Abstract
Background
Remimazolam and dexmedetomidine are commonly used as sedatives. However, the effects and safety of remimazolam alone or in combination with dexmedetomidine have not been investigated.
Aim
We sought to investigate the clinical effects of remimazolam alone or in combination with dexmedetomidine in bronchoscopy, and their influence on cognitive function.
Method
Ninety eligible patients who underwent bronchoscopy under intravenous anesthesia were randomly divided into three groups: propofol control, remimazolam, and remimazolam plus dexmedetomidine. The primary outcome was the incidence of perioperative hypoxemia. Secondary outcomes included induction and maintenance doses of remimazolam, hemodynamic variables, scores for modified Observer's Assessment of Alertness/Sedation (MOAA/S), coughing, limb movement, incidence of adverse events, patient satisfaction, bronchoscopist satisfaction, incidence of post-operative cognitive dysfunction (POCD), time to loss of consciousness (LoC), and time to awake.
Results
The incidence of hypoxemia, hypotension, and bronchoscopist satisfaction score were significantly decreased, and time to LoC and time to awake were markedly longer in the remimazolam and remimazolam plus dexmedetomidine groups than in the propofol control group (p < 0.05). The remimazolam group had significantly decreased induction and maintenance doses of remimazolam and a shorter time to LoC than the remimazolam plus dexmedetomidine group (p < 0.05). Scores for coughing, limb movement, MOAA/S, and post-operative patient satisfaction were comparable among the three groups. POCD was not induced in any of the groups.
Conclusion
Remimazolam is safe and effective for painless bronchoscopy, with a low incidence of adverse reactions, and exhibits a good synergistic effect with dexmedetomidine.
Trial Registration
This trial protocol had been registered on Chinese Clinical Trial Registry (ChiCTR2000041435, date: 2020 12 26
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impact statements
-
Research shows that r emimazolam is safe and effective for sedation during bronchoscopy with a low incidence of adverse reactions providing new drug discovery opportunities for painless clinical bronchoscopy.
-
Dexmedetomidine can accelerate the onset of remimazolam action and reduce the induction and maintenance of remimazolam doses , thereby laying a foundation for the combination of remimazolam and dexmedetomidine in painless clinical bronchoscopy .
Introduction
Bronchoscopy has been used as an important diagnostic and therapeutic tool for respiratory disorders and may cause complications such as hypoxia, coughing, and shortness of breath [1]. These complications may interrupt bronchoscopy [2]. Using sedatives during the procedure could alleviate patient discomfort, decrease complications, and improve procedural tolerance [3].
Various sedative options are available for bronchoscopy. Propofol is a conventional intravenous anesthetic agent in bronchoscopy characterized by rapid induction and recovery [4, 5]. However, this can result in respiratory depression and hypotension [6]. Remimazolam, an ultra-short-acting benzodiazepine sedative acting on GABA receptors, is not only as effective as propofol, but also has superior hemodynamic stability [7]. Remimazolam has been successfully used for the induction and maintenance of general anesthesia [8] and in colonoscopy [9]. A prospective, double-blind, randomized trial conducted at 30 US sites suggested that remimazolam is an effective and safe sedative medication during flexible bronchoscopy, characterized by a more rapid onset of action and recovery [10]. Another study indicated that remimazolam protects against lipopolysaccharide-induced endotoxicity and improves the survival of mice [11]. These reports demonstrate the safety and efficacy of remimazolam for procedural sedation and analgesia.
Dexmedetomidine is a selective alpha 2‑agonist which can cause mild respiratory depression because of its sedative and analgesic properties [12]. Dexmedetomidine plus sufentanil can be safely and efficaciously used in children undergoing flexible bronchoscopy [13]. Dexmedetomidine-propofol-fentanyl provides safer and more effective sedation, faster recovery, higher bronchoscopist satisfaction to sedation, and more transient bradycardia in sedative flexible bronchoscopy compared to midazolam-propofol- fentanyl [14]. However, no studies have compared remimazolam alone or in combination with dexmedetomidine for sedation during bronchoscopy.
Our study compared the efficacy, safety, and incidence of post-operative cognitive dysfunction (POCD) of different sedative regimens in patients undergoing bronchoscopy: propofol alone, remimazolam alone, and remimazolam in combination with dexmedetomidine. This study has implications for the selection and use of sedative regimens for bronchoscopy and provides a reference for the rational use of sedatives in clinical practice.
Aim
This study sought to investigate the clinical effects of remimazolam alone or in combination with dexmedetomidine in painless bronchoscopy, and their influence in terms of POCD to provide a reference for rational clinical drug use.
Ethics approval
The trial protocol was registered with the Chinese Clinical Trial Registry (ChiCTR2000041435, date: 2020–12–26). This study was approved by the Ethics Committee of Tai'an Central Hospital [(2020). (14), date: 2020–8–28]. Written informed consent was obtained from all participants.
Method
Overall design
This was a single-center, prospective, randomized controlled clinical trial, including patients receiving elective bronchoscopy in the outpatient bronchoscopy unit of Tai'an Central Hospital from January 2021 to August 2021. Ninety patients were eligible for the trial and were enrolled in the study. Inclusion criteria were patients aged between 18 and 70 years and classified as American Society of Anesthesiologists (ASA) grade I-III [9, 15]. The exclusion criteria were as follows: hypoxemia (SPO2 < 90% at resting state), bradyarrhythmia (HR < 60/min), and hypotension (MAP < 60 mmHg); patients with asthma, chronic obstructive pulmonary diseases, and obstructive sleep apnea syndrome; New York Heart Association (NYHA) classifications ≥ III; pregnancy; any metal health issues and cognitive impairment assessed pre-operatively based on the Mini Mental Examination Scale (MMSE); long-term drug abuse; and intolerance or allergies to experimental drugs in this trial.
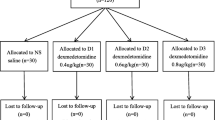
All eligible patients were randomly allocated into three groups by a computer-generated random number table in a sealed envelope: propofol control (C) group, remimazolam (R) group, and remimazolam plus dexmedetomidine (RD) group. Patients were blinded to the grouping, whereas bronchoscopists and investigators were aware of the grouping because propofol and remimazolam have different colors. A flowchart of this trial is shown in Fig. 1.
Anesthesia procedures
All patients fasted for 8 h pre-operatively. In the waiting area, patients received nebulized inhalation of 5 mL of 2% lidocaine, and oxygen inhalation via a mask (4 L/min) and venous channels were established. Five minutes prior to surgery, all patients were intravenously injected with 0.15 μg/kg sufentanil. In group C, the patients were induced with propofol (2 mg/kg), followed by a maintenance dose of 4–6 mg/kg/h. In group R, the patients were treated with 6 mg/kg/h remimazolam (batch number:200211AK, Jiangsu Hengrui Pharmaceutical Co., Ltd., Jiangsu, China), followed by a maintenance dose of 0.6–2 mg/kg/h. For the RD group, 15 min of preoperative intravenous pumping of dexmedetomidine was administrated at a dose of 0.5 μg/kg for 10 min, followed by a dose of 0.5 μg/kg/h until the surgical operation was completed. Remimazolam was administered in the same manner as in group R.
After loss of consciousness (LoC), the nasopharyngeal airway was used, and patients received oxygen inhalation (4 L/min). When the bronchoscope reached the glottis and carina, 2 mL 2% lidocaine was administered. When coughing and body movement were observed, the C group received an intravenous injection of 0.5 mg/kg propofol, while the R and RD groups were administered 0.1 mg/kg remimazolam by intravenous injection. The infusion rates of propofol and remimazolam were spontaneously adjusted. After the bronchoscopic procedure was complete, administration of sedatives was ceased and the patients were transferred to a resuscitation room. All sedation procedures were performed under the supervision of a bronchoscopist.
Two experienced bronchoscopists performed bronchoscopies. Experienced anesthesiologists were responsible for sedation, monitoring, and any necessary interventions. If hypoxemia (SPO2 < 90%) occurred, the oxygen flow rate was increased to 10 L/min, infusion speed of sedatives was decreased, and the breathing bag of the anesthesia machine was squeezed to simulate artificial ventilation. If hypoxemia lasted for > 30 s, these measures were stopped and ventilation via a facial mask was given until SPO2 reached 96%. All data were collected from anesthesia documents and analyzed by investigators who were blinded to the study.
Primary and secondary outcomes
Hypoxemia was defined as an SPO2 value < 90% during the procedure. The primary outcome of the trial was the incidence of perioperative hypoxemia. Secondary outcomes included incidence of POCD; induction and maintenance doses of remimazolam; mean arterial pressure (MAP), heart rate (HR) and SPO2 pre-operatively (T0), at the time of the bronchoscope passing through the vocal cord (T1), 3 min (T2) and 5 min (T3) after T1, at the end of surgery (T4), at the time when patients were awaken successfully (T5), and before patients left the resuscitation room (T6); modified Observer's Assessment of Alertness/Sedation (MOAA/S) Scale score [9, 15] at T1, T2 and T5; patient satisfaction score; bronchoscopist satisfaction score; times of mask ventilation; coughing scores at T1 and T2; time to LoC (time interval from start of drug administration to LoC); time to awake (time interval from the end of administration to awareness); ambulation time (time interval between awareness and leaving the resuscitation room); incidence of intra-operative adverse events, including bradycardia, tachycardia, hypotension, hypertension, nausea, and vomiting.
POCD was assessed using the MMSE scale one day pre-operatively and one day post-operatively. The MMSE scale involves seven aspects: time orientation, place orientation, immediate memory, attention and computation, delayed memory, language, and visual space. There are 30 items in total, one point for each item, and 0 points for incorrect or unknown evaluation outcomes. The total score on the scale ranges from 0 to 30 points. The score is closely related to the level of education, and the standards are as follows: illiteracy > 17, elementary school education > 20, and middle school education and above > 24. Higher scores indicate better cognitive function. An MMSE score < 24 or a decrease from pre-operative to post-operative scores greater than two scores was defined as POCD [16]. Patient satisfaction with the procedure at follow-up (one day post-operative) and bronchoscopist satisfaction with the procedure immediately after surgery were assessed using a satisfaction questionnaire on a 10-point scale by the bronchoscopist. On the 10-point scale, a score of 0 indicated the least satisfaction, whereas a score of 10 indicated the highest [17]. Coughing severity was rated on a 4-point scale: 1, no coughing; 2, slight coughing; 3, moderate coughing; and 4, severe coughing [18].
Sample size estimation
Based on the results of our preliminary experiments, we calculated a sample size in which the incidence of hypoxemia was 35% in group C, 25% in group R, and 20% in the RD group. With a power of 0.95 and alpha error of 0.05, the sample size was calculated to be 85 using G-power software (v.3.1). Considering a drop-out rate of 10%, our study enrolled 95 patients, and 90 sample cases were successfully collected.
Statistical analysis
All statistical analyses were performed using SPSS software (v.25.0). Normally distributed quantitative data are presented as means ± SD, and Student’s t-test or one-way analysis of variance (ANOVA) test followed by Bonferroni’s correction was used to compare data between groups. P < 0.05 was considered to be statistically significant. Enumeration data are reported as percentages (%) and compared between groups using the Chi-square test, adjusted Chi-square test, or Fisher's exact test. In pairwise comparisons, the α segmentation method was used to reduce type I error. Data from the three groups were compared pairwise three times (α = 0.05/3 = 0.017). Therefore, P < 0.017 was considered statistically significant in pairwise comparisons of the enumeration data of the three groups.
Results
This study reviewed 90 patients undergoing bronchoscopy under total intravenous anesthesia, who were randomly allocated to group C (N = 30), group R (N = 30), and group RD (N = 30). The overall demographic and clinical characteristics of the three groups are presented in Table 1. The three groups did not differ significantly in age (P = 0.071), sex (P = 0.351), BMI (P = 0.318), surgery duration (P = 0.382), or ASA grade (P = 1.000). Compared to patients in group C, patients in group R (OR = 0.229, 95% CI = 0.069–0.758, P = 0.012) and group RD (OR = 0.176, 95%CI = 0.049–0.628, P = 0.005) had significantly lower incidences of hypoxemia (Table 1). Similarly, the incidences of simulated artificial ventilation (P = 0.005) and mask ventilation (P = 0.032) were significantly lower in group C than those in groups R and RD (Table 1).
Perioperative hemodynamic variables are shown in Table 2. At T1, T3, and T4, MAP was significantly elevated in groups R and RD compared with that in group C (P < 0.05, Table 2). The HR at T2 and T3 was significantly lower in group RD than in group C (P < 0.05) and at T1, T3, T4, and T5 than in group R (P < 0.05, Table 2). Moreover, SPO2 was significantly lower in groups R and RD than in group C at T1, T2, and T3 (P < 0.05, Table 2).
The adverse events observed in the three groups are shown in Table 3. Groups R (OR = 0.211, 95% CI = 0.070–0.631, P = 0.004) and RD (OR = 0.073, 95% CI = 0.021–0.255, P < 0.001) had markedly lower incidences of hypotension than group C, which agreed with the results obtained for hemodynamic variables. In addition, a significantly higher incidence of tachycardia was observed in group RD (OR = 0.080, 95% CI = 0.009–0.685, P = 0.006) than in group C. Additionally, neither nausea nor vomiting occurred in any patients in the three groups.
Patients in group RD required lower induction and maintenance doses of remimazolam than patients in group R (OR = 0.02, 95% CI = 0.01–0.03, P = 0.002; OR = 0.38, 95% CI = 0.17–0.59, P = 0.001, Table 3). Time to LoC and time to awake were significantly longer in groups R and RD than in group C (P < 0.001). Compared to group C, group RD exhibited a significantly longer ambulation time (P = 0.004), whereas group R had a similar ambulation time (P > 0.05, Table 3). Furthermore, compared with group R, group RD had a significantly prolonged time to LoC and time to awake (P < 0.05). However, differences in ambulation times were not significant between the two groups (P > 0.05).
Table 4 shows that the coughing and limb movement scores at T1 and T2, and the MOAA/S scores at T1, T2, and T5 were comparable among the three groups (P > 0.05). The MMSE scores of the three groups were similar one day pre-operatively and one day post-operatively (P > 0.05, Table 5). POCD was not induced in any of the groups. The difference in patient satisfaction scores was not significant between the three groups one day post-operatively (P > 0.05). Immediately after surgery, groups R and RD had significantly lower bronchoscopist satisfaction scores than group C (8.30 ± 0.84, 8.07 ± 0.91 vs. 9.67 ± 0.71, P < 0.001, Table 5). Moreover, the bronchoscopist satisfaction scores were not significantly different between groups R and RD (P > 0.05, Table 5).
Discussion
Statement of key findings
There exists a consensus that topical anesthesia, analgesics, and sedative agents should be recommended for all patients undergoing bronchoscopy, except in cases of contradictions [19, 20]. Propofol is safe and effective and has been widely used for sedation during bronchoscopy [4, 21]. In the current study, we found that remimazolam alone or in combination with dexmedetomidine obtained similar coughing and limb movement scores at T1 and T2, and similar MOAA/S scores at T1, T2, and T5 points relative to propofol. This suggested that remimazolam had comparable sedative efficacy to that of propofol during bronchoscopy. Consistent with our findings, a multicenter, single-blind, randomized trial reported that remimazolam is well-tolerated and comparable to propofol in terms of efficacy for general anesthesia [22].
Strengths and weaknesses
This study had certain advantages. Remimazolam alone, or in combination with dexmedetomidine, did not cause nausea or vomiting in patients. One day post-operative patient satisfaction scores were not significantly different among the three sedation regimens. Compared with propofol, remimazolam alone or in combination with dexmedetomidine resulted in significantly lower bronchoscopist satisfaction scores. There are three possible reasons for these results. First, dexmedetomidine infusion prior to remimazolam infusion prolongs the waiting time of bronchoscopists. Second, patients receiving remimazolam alone or in combination with dexmedetomidine had a significantly longer time to awake and longer time to ambulation, which consequently reduced the turnover rate because the number of beds in the resuscitation room was limited. Third, bronchoscopists were not blinded to the groupings.
Interpretation
Stable sedation is important, especially for complex and persistent therapeutic endoscopic surgery. Patients’ involuntary movements under sedation can result in surgery-related complications. In addition, the need for higher doses of sedatives to suppress involuntary movements may increase the risk of sedation-related adverse events and prolong the duration of surgery [23]. Lee et al. [24] demonstrated that continuous infusion of propofol during therapeutic endoscopic retrograde cholangiopancreatography has an advantage over intermittent bolus injection in maintaining a constant level of sedation without increasing the frequency of adverse events. Another study explored the pharmacokinetics and pharmacodynamics of remimazolam after continuous infusion and found that remimazolam was characterized by a pharmacokinetic-pharmacodynamic profile with rapid onset, fast recovery, and moderate hemodynamic side effects [25, 26]. Therefore, in our study, we chose continuous infusion of sedatives in order to maintain a constant level of sedation during painless bronchoscopy.
Hypoxemia frequently occurs during flexible bronchoscopy and requires the termination of the bronchoscopy procedure until it is corrected. Previous studies have demonstrated that the incidence of hypoxemia is 30–50% during flexible bronchoscopy with propofol sedation [27, 28]. Similarly, in our study, hypoxemia was reported in 46.7% of patients receiving propofol sedation only. Remimazolam alone or in combination with dexmedetomidine resulted in markedly decreased incidences of hypoxemia, simulated artificial ventilation, and mask ventilation compared with propofol. These observations suggested that remimazolam exerts a milder suppressive effect on the respiratory system than propofol.
Hypotension is a common complication during bronchoscopy using propofol sedation [29, 30]. Remimazolam shows a low incidence of hypotension for the induction and maintenance of general anesthesia, indicating milder circulatory suppression [22]. Consistently, our study found that hypotension was more frequent with propofol than with remimazolam alone or in combination with dexmedetomidine at T1, T3, and T4. The incidences of hypertension and bradycardia were not significantly different between propofol and remimazolam administration.
Dexmedetomidine is an all-in-one drug with sedative, analgesic, anxiolytic, sympatholytic, and opioid-sparing properties, and has been widely applied as an anesthetic adjunct in diverse applications [31]. The onset time of dexmedetomidine is generally 10–15 min, and the peak time is 25–30 min. In clinical practice, we observed that during sedation with dexmedetomidine, the blood pressure of patients generally increased or changed slightly, and the HR did not decrease significantly when the drug load was administered. With the extension of time (after maintaining the dose for more than half an hour), the blood pressure and HR tended to decrease [32, 33], while our experimental operations (painless bronchoscopy) generally ended in approximately 15 min. In this study, the effects of remimazolam on circulation and respiration were much lower than those of propofol. When combined with dexmedetomidine, the dosage of remimazolam was reduced, and the incidence of hypotension in the dexmedetomidine group was lower than that in the propofol group. In our study, although the actual incidence of bradycardia in the dexmedetomidine group was higher than that in the other two groups, the difference was not statistically significant.
Additionally, dexmedetomidine induces less delirium, causes minimal respiratory depression and provides stable hemodynamics [34]. We also found that although remimazolam alone or in combination with dexmedetomidine had a significantly longer time to LoC and time to awake than propofol, the combination with dexmedetomidine led to a shorter time to LoC than remimazolam alone, indicating a more rapid onset of action. Because the half-life of dexmedetomidine during continuous infusion was longer than that of propofol and remimazolam, the waking time and time to leaving the resuscitation room in the dexmedetomidine group were longer than those in the other two groups [26, 33, 34], which could lead to prolonged turnover time and decreased work efficiency, especially in endoscopic rooms of busy grade III, class A, hospitals. Considering that dexmedetomidine (which is inexpensive) can reduce the dosage of remimazolam (an expensive drug) to shorten the onset time, the combination of remimazolam and dexmedetomidine still has clinical significance.
Further research
Our findings suggested that the two drugs described here exhibit a good synergistic effect on sedation during bronchoscopy. Dexmedetomidine improves POCD in elderly patients with colorectal cancer under general anesthesia [35]. However, in our study, POCD was not induced by any of the three sedation regimens. Therefore, further studies are warranted to investigate additional regimens.
Conclusion
Taken together, remimazolam has similar results to propofol in terms of safety and efficacy for sedation during bronchoscopy, without inducing POCD. Although it prolongs time to awake and time to ambulation, dexmedetomidine has a synergistic effect with remimazolam, decreases induction and maintenance doses of remimazolam, and shortens the time to LoC. These findings provide a theoretical basis for remimazolam alone or in combination with dexmedetomidine in painless bronchoscopy.
References
Criner GJ, Eberhardt R, Fernandez-Bussy S, et al. Interventional Bronchoscopy. Am J Respir Crit Care Med. 2020;202(1):29–50.
Nelson ME. Moderate sedation changes for bronchoscopy in 2017. Chest. 2017;152(4):893–7.
McCambridge AJ, Boesch RP, Mullon JJ. Sedation in bronchoscopy: a review. Clin Chest Med. 2018;39(1):65–77.
Wang Z, Hu Z, Dai T. The comparison of propofol and midazolam for bronchoscopy: a meta-analysis of randomized controlled studies. Medicine (Baltimore). 2018;97(36):1–5.
Ho C, Hayes D Jr, Khosravi M, et al. Sedation with propofol for bronchoscopy in cystic fibrosis lung transplant recipients. Lung. 2018;196(4):435–9.
Ferguson I, Bell A, Treston G, et al. Propofol or ketofol for procedural sedation and analgesia in emergency medicine-the POKER study: a randomized double-blind clinical trial. Ann Emerg Med. 2016;68(5):574-82.e1.
Sneyd JR, Rigby-Jones AE. Remimazolam for anaesthesia or sedation. Curr Opin Anaesthesiol. 2020;33(4):506–11.
Doi M, Hirata N, Suzuki T, et al. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020;34(4):491–501.
Rex DK, Bhandari R, Desta T, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018;88(3):427-37.e6.
Pastis NJ, Yarmus LB, Schippers F, et al. Safety and efficacy of remimazolam compared with placebo and midazolam for moderate sedation during bronchoscopy. Chest. 2019;155(1):137–46.
Liu X, Lin S, Zhong Y, et al. Remimazolam protects against lps-induced endotoxicity improving survival of endotoxemia mice. Front Pharmacol. 2021;12: 739603.
Chadha M, Kulshrestha M, Biyani A. Anaesthesia for bronchoscopy. Indian J Anaesth. 2015;59(9):565–73.
Dang X, Hu W, Yang Z, et al. Dexmedetomidine plus sufentanil for pediatric flexible bronchoscopy: a retrospective clinical trial. Oncotarget. 2017;8(25):41256–64.
Wu SH, Lu DV, Hsu CD, et al. The effectiveness of low-dose dexmedetomidine infusion in sedative flexible bronchoscopy: a retrospective analysis. Medicina (Kaunas). 2020;56(4):1–7.
Sharma J, Purohit S, Bhatia S, et al. Awake orotracheal fibre-optic intubation: comparison of two different doses of dexmedetomidine on intubation conditions in patients undergoing cervical spine surgery. Indian J Anaesth. 2017;61(10):811–7.
Gan J, Tu Q, Miao S, et al. Effects of oxycodone applied for patient-controlled analgesia on postoperative cognitive function in elderly patients undergoing total hip arthroplasty: a randomized controlled clinical trial. Aging Clin Exp Res. 2020;32(2):329–37.
Gao Y, Kang K, Liu H, et al. Effect of dexmedetomidine and midazolam for flexible fiberoptic bronchoscopy in intensive care unit patients: a retrospective study. Medicine (Baltimore). 2017;96(25):1–6.
Xue FS, He N, Liao X, et al. Clinical assessment of awake endotracheal intubation using the lightwand technique alone in patients with difficult airways. Chin Med J (Engl). 2009;122(4):408–15.
Wahidi MM, Jain P, Jantz M, et al. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 2011;140(5):1342–50.
Noda N, Hara M, Ise S, et al. Comfort and safety of bronchoscopy performed under sedation and local anesthesia in elderly patients. Medicine (Baltimore). 2020;99(43): e22561.
Maurel V, Legrand M, Bourgeois E, et al. Sedation with remifentanil or propofol for flexible bronchoscopy: a randomised controlled trial. Eur J Anaesthesiol. 2020;37(4):333–4.
Doi M, Morita K, Takeda J, et al. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020;34(4):543–53.
Gralnek IM, Bisschops R, Matharoo M, et al. Guidance for the implementation of a safety checklist for gastrointestinal endoscopic procedures: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Gastroenterology and Endoscopy Nurses and Associates (ESGENA) Position Statement. Endoscopy. 2022;54(2):206–10.
Lee JG, Yoo KS, Byun YJ. Continuous infusion versus intermittent bolus injection of propofol during endoscopic retrograde cholangiopancreatography. Korean J Intern Med. 2020;35(6):1338–45.
Eisenried A, Schüttler J, Lerch M, et al. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part II. Pharmacodynam Electroencephalogram Effects Anesthesiol. 2020;132(4):652–66.
Schüttler J, Eisenried A, Lerch M, et al. Pharmacokinetics and pharmacodynamics of Remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: Part I. Pharmacokinet Clin Pharmacodynam Anesthesiol. 2020;132(4):636–51.
Zha B, Wu Z, Xie P, et al. Supraglottic jet oxygenation and ventilation reduces desaturation during bronchoscopy under moderate to deep sedation with propofol and remifentanil: a randomised controlled clinical trial. Eur J Anaesthesiol. 2021;38(3):294–301.
Zhang H, Fang B, Zhou W. The efficacy of dexmedetomidine-remifentanil versus dexmedetomidine-propofol in children undergoing flexible bronchoscopy: a retrospective trial. Medicine (Baltimore). 2017;96(1):1–6.
Paul M, Rastogi A, Chatterje A, et al. Comparative evaluation of propofol and combination of propofol-dexmedetomidine in adjunct with topical airway anesthesia for rigid bronchoscopy: a randomized double-blinded prospective study. Ann Card Anaesth. 2021;24(1):49–55.
Abulebda K, Abu-Sultaneh S, Ahmed SS, et al. Intensivist-based deep sedation using propofol for pediatric outpatient flexible bronchoscopy. World J Crit Care Med. 2017;6(4):179–84.
Sottas CE, Anderson BJ. Dexmedetomidine: the new all-in-one drug in paediatric anaesthesia? Curr Opin Anaesthesiol. 2017;30(4):441–51.
Li H, Zhang N, Zhang K, et al. Observation of the clinical efficacy of dexmedetomidine in flexible bronchoscopy under general anesthesia: clinical case experience exchange. J Int Med Res. 2019;47(12):6215–22.
Weerink MAS, Struys M, Hannivoort LN, et al. Clinical pharmacokinetics and pharmacodynamics of Dexmedetomidine. Clin Pharmacokinet. 2017;56(8):893–913.
Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72(4):323–30.
Zhang J, Liu G, Zhang F, et al. Analysis of postoperative cognitive dysfunction and influencing factors of dexmedetomidine anesthesia in elderly patients with colorectal cancer. Oncol Lett. 2019;18(3):3058–64.
Acknowledgements
None.
Funding
This study was supported by Scientific Research Foundation of comfort medicine of Shandong Province Medical Association in 2020 (No. YXH2020ZX023).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, S., Wang, T., Cao, L. et al. Clinical effects of remimazolam alone or in combination with dexmedetomidine in patients receiving bronchoscopy and influences on postoperative cognitive function: a randomized-controlled trial. Int J Clin Pharm 45, 137–145 (2023). https://doi.org/10.1007/s11096-022-01487-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-022-01487-4