Abstract
Aim
To ascertain the predictors of remission and relapse in patients of Cushing’s disease (CD) undergoing pituitary transsphenoidal surgery (TSS).
Methods
Patients with CD subjected to TSS over 35 years at a tertiary care center were included. Patients were grouped into remission and persistent disease at 1 year after surgery, and were further followed up for relapse. Demographic, clinical, biochemical, histological, radiological and post-operative follow-up parameters were analyzed.
Results
Of the 152 patients of CD, 145 underwent TSS. Remission was achieved in 95 (65.5%) patients at 1 year. Patients in remission had shorter duration of symptoms prior to presentation (p = 0.009), more frequent presence of proximal myopathy (p = 0.038) and a tumor size of < 2.05 cm (p = 0.016) in comparison to those with persistent disease. Post-TSS, immediate post-operative 0800-h cortisol (< 159.85 nmol/L; p = 0.001), histological confirmation of tumor (p = 0.045), duration of glucocorticoid replacement (median 90 days; p = 0.001), non-visualization of tumor on MRI (p = 0.003), new-onset hypogonadism (p = 0.001), 3-month 0800-h cortisol (< 384.9 nmol/L; p = 0.001), resolution of diabetes (p = 0.001) and hypertension (p = 0.001), and recovery of hypothalamic–pituitary–adrenal axis (p = 0.018) favored remission. In logistic regression model, requirement of glucocorticoid replacement (p = 0.033), and resolution of hypertension post-TSS (p = 0.003) predicted remission. None of the parameters could predict relapse.
Conclusion
The study could ascertain the predictors of remission in CD. Apart from the tumor characteristics, surgical aspects and low post-operative 0800-h cortisol, the results suggest that baseline clinical parameters, longer glucocorticoid replacement, and resolution of metabolic complications post-TSS predict remission in CD. Long-term follow-up is essential to look for relapse.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cushing’s disease (CD) is the most common form of endogenous Cushing’s syndrome (CS) accounting for more than 70% of cases of endogenous hypercortisolism [1, 2]. The chronic, autonomous and unabated release of cortisol by the adrenal glands, under tumoral corticotroph influence, results in characteristic metabolic, musculoskeletal, neuropsychiatric, and dermatological complications [3]. The cause of CD is a microadenoma in 90% cases and a macroadenoma in 10% cases. Despite clinical and biochemical evidence of hypercortisolism, the tumor is not radiologically evident in up to 40% cases [1, 4]. Mortality is increased in patients with CD (crude mortality rate: 4.25), particularly in patients with active CD [5, 6]. Normalization of hypercortisolism improves the mortality rate to near normal by decreasing the risk of cardiovascular disease, metabolic syndrome, infections, and psychiatric disorders, all of which significantly impact the risk of mortality in patients with CD [7, 8]. Pituitary transsphenoidal surgery (TSS) is the first-line treatment of CD with a remission rate of 78% (25–100%) and a relapse rate of 13% (0–65.6%) during 10-year period after surgery [5]. Therefore, up to one-third patients with CD encounter lack of remission (persistent disease) and/or relapse (tumor re-growth) and require further treatment in the form of a second surgery, medical therapy, pituitary radiotherapy and/or adrenalectomy [5, 9].
Despite the plethora of accessible data on CD, the facet of remission and cure in CD remains enigmatic. The terms remission and cure have been used interchangeably in the context of CD. However, although remission can be seen immediately after surgery, the cure is a prospective realization in CD and is often elusive. Isolated low post-operative cortisol due to suppression of surrounding normal corticotrophs in itself is insufficient to predict remission; hence, we require a gamut of predictors to foresee remission in CD. In addition, there is unpredictable relapse of CD in patients who are in remission during the 10 years after surgery [1, 10,11,12,13,14]. Demonstrating remission in patients with CD is crucial as there is evidence that patients with persistent disease and/or relapse after initial surgery have the higher mortality rate [15]. There is no single robust predictor of remission in CD [5, 10, 16,17,18,19,20]. Parameters such as a localized tumor (imaging, intra-operative) [16, 21], histological evidence of tumor [10, 17, 21, 22], low post-operative (day 1–7) cortisol [10, 21, 23,24,25], normal or low urinary free cortisol (UFC) [1, 9, 11, 22, 26], low adrenocorticotrophic hormone (ACTH) [27] and prolonged duration of glucocorticoid replacement [10, 25] favorably predict remission, whereas factors such as male gender [17, 28], macroadenoma [29], cavernous sinus invasion [28, 30], lack of pre-operative or intra-operative localization [5], absence of tumor on histological examination [16, 17, 28] and higher post-operative basal and Corticotrophin releasing hormone (CRH)-stimulated cortisol/ACTH levels [18] are unfavorable for remission. In this analysis of 152 patients of CD, we try to ascertain the factors predicting remission and relapse in CD after TSS.
Materials and methods
This is a single-center study of 152 patients of CD treated at the Department of Endocrinology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India over 35 years (1984–2019) with a prospective follow-up from 2004 and retrospective data retrieval from 1984 to 2003. The study was approved by the Institutional Ethics Committee, PGIMER, Chandigarh (INT/IEC/2017/85). An informed written consent was obtained only from the prospective participants.
Baseline demographic data for this analysis included age, gender, BMI, duration of symptoms prior to presentation, and clinical features of CS (hyperpigmentation, striae, easy bruisability, proximal myopathy, cuticular atrophy, psychiatric manifestations, hypertension and diabetes). Baseline cortisol dynamics included 0800 and 2300-h cortisol and ACTH, overnight dexamethasone suppression test (ONDST), low-dose dexamethasone suppression test (LDDST) and high-dose dexamethasone suppression test (HDDST) as per standard protocol [31]. 2300-h cortisol was collected in a prechilled vial in the awake state, and after centrifugation, plasma was stored at – 20 ºC. Cortisol and ACTH were measured using radioimmuno-assay (RIA) before 2009 and electro-chemiluminescence-immunoassay (ECLIA; Roche Diagnostic, Germany) after 2009. The technique for sample collection, intra-assay and inter-assay coefficient of variation of ECLIA and RIA have been described previously [32, 33]. Pre-operative imaging of the pituitary by contrast-enhanced magnetic resonance imaging (CEMRI; 1.5 and 3 T; Siemens Magnetom) was done in all patients. In subjects having non-visualized pituitary tumor, computed tomography (CT) of thorax and abdomen was performed in all, whereas somatostatin receptor-based scintigraphy and inferior petrosal sinus sampling (IPSS) were performed in select individuals, to rule out an ectopic source of CS. Dual-energy X-ray absorptiometry (DXA) scan done using the HOLOGIC Discovery A machine. Microscopic TSS was performed by two experienced neurosurgeons (K.K.M. and S.D.). After TSS, hydrocortisone succinate 100 mg i.v. thrice daily was administered to patients having hypocortisolism, i.e., 0800-h serum cortisol ≤ 138 nmol/L [9]. If the patient did not achieve hypocortisolism, then 0800-h cortisol was monitored till day 7. Hydrocortisone was tapered to 10–20 mg (per-oral) in three divided doses. Post-operatively, patients were assessed for pituitary hormone deficiencies and relapse. During follow-up, patients with persistent disease or relapse were recommended a second surgery or radiotherapy with or without medical therapy (ketoconazole) on a case to case basis.
Post-operative 0800-h cortisol (day 1–7), histology of the resected tissue including immunohistochemistry (IHC) (wherever possible) for ACTH, post-operative pituitary hormones, cortisol at 3 months (0800- and 2300-h cortisol, and ONDST, if hypercortisolemic), resolution of clinical and metabolic features, pituitary CEMRI, duration of steroid replacement and recovery of the hypothalamic–pituitary–adrenal (HPA) axis were assessed.
Immediate post-operative remission was defined as a post-operative 0800-h (day 1–7) ≤ 138 nmol/L. Delayed remission was defined as 0800-h cortisol < 350 nmol/L at 3 months with a normal 2300-h cortisol (< 207 nmol/L) or a suppressed ONDST (< 50 nmol/L). During follow-up, 0800 h cortisol was measured after stopping hydrocortisone for 24 h. Any patient with a follow-up 0800-h cortisol of ≤ 138 nmol/L was regarded to be in remission. At 6–12 weeks, patients with a 0800-h cortisol ≥ 500 nmol/L and 2300-h cortisol > 207 nmol/L or ONDST ≥ 50 nmol/L were deemed to have persistent CD. Patients with cortisol of > 138 and < 349 nmol/L and an ONDST < 50 nmol/L were either observed or subjected to a 250 μg synacthen stimulation test (6 months onwards) to check for the recovery of HPA axis (normal > 550 nmol/L). Patients with stimulated cortisol < 550 nmol/L were supplemented with hydrocortisone during illness.
Statistical analysis
Data obtained were analyzed by IBM SPSS version 22.0 (IBM Corp., Armonk, NY). Variables were analyzed for descriptive data and normality of distribution. Categorical variables were shown as numbers and percentages. Continuous data were reported as median and interquartile (IQR) range. Inter-group analysis of baseline and post-operative parameters was done using Chi-Square/Fisher’s Exact test for categorical variables and Mann–Whitney U test for continuous variables without normal distribution. Correlation between ≥ 2 continuous variables was done using Spearman’s Rho, and between continuous and categorical variables was done using Kruskal–Wallis H test. Receiver-Operating Characteristic (ROC) curves were calculated to find cutoff values for various parameters as required. A multivariate logistic regression analysis was carried out to find predictors of remission amongst various factors found to be different between the remission and persistent subgroups. For all statistical tests, p < 0.05 was considered significant.
Results
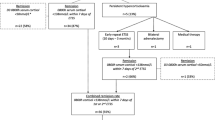
This study comprises 152 patients of CD. Five patients died pre-operatively, while two were lost to follow-up pre-operatively leading to their exclusion from this analysis. A total of 145 patients underwent TSS. The median duration of follow-up was 6 years (3 months-15 years). The post-operative management and outcome of the patients is shown in Figs. 1 and 2. Ninety-four patients achieved remission at 3 months. Of these, 6 patients relapsed within the first year of follow-up while, amongst the 51 patients with persistent disease, 7 went into remission. At the end of the first year of follow-up, 95 patients were in remission. Out of these 95 patients, 58 remained in remission, 23 patients had a relapse and 14 were lost to follow-up. Remission rates at 3 months and 1 year were 64.8% (94/145) and 65.5% (95/145), respectively.
Comparison of baseline parameters amongst remission and persistent subgroups
Demographics and comorbidities of CD were similar between the two groups (Table 1). Patients in remission had shorter duration of symptoms prior to presentation (median 2 years; p = 0.009) and higher prevalence of proximal myopathy (p = 0.038) (Table 1). The presence of diabetes was slightly higher in the remission subgroup, although it did not reach statistical significance (p = 0.063). Conventional microadenoma versus macroadenoma scrutiny did not reveal meaningful results, therefore subgroups were analyzed for tumor diameter at different cutoffs (5, 15, 20, and 25 mm). A tumor size of < 2.05 cm was significantly associated with remission (p = 0.016). Other, discriminatory clinical parameters of protein catabolism were not significantly different between the two groups (Table 1). Cortisol dynamics (baseline cortisol, ONDST, LDDST, HDDST) and ACTH were comparable across the groups (Table 1).
Comparison of post-operative parameters amongst remission and persistent subgroups
Patients in remission had lower immediate post-operative 0800-h cortisol (p = 0.001), greater histological confirmation of tumor (p = 0.045), longer duration of steroid replacement (median 90 days; p = 0.001), greater frequency of non-visualization of tumor on follow-up CEMRI (p = 0.003), new-onset hypogonadism (p = 0.001) and 3-month 0800-h cortisol < 350 nmol/L (p = 0.001), resolution of diabetes (p = 0.001) and hypertension (p = 0.001) along with recovery of HPA axis (p = 0.018) as compared to patients with persistent disease (Table 2).
Predictors of remission
From the ROC curve analysis, the cutoff values for immediate post-operative 0800-h cortisol and 3-month cortisol to predict remission were 159.85 nmol/L and 384.9 nmol/L with sensitivity/specificity of 77%/84.1% and 81.6%/61.4%, respectively. For immediate post-operative 0800-h cortisol, a cutoff of 49.7 nmol/L had a sensitivity and specificity of 33.3% and 93.2%, respectively, to predict remission (p = 0.002). A logistic regression model studying the effects of the aforementioned parameters on the likelihood of remission in CD was statistically significant and explained 70.1% (Nagelkerke R2) of the variance in remission achieved and correctly classified 91.5% of cases in remission. Patients with the duration of steroid replacement > 90 days and resolution of hypertension post-TSS were 15.8 times (p = 0.033) and 12.2 times (p = 0.003) more likely to enter remission, respectively.
Evidence of cortisol burden and its withdrawal
The protein catabolism features of myopathy (p = 0.014), striae (p = 0.006), easy bruisability (p = 0.011), cuticular atrophy (p = 0.007), and low bone density (p = 0.02) correlated with baseline cortisol level. Metabolic complications of hypertension (p = 0.000), diabetes (p = 0.017), and hypokalemia (p = 0.037) correlated with baseline cortisol level, as did psychiatric manifestations (p = 0.005). The duration of symptoms at presentation did not correlate with baseline cortisol levels (p = 0.39). Post-TSS, no correlation was seen between baseline cortisol and immediate post-operative 0800-h cortisol, 3-month 0800-h cortisol, duration of glucocorticoid replacement, and resolution of diabetes and hypertension (p = 0.298, p = 0.384, p = 0.315, p = 0.074, and p = 0.879, respectively). Post-TSS, resolution of hypertension correlated with immediate post-operative 0800-h cortisol (p = 0.003), 3-month 0800-h cortisol (p = 0.024), and duration of glucocorticoid replacement (p = 0.005).
Comparison of baseline and post-operative parameters amongst long-term remission and relapse subgroups
Out of the 95 patients in remission, 58 remained in remission, 23 patients had a relapse and 14 were lost to follow-up. Unlike the previous analyses, no differences were found in either baseline or post-operative parameters of these subgroups (Supplementary Tables 1, 2).
Discussion
In this study, we could ascertain the predictors of remission in CD. The clinical parameters reflecting cortisol burden (shorter duration of symptoms prior to the presentation, presence of proximal myopathy), its withdrawal after surgery (low post-operative cortisol, longer duration of glucocorticoid replacement and resolution of metabolic derangements), tumor characteristics (size, pre- and intra-operative localization, post-surgery non-visualization), and surgical aspects (new-onset hypogonadism, histologically confirmed tumor) predicted remission favorably while none of the parameters could predict relapse. Overall, the remission rates in the present study, are comparable to the earlier studies from India [17, 25], and elsewhere [10, 23, 28, 30].
Remission: baseline parameters
The parameters of shorter duration of symptoms prior to presentation and presence of proximal myopathy significantly favored remission. In an earlier study, none of the discriminatory clinical features could predict remission [34]. The clinical features are a mirror of the cortisol excess in a patient of CD. Yet, other features (hyperpigmentation, striae, easy bruisability, hypokalemia, cuticular atrophy, psychiatric manifestations, low bone density and hypertension) which are also the manifestations of hypercortisolism did not predict remission in this study. Proximal myopathy correlated with cortisol levels (p = 0.014) in our study, and it is one of the most specific manifestation of hypercortisolism (discriminatory index: 8) [35]. A recent study has shown that certain glucocorticoid receptor polymorphisms are associated with reduced handgrip strength in patients with active CS [36]. Thus, a shorter duration of symptoms and the presence of proximal myopathy in patients who achieved remission suggests higher initial cortisol burden.
An adenoma size < 2.05 cm favored remission. Similar to findings of the current study, a previous study has shown an increased risk (odds ratio: 3.5) of residual disease at an tumor size of > 2 cm [29]. Conversely, the results of a meta-analysis have shown that tumor size and macroscopic invasion are not associated with relapse in CD, providing conflicting evidence [19].
Remission: post-operative parameters
In the current study, immediate post-operative 0800-h cortisol, 3-month 0800-h cortisol, non-visualization of tumor on follow-up CEMRI, histological confirmation of tumor, new-onset hypogonadism, duration of steroid replacement, resolution of diabetes and hypertension and recovery of HPA axis significantly favored remission.
In CD, the HPA axis is functional despite the presence of an autonomous tumor. Cortisol negatively feedbacks to the normal (surrounding) corticotrophs as well as tumoral corticotrophs. Hence, the remission in CD is predicted by a low post-operative 0800-h cortisol due to the prolonged and intense suppression of the surrounding normal corticotrophs after adenoma removal. A low post-operative 0800-h cortisol is a well-established predictor of remission [9]. It is usually measured serially from day 1–7 after surgery [9]. Two cutoffs, < 138 nmol/L [21, 22, 24, 28] and < 50 nmol/L [10, 23, 30] have been used, and both are predictive of remission. Through the ROC, the values obtained in our study were < 49.7 nmol/L and < 159.85 nmol/L, respectively. Latter had a sensitivity/specificity of 77%/84.1%, suggesting that patients with a borderline value may enter delayed remission or relapse during follow-up and thus, watchful waiting for 6–12 weeks is warranted in every case. At 3-months 0800-h cortisol of < 384.9 nmol/L also favored remission. There are multiple caveats in the interpretation of post-operative cortisol. First, delayed remission of hypercortisolism is well known. This may be a result of factors such as vascular insufficiency and late necrosis of residual tumor, amelioration of autonomous cortisol production from the hyperplastic adrenals and stress of the surgery along with cortisol estimated on hydrocortisone replacement [37, 38]. Second, low cortisol does not necessarily imply remission. This can be due to pre-operative ketoconazole use [9], partial resection causing transient secondary adrenal insufficiency followed by gradual resumption of ACTH secretion [37, 39] or cyclicity of the residual adenoma [40]. Further, the use of glucocorticoids post-operatively can suppress residual tumoral tissue resulting in a false negative result [41].
Patients in remission had more frequent non-visualization of tumor on follow-up CEMRI. Yet, this finding cannot be considered specific. Just like a non-visualized pre-operative adenoma, a small remnant is good enough to keep the patient symptomatic, necessitating further management [39]. In our cohort, 15 patients with persistent disease had a non-visualized adenoma on follow-up CEMRI. This scenario may be due to the inability of the MRI to delineate a microscopic remnant or dural invasion. Conversely, in 20% of patients with clinical and biochemical remission, CEMRI sella was reported to have a residue. This possibly happened due to post-operative changes being misconstrued as remnant adenoma.
Histological presence of tumor has been reported to have a high remission rate of 82.1% [5]. Our findings were similar (88%), but were not specific as adenoma was identified in 72% of patients with persistent disease. It is likely that microscopic or macroscopic, functionally active remnants of adenoma or capsule led to persistent disease or relapse. Thus, presence of tumor tissue on histology is not confirmatory of a surgical remission. Patients in remission without histological confirmation are likely to have had loss of tissue due to tumor necrosis or tissue handling [42].
CD is a state of functional and/or anatomical suppression of hypothalamic-pituitary–gonadal (HPG) axis. The surgical intervention can further result in structural damage to residual gonadotrophs as they are diffusely scattered in the pituitary. In the current study, frequency of hypogonadism was higher post-operatively than pre-operatively (65.9% vs. 30.9%; p = 0.001). This could be a result of extensive surgical exploration and wide excision of tumor (including pseudocapsule). New-onset hypogonadism was significantly associated with remission contrary to the findings of a previous study [23].
HPA axis is likely to remain suppressed in the event of successful surgery, leading to low post-operative cortisol. The duration of this suppression and subsequent steroid replacement is of clinical importance as far as remission is concerned. Both these factors have been reported as predictors in multiple studies [10, 21, 25]. Our result agrees with previous studies suggesting that glucocorticoid replacement for > 90 days duration predicts remission in CD.
Resolution of diabetes (77%) and hypertension (71%) at 3 months predicted remission at 1 year in our cohort. The protean metabolic manifestations of CD are a result of hypercortisolism. Previous studies have shown either resolution or reduction in doses of medications for both diabetes and hypertension after remission in CD [23, 43, 44]. However, another study failed to correlate metabolic profile at 1-year follow-up with remission [34]. It is likely that sudden withdrawal of glucocorticoids leads to resolution of metabolic parameters and predicts remission. Post-operative cortisol significantly correlated with resolution of hypertension (p = 0.003) in our study.
The factor underlying most of the predictors of remission in this study is cortisol burden and its withdrawal following surgery. Patients in remission had higher cortisol burden indicated by more frequent presence of proximal myopathy and shorter duration of symptoms prior to presentation and following surgery, possibly, withdrawal of this cortisol burden led to lower cortisol level and prolonged duration of glucocorticoid replacement. We hypothesize that cortisol burden is a product of cortisol levels and the tissue sensitivity to cortisol. Since cortisol levels were comparable across the remission and persistence groups, a greater tissue sensitivity to cortisol probably resulted in lesser lag time, differences in myopathy and low post-operative cortisol due to suppression of surrounding corticotrophs. We also propose that the cortisol sensitivity at the pituitary could have resulted in a smaller tumor size. Corticotroph tumors have an acquired relative resistance to feedback inhibition by cortisol, thus implying that they are still partly sensitive to glucocorticoid negative feedback. This is evident from cortisol/ACTH suppressibility on HDDST in 60–70% adenomas [1] and cyclicity in some adenomas [40]. Molecular mechanisms (e.g., Heat-shock protein 90, testicular nuclear receptor 4) leading to this relative resistance have now been elucidated [45]. Some of these are now targets for medical management of CD at the level of pituitary [46]. Although these alterations are pituitary specific, the phenotype of CS is dependent on individual sensitivity to the effects of glucocorticoids. The mechanisms responsible for variations in phenotype of CS are not yet elucidated. The differences in glucocorticoid levels and sensitivity determine the severity of disease and tumor size as well as prediction of remission.
Relapse
A total of 23 patients (15.8%) had a relapse of CD. After scrutinizing various clinical, biochemical, radiological and post-operative parameters, we could not find any factor indicating relapse in this cohort. No absolute value of cortisol can give a 100% assurance of not having a relapse [47]. Even the stringent criteria of < 50 nmol/L has not been found to be superior to predict relapse compared to post-operative eucortisolemia [10]. Aforementioned clinical and post-operative factors predicting remission in CD were all comparable in the relapse subgroup. This affirms the theory that TSS may lead to either stunning of the adenoma tissue (for a variable time) or partial removal of the adenoma, resulting in transient biochemical and clinical remission of CD. In the latter scenario, the doubling of remnant adenoma is a very slow process and thus relapse is possible even after 10 years [1, 10,11,12,13,14].
Strengths and limitations
The current study had many strengths including the data collection of a large cohort of patients of CD at a single center, long-term follow-up, pre-defined criteria for cortisol dynamics and post-operative cortisol, two experienced neurosurgeons throughout the duration of the study (K.K.M., S.D.), two continuing investigators (R.W. and A.B.) and its ability to ascertain multiple predictors of remission in CD, few of which (presence of proximal myopathy, resolution of hypertension) have not been previously reported. The cutoff values of immediate 0800-h cortisol and 3-month 0800-h cortisol are similar to previously described values in the literature.
Retrospective data collection for a significant duration and the consequent data unavailability for various clinical, biochemical and radiological parameters for that period represents the major limitation of this study. Certain tests such as 24-h UFC, CRH stimulation, IGF-1 and prolactin could not be performed in participants of this study.
Conclusion
Overall, our study has laid out a conglomeration of clinical, biochemical and radiological parameters predicting remission in CD, with a competent rate of remission in comparison to national and international centers. The results suggest that the parameters of cortisol burden such as shorter duration of symptoms prior to presentation, presence of proximal myopathy and cortisol withdrawal such as low post-operative cortisol and prolonged requirement of steroid supplementation, and resolution of diabetes and hypertension post-TSS can possibly predict remission in CD. In addition, previously described factors such as adenoma size, pre-operative localization, histologically confirmed adenoma, and new-onset hypogonadism could also predict remission. However, no parameter could predict relapse and thus, long-term and regular follow-up of patients in remission is mandatory. Further studies are warranted to explore the effect of cortisol sensitivity to better understand its possible impact on the disease course and remission.
References
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367:1605–1617. https://doi.org/10.1016/S0140-6736(06)68699-6
Lacroix A, Feelders RA, Stratakis CA, Nieman LK (2015) Cushing’s syndrome. Lancet Lond Engl 386:913–927. https://doi.org/10.1016/S0140-6736(14)61375-1
Pivonello R, Isidori AM, De Martino MC et al (2016) Complications of Cushing’s syndrome: state of the art. Lancet Diabetes Endocrinol 4:611–629. https://doi.org/10.1016/S2213-8587(16)00086-3
Pivonello R, De Martino MC, De Leo M et al (2008) Cushing’s Syndrome. Endocrinol Metab Clin North Am 37(135–149):ix. https://doi.org/10.1016/j.ecl.2007.10.010
Pivonello R, De Leo M, Cozzolino A, Colao A (2015) The treatment of Cushing’s disease. Endocr Rev 36:385–486. https://doi.org/10.1210/er.2013-1048
Pivonello R, Simeoli C, De Martino MC, Colao A (2016) Is mortality in Cushing’s disease reversible with remission? Lancet Diabetes Endocrinol 4:551–552. https://doi.org/10.1016/S2213-8587(16)30044-4
Feelders RA, Pulgar SJ, Kempel A, Pereira AM (2012) The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur J Endocrinol 167:311–326. https://doi.org/10.1530/EJE-11-1095
Graversen D, Vestergaard P, Stochholm K et al (2012) Mortality in Cushing’s syndrome: a systematic review and meta-analysis. Eur J Intern Med 23:278–282. https://doi.org/10.1016/j.ejim.2011.10.013
Nieman LK, Biller BMK, Findling JW et al (2015) Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 100:2807–2831. https://doi.org/10.1210/jc.2015-1818
Alexandraki KI, Kaltsas GA, Isidori AM et al (2013) Long-term remission and recurrence rates in Cushing’s disease: predictive factors in a single-centre study. Eur J Endocrinol 168:639–648. https://doi.org/10.1530/EJE-12-0921
Biller BMK, Grossman AB, Stewart PM et al (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462. https://doi.org/10.1210/jc.2007-2734
Pivonello R, De Martino MC, De Leo M et al (2008) Cushing’s Syndrome. Pituit Disord 37:135–149. https://doi.org/10.1016/j.ecl.2007.10.010
Atkinson AB, Kennedy A, Wiggam MI et al (2005) Long-term remission rates after pituitary surgery for Cushing’s disease: the need for long-term surveillance. Clin Endocrinol (Oxf) 63:549–559. https://doi.org/10.1111/j.1365-2265.2005.02380.x
Tritos NA, Biller BMK, Swearingen B (2011) Management of Cushing disease. Nat Rev Endocrinol 7:279–289. https://doi.org/10.1038/nrendo.2011.12
Lindholm J, Juul S, Jørgensen JOL et al (2001) Incidence and late prognosis of Cushing’s syndrome: a population-based study1. J Clin Endocrinol Metab 86:117–123. https://doi.org/10.1210/jcem.86.1.7093
Chee GH, Mathias DB, James RA, Kendall-Taylor P (2001) Transsphenoidal pituitary surgery in Cushing’s disease: can we predict outcome? Clin Endocrinol (Oxf) 54:617–626. https://doi.org/10.1046/j.1365-2265.2001.01261.x
Ammini AC, Bhattacharya S, Sahoo JP et al (2011) Cushing’s disease: results of treatment and factors affecting outcome. Horm Athens Greece 10:222–229. https://doi.org/10.14310/horm.2002.1312
Alwani RA, de Herder WW, van Aken MO et al (2010) Biochemical predictors of outcome of pituitary surgery for Cushing’s disease. Neuroendocrinology 91:169–178. https://doi.org/10.1159/000258677
Roelfsema F, Biermasz NR, Pereira AM (2012) Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: a structured review and meta-analysis. Pituitary 15:71–83. https://doi.org/10.1007/s11102-011-0347-7
Pendharkar AV, Sussman ES, Ho AL et al (2015) Cushing’s disease: predicting long-term remission after surgical treatment. Neurosurg Focus FOC 38:E13. https://doi.org/10.3171/2014.10.FOCUS14682
Ciric I, Zhao J-C, Du H et al (2012) Transsphenoidal surgery for Cushing disease: experience with 136 patients. Neurosurgery 70:70–80. https://doi.org/10.1227/NEU.0b013e31822dda2c
Jagannathan J, Smith R, DeVroom HL et al (2009) Outcome of using the histological pseudocapsule as a surgical capsule in Cushing disease. J Neurosurg JNS 111:531–539. https://doi.org/10.3171/2008.8.JNS08339
Hassan-Smith ZK, Sherlock M, Reulen RC et al (2012) Outcome of Cushing’s disease following transsphenoidal surgery in a single center over 20 years. J Clin Endocrinol Metab 97:1194–1201. https://doi.org/10.1210/jc.2011-2957
Esposito F, Dusick JR, Cohan P et al (2006) Early morning cortisol levels as a predictor of remission after transsphenoidal surgery for Cushing’s Disease. J Clin Endocrinol Metab 91:7–13. https://doi.org/10.1210/jc.2005-1204
Bansal P, Lila A, Goroshi M et al (2017) Duration of post-operative hypocortisolism predicts sustained remission after pituitary surgery for Cushing’s disease. Endocr Connect 6:625–636. https://doi.org/10.1530/EC-17-0175
Prevedello DM, Pouratian N, Sherman J et al (2008) Management of Cushing’s disease: outcome in patients with microadenoma detected on pituitary magnetic resonance imaging. J Neurosurg JNS 109:751–759. https://doi.org/10.3171/JNS/2008/109/10/0751
Acebes JJ, Martino J, Masuet C et al (2007) Early post-operative ACTH and cortisol as predictors of remission in Cushing’s disease. Acta Neurochir (Wien) 149:471–479. https://doi.org/10.1007/s00701-007-1133-1
Hammer GD, Tyrrell JB, Lamborn KR et al (2004) Transsphenoidal microsurgery for Cushing’s disease: initial outcome and long-term results. J Clin Endocrinol Metab 89:6348–6357. https://doi.org/10.1210/jc.2003-032180
Blevins LS Jr, Christy JH, Khajavi M, Tindall GT (1998) Outcomes of therapy for Cushing’s disease due to adrenocorticotropin-secreting pituitary macroadenomas1. J Clin Endocrinol Metab 83:63–67. https://doi.org/10.1210/jcem.83.1.4525
Wagenmakers MAEM, Boogaarts HD, Roerink SHPP et al (2013) Endoscopic transsphenoidal pituitary surgery: a good and safe primary treatment option for Cushing’s disease, even in case of macroadenomas or invasive adenomas. Eur J Endocrinol 169:329–337. https://doi.org/10.1530/EJE-13-0325
Nieman LK, Biller BMK, Findling JW et al (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93:1526–1540. https://doi.org/10.1210/jc.2008-0125
Jarial KDS, Bhansali A, Mukherjee KK et al (2018) Utility of a single late-night plasma cortisol and ACTH for the diagnosis of Cushing syndrome. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol 24:156–162. https://doi.org/10.4158/EP171803.OR
Das L, Bhansali A, Pivonello R et al (2020) ACTH increment post total bilateral adrenalectomy for Cushing’s disease: a consistent biosignature for predicting Nelson’s syndrome. Pituitary. https://doi.org/10.1007/s11102-020-01047-x
Stieg MR, Auer MK, Berr C et al (2019) Clinical score system in the treatment of Cushing’s disease: failure to identify discriminative variables from the German Cushing’s Registry. Pituitary 22:129–136. https://doi.org/10.1007/s11102-019-00942-2
Melmed S, Polonsky KS, Larsen PR, Kronenberg HM (2016) Williams textbook of endocrinology, 13th edn. Elsevier, Philadelphia
Müller LM, Kienitz T, Deutschbein T et al (2019) Glucocorticoid receptor polymorphisms influence muscle strength in Cushing’s syndrome. J Clin Endocrinol Metab 105:305–313. https://doi.org/10.1210/clinem/dgz052
Pereira AM, van Aken MO, van Dulken H et al (2003) Long-term predictive value of postsurgical cortisol concentrations for cure and risk of recurrence in Cushing’s disease. J Clin Endocrinol Metab 88:5858–5864. https://doi.org/10.1210/jc.2003-030751
Valassi E, Biller BMK, Swearingen B et al (2010) Delayed remission after transsphenoidal surgery in patients with Cushing’s disease. J Clin Endocrinol Metab 95:601–610. https://doi.org/10.1210/jc.2009-1672
Fitzgerald PA, Aron DC, Findling JW et al (1982) Cushing’s disease: transient secondary adrenal insufficiency after selective removal of pituitary microadenomas; evidence for a pituitary origin. J Clin Endocrinol Metab 54:413–422. https://doi.org/10.1210/jcem-54-2-413
Atkinson AB, McCance OR, Kennedy L, Sheridan B (1992) Cyclical Cushing’s syndrome first diagnosed after pituitary surgery: a trap for the unwary. Clin Endocrinol (Oxf) 36:297–299. https://doi.org/10.1111/j.1365-2265.1992.tb01447.x
Ayala A, Manzano AJ (2014) Detection of recurrent Cushing’s disease: proposal for standardized patient monitoring following transsphenoidal surgery. J Neurooncol 119:235–242. https://doi.org/10.1007/s11060-014-1508-0
Arnott RD, Pestell RG, McKelvie PA et al (1990) A critical evaluation of transsphenoidal pituitary surgery in the treatment of Cushing’s disease: prediction of outcome. Acta Endocrinol (Copenh) 123:423–430. https://doi.org/10.1530/acta.0.1230423
Fallo F, Sonino N, Barzon L et al (1996) Effect of surgical treatment on hypertension in Cushing’s syndrome. Am J Hypertens 9:77–80. https://doi.org/10.1016/0895-7061(95)00299-5
Magiakou MA, Smyrnaki P, Chrousos GP (2006) Hypertension in Cushing’s syndrome. Best Pract Res Clin Endocrinol Metab 20:467–482. https://doi.org/10.1016/j.beem.2006.07.006
Ciato D, Albani A (2020) Molecular mechanisms of glucocorticoid resistance in corticotropinomas: new developments and drug targets. Front Endocrinol 11:21–21. https://doi.org/10.3389/fendo.2020.00021
Rubinstein G, Osswald A, Zopp S et al (2019) Therapeutic options after surgical failure in Cushing’s disease: a critical review. Best Pract Res Clin Endocrinol Metab 33:101270. https://doi.org/10.1016/j.beem.2019.04.004
Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK (2011) The Postoperative basal cortisol and CRH tests for prediction of long-term remission from Cushing’s disease after transsphenoidal surgery. J Clin Endocrinol Metab 96:2057–2064. https://doi.org/10.1210/jc.2011-0456
Acknowledgements
We are highly indebted to late Prof Kanchan Kumar Mukherjee, senior neurosurgeon who performed transsphenoidal surgery for the patients of Cushing’s disease at our center. He was instrumental in encouraging us to write this article before his demise. We acknowledge Mrs. Kusum Chopra for statistical analysis.
Funding
This research did not receive any specific Grant from any funding agency in the public, commercial or not-for-profit sector.
Author information
Authors and Affiliations
Contributions
AD and NG did the data analysis, drafted and edited the manuscript and reviewed the literature. RW conceived the study, managed the patients, collected the data, performed interpretation of data and statistical analysis and edited the manuscript. AB managed the patients, collected the data, edited the manuscript and supervised patient management. PD edited the manuscript and supervised patient management. SKB supervised patient management. RP performed data interpretation and edited the manuscript. CA provided neuro-radiological expertise in terms of imaging interpretation and inferior petrosal sinus sampling. SD performed the pituitary surgery. AH managed the patients and collected the data. CS performed data interpretation and edited the manuscript. NS provided laboratory expertise for biochemical analysis. UNS performed histopathological analysis.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all the authors, the corresponding author states that there is no conflict of interest.
Ethical approval
Institutional ethics committee approval was obtained.
Research involving human participants and/or animals
The study was approved by the Institutional Ethics Committee, PGIMER, Chandigarh (INT/IEC/2017/85).
Informed consent
Written informed consent for enrolment was obtained from participants or their relatives as applicable.
Consent to participate
Written informed consent for enrollment was obtained from participants or their relatives as applicable.
Consent for publication
Written informed consent for enrollment was obtained from participants or their relatives as applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dutta, A., Gupta, N., Walia, R. et al. Remission in Cushing’s disease is predicted by cortisol burden and its withdrawal following pituitary surgery. J Endocrinol Invest 44, 1869–1878 (2021). https://doi.org/10.1007/s40618-020-01495-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-020-01495-z