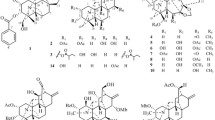
Phytochemical investigation of the flowers of Anthemis odontostephana Boiss. var. odontostephana (Asteraceae) resulted in the isolation of a natural cyclohexenone sesquiterpenoid with a bisabolene skeleton, 1-hydroxydelobanone (1), for the first time as a natural product, and a previously reported cyclohexenone derivative, antheminone A (2), along with two known flavonoids, pectolinaringenin (3), and salvigenin (4). The stereochemistry at positions C-4 and C-5 of the cyclohexenone ring of compound 2 was established based on 1H–1H spin-spin coupling constants and ROESY spectra. Compounds 2 and 3 were reported previously as antileishmanial and cytotoxic agents by other authors. The structures of the compounds were elucidated using NMR spectroscopic methods including 1H NMR, 13C NMR, APT, COSY, HSQC, and HMBC, and mass spectrometry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The genus Anthemis belongs to the Asteraceae family and consists of about 200 species [1] of which 39 are found in Iran, with 15 endemic species showing a rate of endemism of ca. 38% [2]. The most important biological activity of plants of the genus Anthemis is their use in gastrointestinal ailments [3]. Anthemis spp. or their constituents have shown antimicrobial [4–6], anti-inflammatory, sedative [7], and antiprotozoal [8] effects. In Iranian traditional medicine, some Anthemis spp. are regarded as appetizers and as wound healers [3].
The essential oil of A. odontostephana var. odontostephana has been investigated previously, and 47 compounds have been identified of which spathulenol, hexadecanoic acid, and germacrene D were the most abundant compounds [9]. The amounts of the first two compounds reported in the oil of A. odontostephana were more than what have been reported from Anthemis spp. so far [10]. Different solvent extracts of A. odontostephana showed antileishmanial effect against Leishmania promastigotes, among which the hexane extract was the most potent extract [11]. In this paper, we report a bisabolol sesquiterpenoid with cyclohexenone functionality, 1-hydroxydelobanone (1), and its known hydroxylated derivative antheminone A (2), in addition to two flavonoids (3 and 4).
Using a series of normal and reversed-phase (RP) column chromatographies and HPLC purifications, we isolated two sesquiterpene cyclohexenones (1 and 2) and two flavonoids (3 and 4) and elucidated their structures by extensive NMR spectroscopy and mass spectrometry experiments.

The HR-ESI-MS suggested the molecular formula C15H24O3 for compound 1 by virtue of an ion at m/z 252.1733 (calcd 252.1725). The results of the attached proton test (ATP) and the 13C NMR and 1H NMR (Table 1) data agreed with those reported for antheminone A (2) previously isolated from Anthemis maritime [12]. The planar structure of compound 1 was determined by comparing the NMR and MS data with those previously reported [12]. In addition, HSQC and HMBC experiments were performed to correlate the protons to their corresponding carbon atoms and to establish the 1H–13C long-range correlations (Table 1, Fig. 1). The 1H NMR signals at δ 6.75 (1H, dd, J = 1.4, 6.0 Hz, H-3), 4.68 (1H, dd, J = 3.0, 6.0 Hz, H-4), 1.98 (ddd, J = 3.0, 3.7, 13.5 Hz, H-5), 2.87 (dd, J = 13.5, 16.5 Hz, H-6ax), and 2.52 (dd, J = 3.7, 16.5 Hz, H-6eq) are attributed to the cyclohexenone ring spin system by virtue of the 1H–1H coupling constants and 1H–1H COSY cross signals. The position of the exo-methyl group (Me-13) was established at C-2 by the observation of cross peaks between the signal at δH 1.82 (3H, br.d, J = 1.4 Hz, H-13) and the carbonyl signal C-1 (δC 200.4), and the olefinic carbons C-2 (δ 137.5) and C-3 (δ 141.9) in the HMBC spectrum (Fig. 1). The two small coupling constants of JH-4–H-5 = 3.0 Hz and JH-5–H-6eq = 3.7 Hz indicated that the alkenyl side chain at C-5 and the hydroxyl at C-4 are in pseudoequatorial and pseudoaxial orientation, respectively (cis-configuration), while H-5ax and H-6ax are in trans antiparallel orientation, having a large coupling constant JH-5ax–H-6ax = 13.5 Hz. The above conclusion concerning the relative configuration was supported by the observation of cross peaks of H-4 with H-5 and H-3 in the ROESY spectrum (Fig. 1). The two signals of the olefinic methyl groups at δH 1.69 (3H, s, H-12) and 1.61 (3H, s, H-15) and one signal of a methyl group attached to the hydroxylated quaternary carbon atom (C-7) at δ 1.41 (3H, s, H-14) were attributed to an isoprenoid moiety attached to C-5. The HMBC cross peaks confirmed the connectivity of H-14 and C-7 and H-12 and H-15 to C-10 (Fig. 1). Based on 1H–1H-COSY data, the proton signals at δ 1.55 (2H, ddd, J = 1.5, 6.6, 9.2, H-8), 1.95 (2H, m, H-9), and 5.10 (br.t, J = 7.5 Hz, H-10) are connected to each other in a three-spin system.
The connection of the alkenyl side chain to the cyclohexenone ring was confirmed by observation of cross peaks between H-5 (δH 1.98) and C-7 (δ 74.9) in the HMBC spectrum (Fig. 1). From that data, compound 1 was identified to be 1-hydroxydelobanone, a compound reported as a hydrolysis product of 1-acetoxydelobanone [13]. 1-Hydroxydelobanone (1) was found here from Anthemis odontostephana for the first time as a natural product.
Antheminone A (2) is a 13-hydroxy derivative of compound 1 showing two doublets at δ 4.13 (d, J = 14.5 Hz, H-13a) and 4.16 (d, J = 14.5 Hz, H-13b) in the 1H NMR spectrum, while the rest of the signals are similar to those recorded for compound 1 (Table 1) and those reported previously [12]. Although we found similar 1H NMR data for antheminone A (2) (Table 1), as reported by [12], we concluded that the stereocenters C-4 and C-5 have the opposite trans-configuration. The stereochemistry at these positions is confirmed by observing the same spin system at δ 4.78 (1H, dd, J = 3.0, 5.9 Hz, H-4), 2.01 (ddd, J = 3.0, 3.7, 13.5 Hz, H-5), 2.89 (dd, J = 13.5, 17.0 Hz, H-6ax), and 2.55 (dd, J = 3.7, 17.0 Hz, H-6eq) as we already described for compound 1. The configuration proposed here was compatible with that suggested for compound 1 by [13]. The absolute configuration of acetoxydelobanone was determined by ORD [13].
Experimental
General Experimental Procedures. High-performance liquid chromatography (HPLC) was performed on a Waters apparatus equipped with a pump module 600 and a dual wavelength (254, 366 nm) UV detector using a Shimpack Si 20 × 250 mm column.
The 1H and 13C NMR were recorded on a Bruker DRX 500 and a Bruker AV 400 MHz, respectively. CDCl3 and CD3OD were used as a solvent and TMS as an internal standard. Homonuclear 1H–1H connectivity was determined by the COSY experiment. One-bond heteronuclear1H–13C connectivity was determined with the HSQC experiment. Through-space 1H–1H connectivity was established using ROESY experiments with a mixing time of 300 ms. Two- and three-bond 1H–13C connectivity was determined by gradient 2D HMBC experiments optimized for JH-C = 10 Hz. Electron-impact mass spectra (EI-MS) were recorded on a MasSpec sector field mass spectrometer (Micromass Ltd., Manchester, UK) with a direct insertion of probe.
Silica gels in different particle sizes (70–230 mesh) were used for gravity column chromatography. TLC plates (silica gel 60 GF254 pre-coated plates, Merck) were visualized by UV observation at 254 and 365 nm and by spraying with cerium sulfate-sodium molybdate reagent. All reagent solid materials and solvents were purchased from Merck (Germany).
Plant Material. Aerial parts of A. odontostephana var. odontostephana were collected from Mazraehno Village, Yazd, Iran, in May 2009 at an altitude of 1370 m above sea level. The plant was identified by Prof. Asghar Mosleh-Arani, Department of Agriculture and Natural Resources, Yazd University, Yazd, Iran, and a voucher specimen of the source plant was kept (No. 2411) at the School of Pharmacy, Isfahan, Iran. Flowers were separated, shadow dried, and well grounded.
Extraction and Isolation of Compounds. Grounded flowers (335 g) were extracted with acetone (3 × 3.3 L) with constant stirring for 2 days to render Ext-1 (20.7 g). The acetone extract was suspended in MeOH and kept at –20°C; after 2 days it was chilled filtered, and the filtrate was dried in vacuum to get Ext-2 (10.8 g). The latter extract was eluted on silica gel using EtOAc to obtain Ext-3 (7.5 g), which was then fractionated on RP18 using MeOH–H2O (5:5 to 10:0) to get four fractions (F1–F4). Fraction F2 was then further purified using normal-phase chromatography [silica gel, heptane–EtOAc (6:4 to 3:7)] to get pure compound 1 (257 mg) and a nonpolar fraction that yielded compound 2 (2 mg) after finally purifying by HPLC (silica gel column, CH2Cl2–MeOH, 99:1 to 90:10, flow 10 mL/min). Fraction F3 was further fractionated on a silica gel open column eluted with heptane–EtOAc (8:2 to 3:7, v:v) and then purified by HPLC in the aforementioned conditions to get pure compounds 3 (2 mg) and 4 (13 mg).
Compound 1. Pale yellow liquid. UV quenching and carbonized with CeSO4/Na2MoO4 reagent on TLC. EI-MS m/z 252 [M]+, HR-EI-MS m/z 252.1733 [M]+ (calcd for C15H24O3, 252.1725). For 1H and 13C NMR (500 and 125 MHz, respectively, CDCl3) data, see Table 1.
Compound 2. Pale yellow liquid. For 1H NMR (500 MHz, CDCl3) data, see Table 1.
Pectolinaringenin (3). Pale yellow crystals.1H NMR (400 MHz, CD3OD, δ, ppm, J/Hz): 3.93, 4.08 (each 3H, s, OMe), 6.58 (1H, s, H-8), 6.62 (1H, s, H-3), 7.04 (2H, d, J = 9.0, H-3′, 5′), 7.87 (2H, d, J = 9.0, H-2′, 6′), 13.3 (1H, s, OH).
Salvigenin (4). Pale yellow crystals. MS [M+] 328. 1H NMR (400 MHz, CD3OD, δ, ppm, J/Hz): 3.92, 3.96, 4.00 (each 3H, s, OMe), 6.57 (1H, s, H-8), 6.61 (1H, s, H-3), 7.05 (2H, d, J = 9.0, H-3′, 5′), 7.86 (2H, d, J = 9.0, H-2′, 6′).
References
W. C. Evans, Trease and Evans’ Pharmacognosy, 14 Ed., Saunders, London, 1996.
V. Mozaffarian, A Dictionary of Iranian Plant Names, Farhang Moaser, Tehran, 1996, p. 45.
H. Samsam-Shariat, Collection of Medicinal Plants, Mani, Tehran, 2007.
M. Quarenghi, M. Tereschuk, M. Baigori, and L. Abdala, Fitoterapia, 71, 710 (2000).
I. Uysal, S. Celik, and M. Oldacay, J. App. Sci., 5, 639 (2005).
A. Kurtulmus, T. Fafal, T. Mert, H. Saglam, B. Kivcak, T. Ozturk, B. Demirci, and K. H. C. Baser, Chem. Nat. Compd., 45, 900 (2009).
T. Rossi, M. Melegari, A. Bianchi, A. Albasini, and G. Vampa, Pharmacol. Res. Commun., 20, 71 (1988).
A. Karioti, H. Skaltsa, A. Linden, R. Perozzo, R. Brun, and D. Tasdemir, J. Org. Chem., 72, 8103 (2007).
S. E. Sajjadi, N. Ghassemi, Y. Shokoohinia, and H. Moradi, J. Essent. Oil Bear. Plants, 16, 247 (2013).
N. S. Radulovic, P. D. Blagojevic, B. K. Zlatkovic, and R. M. Palic, J. Chin. Chem. Soc., 56, 642 (2010).
G. Asghari, Res. Pharm. Sci., 7, S800 (2012).
F. Collu, L. Bonsignore, M. Casu, C. Floris, J. Gertsch, and F. Cottiglia, Bioorg. Med. Chem. Lett., 18, 1559 (2008).
K. Takeda, K. Sakurawi, and H. Ishii, Tetrahedron, 27, 6049 (1971).
Acknowledgment
The financial support from Isfahan and Kermanshah Universities of Medical Sciences and Alexander von Humboldt foundation is gratefully acknowledged. We are grateful to Dr. Renate Ellinger and Sybille Lorenz for recording NMR and MS spectra. We are also grateful to Prof. Asghar Mosleh-Arani for identification of plant material.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2015, pp. 427–429.
Rights and permissions
About this article
Cite this article
Shokoohinia, Y., Sajjadi, SE., Jassbi, A.R. et al. Sesquiterpenes and Flavonoids of Anthemis odontostephana var. odontostephana . Chem Nat Compd 51, 491–494 (2015). https://doi.org/10.1007/s10600-015-1322-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-015-1322-8