hagiwara D, Lu W et al (2014) Activating transcription factor 6α is required for the vasopressin neuron system to maintain water balance under dehydration in male mice. Endocrinology 155:4905–4914. https://doi.org/10.1210/en.2014-1522
Tochiya M, Hagiwara D, Azuma Y et al (2018) Chemical chaperone 4-phenylbutylate reduces mutant protein accumulation in the endoplasmic reticulum of arginine vasopressin neurons in a mouse model for familial neurohypophysial diabetes insipidus. Neurosci Lett 682:50–55. https://doi.org/10.1016/j.neulet.2018.06.013
Cryns K, Thys S, Van Laer L et al (2003) The WFS1 gene, responsible for low frequency sensorineural hearing loss and Wolfram syndrome, is expressed in a variety of inner ear cells. Histochem Cell Biol 119:247–256. https://doi.org/10.1007/s00418-003-0495-6
Ben-Barak Y, Russell JT, Whitnall M et al (1984) Phylogenetic cross-reactivities of monoclonal antibodies produced against rat neurophysin. Cell Mol Neurobiol 4:339–349. https://doi.org/10.1007/BF00733596
Ben-Barak Y, Russell JT, Whitnall MH et al (1985) Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci 5:81–97. https://doi.org/10.1523/jneurosci.05-01-00081.1985
Kõks S, Soomets U, Paya-Cano JL et al (2009) Wfs1 gene deletion causes growth retardation in mice and interferes with the growth hormone pathway. Physiol Genom 37:249–259. https://doi.org/10.1152/physiolgenomics.90407.2008.-The
Kawano J, Tanizawa Y, Shinoda K (2008) Wolfram syndrome 1 (WFS1) gene expression in the normal mouse visual system. J Comp Neurol 510:1–23. https://doi.org/10.1002/cne.21734
Kawano J, Fujinaga R, Yamamoto-Hanada K et al (2009) Wolfram syndrome 1 (Wfs1) mRNA expression in the normal mouse brain during postnatal development. Neurosci Res 64:213–230. https://doi.org/10.1016/j.neures.2009.03.005
Babey M, Kopp P, Robertson GL (2011) Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol 7:701–714. https://doi.org/10.1038/nrendo.2011.100
Bonnet Wersinger D, Benkafadar N, Jagodzinska J et al (2014) Impairment of visual function and retinal ER stress activation in Wfs1-deficient mice. PLoS ONE. https://doi.org/10.1371/journal.pone.0097222
Riggs AC, Bernal-Mizrachi E, Ohsugi M et al (2005) Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia 48:2313–2321. https://doi.org/10.1007/s00125-005-1947-4
Lee AS (2001) The glucose-regulated proteins: stress induction and clinical applications. Trends Biochem Sci 26:504–510. https://doi.org/10.1016/S0968-0004(01)01908-9
Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35:373–381. https://doi.org/10.1016/j.ymeth.2004.10.010
Acknowledgements
We thank Michiko Yamada for her helpful technical assistance and the staff of the Division of Experimental Animals, Nagoya University Graduate School of Medicine for their technical support.
Funding
This work was supported by the Acceleration Program for Intractable Diseases Research utilizing Disease-specific iPS cells of the Research Center Network for Realization of Regenerative Medicine from the Japan Agency for Medical Research and Development (to H. Suga) and the Suzuken Memorial Foundation (to H. Arima.).
Author information
Authors and Affiliations
Contributions
HT and HA designed the project. JK and HT performed the experiments and analyzed the data with technical help and advice from TM, YH, YK, DH, HS, TK, MS, TO, YI, SI, RB, and KT. JK, HT, KT, YT, and HA wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All procedures were approved by the Animal Experimentation Committee of the Nagoya University Graduate School of Medicine, and performed in accordance with the institutional guidelines for animal care and use.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurimoto, J., Takagi, H., Miyata, T. et al. Deficiency of WFS1 leads to the impairment of AVP secretion under dehydration in male mice. Pituitary 24, 582–588 (2021). https://doi.org/10.1007/s11102-021-01135-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-021-01135-6