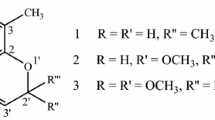Abstract
Malaria is a major parasitic infection in many tropical and subtropical regions with the most severe forms of the disease being caused by Plasmodium falciparum. Dramatic increases in the resistance of this mosquito-transmitted parasite to classical treatments have been observed in recent years, and much research effort is now aimed at the discovery of novel natural products with antiplasmodial activities. On the basis of its use in popular medicine, Aspidosperma pyrifolium Mart. (Apocynaceae), a tree popularly known as “pereiro-do-sertão,” was selected for detailed study. A phytochemical investigation of the aqueous extract of its stem bark revealed the presence of two known monoterpenoid indole alkaloids, 15-demethoxypyrifoline and aspidofractinine, together with the novel compound N-formylaspidofractinine. The structures of these compounds were established from UV, IR, MS and NMR data, and their 1H- and 13C-NMR spectra have been unambiguously assigned for the first time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For thousands of years, plants and plant-derived preparations have been employed by man in the treatment of numerous and diverse medical conditions. Plants remain to this day one of the most important sources of biologically active compounds and continue to provide many of the lead molecules that facilitate the development of new drugs.
It is believed that more than half of the 500,000 plant species estimated to exist worldwide are located in tropical forests, but that less than 1% of such species have been analysed for potential biological activity (Conte 1996). Moreover, tropical plants are typically three to four times more prolific in their production of biologically active molecules than their temperate counterparts (Rodriguez and West 1995). However, as a result of extensive deforestation occasioned by increasing agricultural activity and cattle farming, the irreplaceable tropical botanical resource is under severe threat, and there is an urgent need for the collection, conservation, documentation and analysis of the endangered species before access to this knowledge base is lost forever.
Amongst diseases that give rise to major health concern worldwide, those mediated by parasitic protozoa and helminths, including malaria, African trypanosomiasis, amebiasis, leishmaniasis, schistosomiasis and lymphatic filariasis, are particularly problematic (Nyame et al. 2004). Malaria, which is responsible for more deaths than any other communicable diseases apart from tuberculosis, is a public health problem in more than 90 countries inhabited by some 2,400 million people, i.e. 40% of the world’s population. An estimated 300–500 million new cases of malarial infection occur each year resulting in the death of over 1 million individuals (Breman 2001; Greenwood et al. 2005), most of whom are African children under the age of 5 years (Winstanley 2000).
There is presently no effective vaccine available with which to treat malaria, and therapy relies mainly on two classes of antimalarial, both of which were originally based on plant secondary metabolites. The alkaloid quinine, isolated from Cinchona trees (Foley and Tilley 1997), was the lead molecule for the synthetic compounds chloroquine and mefloquine (Wright 2005), whilst the sesquiterpene endoperoxide artemisinin from Artemisia annua was the natural prototype for the development of a number of potent antimalarial drugs including artesunate (Olliaro et al. 2001) and artemether (Pittler and Ernst 1999; Fig. 1). However, the most deadly of the Plasmodium species that causes human malaria, Plasmodium falciparum, is becoming increasingly resistant to the currently available antimalarial agents. Moreover, few new antimalarial drugs are under clinical trials at this time and, hence, the identification of new compounds with activity against P. falciparum is of considerable importance (Ridley 2002). More specifically, there is an urgent need for the development of therapies based on novel mechanisms of action (Olliaro and Yuthavong 1999; Berry 2000).
As part of our continuing research programme concerning medicinal plants used for treatment of tropical diseases, we have carried out ethnopharmacological and chemosystematic surveys of a wide variety of Brazilian species in an attempt to discover new sources of antimalarial compounds. We here report on a study of the alkaloids from Aspidosperma pyrifolium Mart. (family Apocynaceae), a tree that is widely distributed in the Northeast of Brazil (Craveiro et al. 1983) and popularly known as “pereiro-preto” or “pereiro-do-sertão.”
The family Apocynaceae is of considerable economic and medicinal importance. Although most of the species are distributed within the tropics, a few are to be found in temperate regions, and a number are cultivated on a worldwide basis as house (e.g. Nerium oleander L.), garden (e.g. species of Catharanthus) or greenhouse (e.g. species of Allemanda) plants. Whilst some tree species of this family are prized for their timber, many more are of therapeutic value by virtue of their accumulation of cardiac glycosides and alkaloids (Botanical Dermatology Database 2006). Along with several other families of the order Gentiales, including Loganiaceae and Rubiaceae, the Apocynaceae is particularly rich in monoterpenoid indole alkaloids, a chemically diverse group of compounds accounting for ca 25% of the 12,000 or so alkaloids that have been identified. The indole alkaloids are formed by the condensation of tryptophan with secologanin to yield strictosidine, and the latter may be further elaborated to produce an impressive array of structural variants including such sub-groups as the kopsane, quebrachamine, strychnos, cinchona, ajmaline, yohimbine, oxindole, aspidospermine and ajmalicine alkaloids (De Luca and St Pierre 2000; Lorence and Nessler 2004).
Amongst the genera of the Apocynaceae that have been found to accumulate indole alkaloids, the most important are Catharanthus, Rauwolfia, Alstonia, Kopsia and Aspidosperma. Several species of Alstonia are used in traditional medicine throughout Southeast Asia for the treatment of malaria and dysentery (Kam et al. 1999), whilst a number of indole alkaloids have been isolated from the root bark of Alstonia scholaris (L.) R. Brown (Makabeo et al. 2005).
The genus Aspidosperma is known to accumulate a wide variety of indole alkaloids including those with quebrachamine and aspidospermane skeletons (Deutsch et al. 1994), the latter being particularly prolific in A. pyrifolium Mart. and Aspidosperma megalocarpon Mull. Arg. (Craveiro et al. 1983; Mitaine et al. 1996, 1998). A number of species of Aspidosperma are used in folk medicine to treat fever in general, for example, A. album (Grenand et al. 1987), A. cuspa (Pittier 1978), A. excelsium (Brandão et al. 1985) and A. vargasii (Rutter 1990), whilst others are used specifically against malaria, for example, A. quebracho-blanco (Dominguez 1932; Loizaga and Sagastume 1935) and, in Brazil, A. nitidum (Brandão et al. 1992). The antiplasmodial properties of the aspidospermane indole alkaloids isolated from Bolivian and Colombian species of Aspidosperma have been reported by Mitaine et al. (1996, 1998), and the same authors have determined the in vitro activities of 12 such indole alkaloids against chloroquine-resistant and sensitive strains of P. falciparum (Mitaine-Offer et al. 2002).
Materials and methods
Aspidosperma pyrifolium was collected in October 2001 in São José da Tapera, AL, Brazil, and identified by Dr J.E. de Paula (Universidade de Brasília, Brasília, DF, Brazil). A voucher specimen [number JEP3686 (UB)] was deposited in the herbarium at the Universidade de Brasília.
The dried and powdered stem bark of A. pyrifolium (3 kg) was extracted exhaustively with ethanol (5.5 l) in a Soxhlet apparatus for 72 h, and the methanolic solution was concentrated under reduced pressure to yield 150 g of a brownish viscous liquid. This crude methanol extract was dissolved in methanol (300 ml), water (450 ml) was added, and the resulting mixture partitioned against ethyl acetate (5 × 350 ml). The ethyl acetate layer was concentrated under reduced pressure to yield 91 g of a residue that was retained for further studies, whilst the hydromethanolic phase was lyophilised to furnish 55 g of an alkaloid-rich extract. An aliquot (2.0 g) of this extract was submitted to column chromatography of Sephadex LH-20 and eluted with methanol to afford 43 fractions of 10 ml each. The bulk of the alkaloids were present in fractions 8–14 (as determined by TLC analysis with detection by Draggendorf-reagent), and these were combined and concentrated under vacuum to yield 1.0 g of a crude alkaloid mixture. The alkaloids were separated using a Biotage SP1 Automated Flash Chromatography system (TLC-to-gradient method) employing a Flash40™ + M cartridge eluted with a gradient from dichloromethane to dichloromethane:methanol (1:1) at a flow rate of 40 ml/min. The collected fractions (75 × 19 ml) were analysed by TLC and those containing a single component were combined and concentrated under reduced pressure as follows: fractions 15–25 yielded 100 mg of 2 (viscous mass), fractions 39–47 yielded 150 mg of 3 (viscous mass) and fractions 61–74 yielded 200 mg of 1 (viscous mass).
The structures of compounds 1–3 (Fig. 2) were elucidated from UV (Cary 100 Bio UV–Vis spectrophotometer), IR (Nicolet 380 with ATR), electrospray ionisation MS (Mariner Perspective Biosystems) and HRMS (Micro-TOF, Bruker) data, in conjunction with 1H- and 13C-NMR spectral information determined using Bruker Avance 300 (300 MHz for 1H and 75 MHz for 13C) and Avance 500 (500 MHz for 1H and 125 MHz for 13C) instruments equipped with a 2.5-mm dual or a 5-mm BBi probe head, as appropriate. One- and two-dimensional NMR experiments were performed at a constant temperature of 25 °C with the aid of Bruker software library and modified pulse programs. Analytes were dissolved in 0.5 or 0.2 ml of deuterochloroform, and spectra were calibrated using chloroform (residual non-deuterated solvent) as internal standard.
Results and discussion
The 1H- and 13C-NMR data for compounds 1–3, derived from one- and two-dimensional (DEPT, HSQC, TOCSY and ROESY) experiments, are presented in Table 1. On the basis of this information, compounds 1 and 3 could be identified as the known monoterpenoid indole alkaloids aspidofractinine and 15-demethoxypyrifoline, respectively (Fig. 2). Although the NMR spectra of these compounds have been published previously (Craveiro et al. 1983; Cartier et al. 1989; Dufour et al. 1990), these data were obtained using low-resolution instruments and hence the chemical shift values for the methylene protons and for the carbon atoms could not be assigned unequivocally.
Compound 2 (Fig. 2) presented a molecular formula of C20H25N2O (M + 1) cal.: 309.1961, Ob.: 309.1959 by HRMS and a UV spectrum that was quite similar to those of 1 and 3. The IR spectra of 1–3 were comparable, except that those of 2 and 3 exhibited signals at 1,678 and 1,674 cm−1, respectively, associated with carbonyl groups. The 1H and 13C-NMR spectra of 2 were very similar to those of 1 except for the proton and carbon signals at δ 8.53 s and δ 158.1, respectively (Table 1). Compound 2 was identified as N-formylaspidofractinine. The occurrence of formyl derivatives in this class of alkaloids is already known (Bolzani et al. 1987; Djerassi et al. 1963; Gilbert et al. 1962).
Conclusion
A phytochemical investigation of the alkaloids of A. pyrifolium furnished aspidofractinine (1), 15-demethoxypyrifoline (3) and the new derivative N-formylaspidofractinine (2). Although the NMR data for 1 and 3 have been previously reported, the 1H and 13C have been unambiguously assigned for the first time in the present work. The antiplasmodial activities of these compounds are currently being determined in our laboratories and the results will be presented soon.
References
Berry C (2000) Plasmepsins as antimalarial targets. Curr Opin Drug Discov Devel 3:624–629
Bolzani VS, Serur LM, Matos FJA, Gottlieb OR (1987) Indole alkaloids evolution in Aspidosperma. Biochem Syst Ecol 15:187–200
Botanical Dermatology Database (2006) BoDD index to plant families. http://bodd.cf.ac.uk/Indexes/PlantFamilies.html (last accessed August 2006)
Brandão M, Botelho M, Krettli E (1985) Antimalarial experimental chemotherapy using natural products. Cienc Cult 37:1152–1163
Brandão M, Grandi T, Rocha E et al (1992) Survey of medicinal plants used as antimalarials in the Amazon. J Ethnopharmacol 36:175–182
Breman JG (2001) The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg 64:1–11
Cartier D, Ouahrani M, Lévy J (1989) Cyclization of oxindolic methylketones with acid: a rapid synthesis of (±)-aspidofractinine. Tetrahedron Lett 30:1951–1954
Conte LA (1996) Shaman pharmaceuticals’ approach to drug development. In: Balick MJ, Elisabetsky E, Laird AS (eds) Medicinal resources of the tropical forest-biodiversity and its importance to human health. Columbia University Press, New York
Craveiro AA, Matos FJA, Serur LM (1983) Alkaloids of Aspidosperma pyrifolium. Phytochemistry 22:1526–1528
De Luca V, St Pierre B (2000) The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci 5:168–173
Deutsch HF, Evenson MA, Drescher P et al (1994) Isolation and biological activity of aspidospermine and quebrachamine from an Aspidosperma tree source. J Pharm Biomed Anal 12:1283–1287
Djerassi C, Budzikiewicz H, Owellen RJ et al (1963) Die Massenspektren von Alkaloiden der Refractin-Pleiocarpin-Klasse und die Struktur von Aspidofractinin, einem Nebenalkaloid aus Aspidosperma refractum Mart. Helv Chim Acta 46:743–751
Dominguez JA (1932) Malaria treatment with Aspidosperma quebracho-blanco. Rev Farmacéutica (Buenos Aires) 73:82
Dufour M, Gramain J-C, Husson H-P et al (1990) Total synthesis of indole alkaloids. A new strategy for (±)-19-oxoaspidospermidine and (±)-19-oxoaspidofractinine. J Org Chem 55:5483–5490
Foley M, Tilley L (1997) Quinoline antimalarials: mechanisms of action and resistance. Int J Parasitol 27:231–240
Gilbert B, Ferreira JM, Owellen RJ et al (1962) Mass spectrometry in structural and stereochemical problems. Pyrifoline and refractidine. Tetrahedron Lett 3:59–67
Greenwood BM, Bojang K, Whitty CJM et al (2005) Malaria. Lancet 365:1487–1498
Grenand P, Moretti C, Jacquemin H (1987) Pharmacopées traditionelles en Guyane. Orstom, Paris
Kam T-S, Iek I-H, Choo Y-M (1999) Alkaloids from the stem-bark of Alstonia macrophylla. Phytochemistry 51:839–844
Loizaga NS, Sagastume LC (1935) Malaria treatment with “Quechuol-dominguez”. Sem Méd (Buenos Aires) 2:562–566
Lorence A, Nessler CL (2004) Camptothecin, over four decades of surprising findings. Phytochemistry 65:2735–2749
Makabeo APG, Krohn K, Gehle D et al (2005) Indole alkaloids from the leaves of Philippine Alstonia scholaris. Phytochemistry 66:1158–1162
Mitaine AC, Mesbah K, Petermann C et al (1996) Alkaloids from Aspidosperma species from Bolivia. Planta Med 62:458–461
Mitaine AC, Weniger B, Sauvain M et al (1998) Indole alkaloids from the trunk bark of Aspidosperma megalocarpon. Planta Med 64:487
Mitaine-Offer AC, Sauvain M, Valentin A et al (2002) Antiplasmodial activity of Aspidosperma indole alkaloids. Phytomedicine 9:142–145
Nyame AK, Kawar ZS, Cummings RD (2004) Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch Biochem Biophys 426:182–200
Olliaro PL, Yuthavong Y (1999) An overview of chemotherapeutic targets for antimalarial drug discovery. Pharmacol Ther 81:91–110
Olliaro PL, Nair NK, Sathasivam K et al (2001) Pharmacokinetics of artesunate after single oral administration to rats. BMC Pharmacol 1:1–4
Pittier H (1978) Las plantas usuales de Venezuela. Fundación Eugenio, Mendonza
Pittler MH, Ernst E (1999) Artemether for severe malaria: a meta-analysis of randomized clinical trials. Clin Infect Dis 28:597–601
Ridley RG (2002) Medical need, scientific opportunity and the drive for antimalarial drugs. Nature 415:686–693
Rodriguez E, West JE (1995) International research on biomedicines from the tropical rainforest. Interciencia 20:140–143
Rutter RA (1990) Catalogo de plantas utiles de la Amazonia Peruana. Instituto Linguístico de Verano, Pucallpa, Peru
Winstanley PA (2000) Chemotherapy for falciparum malaria: the armoury, the problems and the prospects. Parasitol Today 16:146–153
Wright CW (2005) Plant derived antimalarial agents: new leads and challenges. Phytochem Rev 4:55–61
Acknowledgements
The authors wish to thank Frederic Meistermann and Benoît Portron (Biotage, France) for their kind help during the Flash Chromatography experiment, Patrick Wehrung (LSMBO, Campus CNRS, Cronenbourg, France) for providing the MS analysis and Roland Graff (SCRMN, ULP, Strasbourg, France) for providing access to the 500 MHz NMR spectrometer. The authors are indebted to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Banco do Nordeste do Brasil (BNB) for financial support in Brazil. One of us (J.X.A-J) wishes to thank CAPES for sponsorship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Araújo, J.X., Antheaume, C., Trindade, R.C.P. et al. Isolation and characterisation of the monoterpenoid indole alkaloids of Aspidosperma pyrifolium . Phytochem Rev 6, 183–188 (2007). https://doi.org/10.1007/s11101-006-9044-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11101-006-9044-y