Abstract
Background Successful deprescribing practices are required to address issues associated with polypharmacy but are hindered by minimal interprofessional collaboration, time constraints, concern for negative outcomes, and absence of a systematic and evidence-based approach. Objective Determine the impact of pharmacist-led deprescribing rounds within a clinical teaching unit (CTU) the number of home medications discontinued upon hospital discharge. Setting Canadian tertiary care hospital. Methods Prospective, dual-arm, interventional study conducted in a single centre, from November 23rd, 2015 to August 30th, 2016. All patients ≥ 19 years old admitted under the CTU were considered for enrolment if on medication(s) prior to admission and patients were excluded if not taking any medications. Study arm allocation alternated daily between the two teams. The control arm operated as per standard whereas the intervention arm’s pharmacist used a deprescribing guide and medication review to identify medications eligible for discontinuation prior to discussing during daily rounds. Discharge documents communicated medication changes to patient and primary healthcare providers. The study was sufficiently powered. Main outcome measure The difference of number of home medications discontinued at discharge between the intervention and control groups. Results 171 and 187 patients were allocated to the intervention and control arms, respectively. No significant differences of baseline characteristics existed between groups. Main outcome measure results showed that deprescribing rounds resulted in significantly more medications deprescribed compared to control (65% vs. 38%; p = 0.001). The rates of readmission and emergency department visits were reduced in the intervention arm. Conclusions Incorporating deprescribing rounds into routine care led to significantly greater discontinuation of medications without increasing rate of emergency department visits or hospital admissions.
Trial registration ISRCTN11751440
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impacts on practice
-
The specialized care of interdisciplinary internal medicine teams in acute care settings can reduce the risk of polypharmacy by optimizing all patient medications, not just those relating to hospital admission.
-
A large deprescribing effect can be seen with a pharmacist-driven intervention in an acute care setting.
-
Patients with medications discontinued by way of deprescribing rounds return home with fewer medicines and stayed off more medications, which may enable cost savings, lower tablet burden, and reduced adverse drug events.
-
Medical learners participating in deprescribing rounds can gain the knowledge and rationale for deprescribing principles and the importance of pharmacist involvement with the process.
Introduction
With an increasing number of clinical practice guidelines, the average number of prescribed medications continues to rise [1, 2]. While it is important to consider the benefits and risks of all prescribed medications, it becomes increasingly important to critically evaluate the benefits and risks in the context of patients with multiple comorbidities. In recent years, the number of medications a patient takes has been identified as the single-most important predictor of harm [3,4,5,6,7,8]. Though the definition of polypharmacy has historically been defined by the number of medications an individual takes, there has been a shift to categorize polypharmacy as appropriate or inappropriate, given that numbers do not comment on the clinical appropriateness of therapies [9]. However, to date, studies have generally identified polypharmacy as a growing concern due to its increasing association with adverse drug events such as impaired cognition, falls, hospitalization, and mortality [10]. Though a global issue, in Canada alone nearly 70% of patients over the age of 65 are taking more than five medications regularly, in which approximately one in five medications may be inappropriate [5, 6, 11]. In general, attempts to reduce inappropriate medication use is challenging due to barriers surrounding lack of interprofessional collaboration, time constraints, concern for negative outcomes after medication cessation, and scarcity of an evidence-based approach to medication discontinuation [3, 12,13,14,15,16]. The same barriers apply to the inpatient setting with the addition of clinicians’ focus on acute medical issues, limited time for follow-up, and lack of collaborative communication between different levels of care. Often, when a patient is admitted to the hospital, the reason for admission and all contributing factors are the primary focus and issues pertaining to polypharmacy are put aside for the patient’s ambulatory care provider(s) to address [10]. In an effort to help relieve the burden of polypharmacy on patients and colleagues in ambulatory care, this trial proposes a way to incorporate the assessment of all patients’ home medications, or medications taken prior to admission in patients’ home setting, rather than just those leading to hospital admission.
With the rising prevalence of polypharmacy, the role of deprescribing has emerged as an intervention within healthcare. Deprescribing is defined as a holistic and encompassing process that re-evaluates the risk-benefit ratio of medications in the context of individualized patient care goals, preferences, and values [3, 6, 17]. A cornerstone of deprescribing is the interprofessional collaboration between pharmacists and physicians. Literature has indicated that this partnership leads to significant reductions in the number of medications, associated costs, and has neutral or positive clinical outcomes in most patients [11, 14, 15, 18, 19].
Though available data illustrates that patient-specific approaches to deprescribing are generally safe, effective, and feasible, there is still absence of a guideline to assist with the implementation of deprescribing into clinicians’ practice [10, 20]. Furthermore, the available deprescribing literature primarily focuses on elderly patients and utilises an approach that may not be as pragmatic in a fast-paced inpatient setting. The methodology of this trial offers an interprofessional approach to incorporate deprescribing as part of routine care for all multimorbid patients on an inpatient ward and helps introduce deprescribing basics to existing and future prescribers.
Aim of study
This study aimed to determine if the number of home medications taken prior to hospital admission would be impacted by inpatient, pharmacist-led, and patient-specific deprescribing rounds and result in a reduction of the number of medications prescribed at hospital discharge.
Ethics approval
The Health Research Ethics Board (HREB) at Island Health approved the study. The authors confirm that all ongoing and related trials for this intervention are registered with ISRCTN (ISRCTN11751440).
Methods
Study design and population
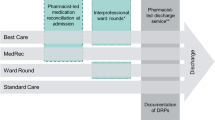
This was a prospective, single centre, unblinded, dual arm interventional study. Enrolled patients were 19 years and older admitted to and discharged from the internal medicine clinical teaching unit (CTU) service at a tertiary care hospital in Victoria, British Columbia between November 23rd, 2015 to July 31st, 2016. Patients were excluded if they were not taking medications prior to admission (i.e. home medications). The medications evaluated were inclusive of all prescription and non-prescription (i.e. over-the-counter) formulations (e.g. topical preparations, tablets) that a patient was taking at home prior to hospital admission. Allocation was through cluster randomization by admission date; allocation to the intervention or control arm was based on which CTU team the patient was admitted under, which alternated between the two teams on a daily basis. Study investigators had no influence on CTU team patient intake (Fig. 1). Patients admitted under the CTU consulting service met the CTU admission requirements, which predated study conceptualization. Each CTU team is comprised of an internist, a senior internal medicine resident, two to three junior resident physicians, two to three medical students, and a clinical pharmacist. The follow-up period for secondary outcomes took place 30 days after discharge from the CTU.
Intervention
Patients allocated to the intervention arm underwent a formal review of all home medications by the clinical pharmacist assigned to that CTU team (Supplemental Material). Home medications are those that the patients were taking at home prior to hospital admission. During the patient’s stay, the intervention pharmacist used a non-standardized deprescribing guide created for the study and medication review to identify medications eligible for discontinuation (Supplemental Material). No additional training was required for the intervention pharmacist to fulfill this role on the CTU team. The evidence-based deprescribing guide was developed by study investigators for the purpose of this study and included common clinical scenarios seen by clinicians on the study site’s CTU. During daily patient care rounds, dedicated time was given to the clinical pharmacist to discuss the patient-specific proposed medication changes with the CTU team. Prior to discontinuation of a medication during the patient’s admission, changes were discussed with the patient to explain the rationale and ensure agreement. Changes to home medications were communicated to both the patient and outpatient healthcare provider as per standard CTU discharge documents.
Control
Patients allocated to the control arm received the same quality of care as the intervention arm. However, the control team’s clinical pharmacist, who is different from the intervention team’s pharmacist, was not given the study’s deprescribing guide nor dedicated time during daily patient care rounds to discuss potential candidates for medication discontinuation with the CTU control team. As the CTU teams operate independently, no specific measures were taken to prevent control group contamination.
Participant follow-up
Patients included in the study who had at least one medication deprescribed were approached to consent to a 30-day post discharge follow-up telephone survey. Using a predetermined survey template created by study investigators, two authors called participants to determine if they remained off the deprescribed medication and to assess the participant’s perceived impact of medication discontinuation. Though the collected survey results were used to quantify the patient’s perceived outcomes, the template allowed for the patient to provide any additional comments not included in the survey answer selection (Supplemental Material).
All study patients were reviewed in Island Health electronic records to assess for emergency department visits or readmission to hospital within 30 days of discharge. The reason for emergency department visits or hospital readmission was not recorded. The participant follow-up period commenced December 23rd, 2015 and continued until August 30th, 2016.
Outcome measures
The primary outcome measure was the number of discontinued home medications at hospital discharge. Home medications may have been discontinued throughout CTU stay however this outcome was summated at discharge. Data was collected using information provided in the standard CTU discharge documents. Secondary outcomes included readmission or emergency department visits within 30 days of discharge, the proportion of medications remaining deprescribed at 30 days after discharge in addition to patient perception of deprescribed medications, and physician impressions of deprescribing rounds. Physician impressions were elucidated through an anonymous, self-administered, web-based questionnaire (Supplemental Material). The questionnaire used to understand physician impressions was not validated.
Statistical analysis
An a priori calculation was performed to determine the required sample size to power the primary objective. A sample size of at least 64 patients in each study arm was required to detect a 20% difference (α = 0.05). Primary outcome results were considered significant if the p value was < 0.05. All patients enrolled allocated to the treatment and control arms were included in the pre-specified analyses and analysed in the groups to which they were allocated. A Levene’s test assessed the variance in baseline demographics between the study groups. Assuming equal variance between groups, a t test was performed to determine the statistical significance of the primary outcome. For the secondary outcomes, a descriptive analysis was performed on categorical and continuous variables to calculate 95% confidence intervals. Statistical analyses were performed by an Island Health Authority statistician with SPSS software using data extracted from REDCap™ (Research Electronic Data Capture).
Results
Participant characteristics
Of the 534 patients admitted to CTU during the study period, 358 were enrolled in the study (Fig. 2). The mean age of enrolled patients was 69 years old, 185 (51.6%) were males, and the mean number of medications prior to admission was 7.5. There were no statistically significant differences between the two groups (Table 1).
Deprescribed medications
Upon hospital discharge, 111 of 171 (0.65, 95% CI 0.58, 0.72) patients had medications deprescribed in the intervention group whereas only 71 of 187 (0.38, 95% CI 0.31, 0.45) control group patients had medications deprescribed (p = 0.001; Table 2). A total of 244 of 1248 (19.6%) home medications were discontinued in patients admitted to the intervention group versus 137 of 1421 (9.6%) in the control group (p = 0.0001; Table 2). Antihypertensives (27.8%), diuretics (20.7%), antithrombotics (8.8%), antilipidemics (4.2%), and benzodiazepines (4.2%) were the most commonly deprescribed groups of medications, comprising just over 65% of all discontinued medications (Supplemental Material).
30-Day follow-up
For patients with medications deprescribed, 30 (27%) in the intervention group and 30 (43%) in the control group were readmitted to hospital or sought medical attention in the emergency department within 30 days of discharge (Table 3). There were similar rates of 30-day hospital readmissions (16% vs. 19%) but fewer rates of emergency department visits between the two groups (11% vs. 24%). Furthermore, there was no statistical difference in readmission rates or emergency department visits between patients in the intervention group with deprescribed medications versus patients without deprescribed medications (16% vs. 12%, 11% vs. 14%).
Of the very few patients who consented to receive and participate in a 30-day follow-up phone call survey, responses were received regarding the 84 deprescribed medications from 41 intervention team patients and 14 deprescribed medications from 9 control team patients; nine patients total from both teams were lost to follow up (Fig. 2, Table 3). Patient responses indicate that a greater percentage of medications remained deprescribed at 30 days in the intervention group compared to the control group (90.3% vs. 73.7%) and the most common reported reason for restarting a deprescribed medication was the primary healthcare provider advised the patient to do so (Supplemental Material). When questioned on their perception of their deprescribed medications, majority of the intervention group reported feeling no different since the home medication was deprescribed but appreciated the reduced tablet burden whereas the control group felt no different or worse (e.g. return of symptoms) since a medication was deprescribed (Supplemental Material).
Physician impression
All 36 clinicians who rotated through the intervention team were invited to complete the self-administered web-based survey at the end of their rotation and the survey was anonymously completed by 22 individuals (Supplemental Material). All respondents thought deprescribing rounds were beneficial and believe that pharmacist involvement is integral to deprescribing. Furthermore, 91% (n = 20) support the notion that deprescribing rounds should be implemented as standard practice on the CTU however 50% (n = 11) did not feel that they learned enough about deprescribing to implement into daily practice.
Discussion
The implementation of pharmacist-driven and patient specific deprescribing rounds on a CTU ultimately led to significantly greater number of home medications deprescribed at discharge. Although the data set was not robust enough to prove clinical significance, trends indicate that despite stopping medications there was no increase in negative outcomes within 30 days of discharge.
There has been a surge of both literature supporting deprescribing practices and task forces offering drug-specific deprescribing guidelines, yet there is still lingering clinician perception of barriers to implementing deprescribing in practice and suspicion to resulting clinical outcomes [3, 7, 8, 12, 15, 16, 18, 21,22,23,24,25]. Reviews compiling clinical outcome data reported similar non-significant outcomes when analyzing the impact of polypharmacy reduction on clinically relevant endpoints and questions the efficacy of deprescribing on the level of polypharmacy or inappropriate medication use during hospitalization [10, 20, 26, 27]. These results may support a hypothesis that polypharmacy may be a marker for poor health in which discontinuation of medications may not be enough to alter trajectory of health [12]. Our trial, however, demonstrates that dedicating time for deprescribing rounds significantly reduces the number of medications a patient is discharged home on and may reduce further hospital visits. Though data showed a trend of reduced hospital readmissions for intervention arm patients with deprescribed medications, we were not able to demonstrate statistical significance in hospital readmission rates. It should be noted however, if we were able to demonstrate a small 1% reduction in readmissions, we estimate a 1.2 million dollars (CAD) cost avoidance annually in our 500 bed hospital.
Despite the growing popularity of deprescribing, evidence indicates that deprescribing is at least as complex as treatment initiation [3, 6]. Like the process of treatment initiation, deprescribing should be viewed as a positive, patient-centred intervention, with inherent uncertainties that requires a shared decision making process, informed consent, and close monitoring [6, 28]. Unlike treatment initiation, however, there is a relative absence of deprescribing-specific guidelines [7, 29]. Despite the lack of guidelines, studies including our own have shown that pharmacist involvement through information collection and distillation can augment rates of medication cessation and the associated reduction of adverse drug reactions (ADRs), medication errors, length of hospital stay, and medication costs [4, 15, 17,18,19, 30]. A key strength of our study is that we implemented a dual-arm comparison between interventional and non-interventional methods in a real-world setting to describe a way for motivated pharmacists and physicians to effectively and safely incorporate deprescribing rounds into their current model of care while instilling the CTU learners with deprescribing basics. While clinical pharmacist involvement assists in the deprescribing process, this study is not commenting on pharmacist practice but is rather supporting the importance of an interprofessional team approach to dedicate time for deprescribing. The benefits of similar pharmacist-physician collaboration will be further elucidated in the Canadian D-PRESCRIBE trial exploring the effectiveness, feasibility, and sustainability of another pharmacist-mediated approach to deprescribing in older community-dwelling adults [31]. Compared to other studies, including the aforementioned trial, ours stands apart from others as it shifted the focus of deprescribing candidates from older adults to adults of any age with any number of medications [10]. Furthermore, it offers a way to use a patient’s hospital stay, where there is a dedicated and specialised medical team and monitoring modalities that are otherwise unavailable in the community setting, to help optimize pharmacologic therapy.
Limitations
While this was a prospective, dual-arm interventional study with objective measures, three of the authors were active members on the intervention team, increasing the likelihood of performance bias. We believe that the involvement of clinicians committed to deprescribing practices, deprescribing champions, helped influence positive intervention outcomes and are integral for successful deprescribing practices. Furthermore, given the variability of clinical pharmacist practice and judgement, this trial can be criticised for differing levels of care provided by the study’s pharmacists. However, it was not feasible or reflective of a real-world clinical setting for the same pharmacist to attend rounds of both teams and we believe that having two different pharmacists helped limit cross-contamination between the two study arms and supported the value of a pharmacist deprescribing champion. Though the study methodology relied on the convenience of an existing allocation method to either clinical teaching team within the CTU, it appeared the two patient groups were well matched. The single centre nature and sample size of this study may limit the external validity and overestimate intervention effects beyond the primary outcome. However, the principles of deprescribing utilized in the study should be applicable to any acute care ward with interdisciplinary collaboration. Given the sample size, we did not proceed with a full economic analysis beyond hospitalization costs but believed hospitalization to be the largest cost driver. Negative outcomes of deprescribing were evaluated within a limited follow-up period of 30 days and yielded low consent and participation rates. As such, the study was underpowered for these outcomes and results of these analyses should be interpreted cautiously given the wide confidence intervals. Though the information would have been useful, due to time constraints, study investigators were unable to collect patient information regarding length of stay on the CTU, qualitative data to determine quality of life and symptom burden and, if applicable, the reason for emergency department visit or hospital readmission with the follow up period. With considerations of the limitations identified in this study, further research encompassing more sites and a larger sample size need to be conducted to explore and qualify the sustainability and long-term implications of deprescribing.
Conclusion
Dedicated time for deprescribing leads to more medications being discontinued without an increase in need for medical attention. Medication-specific monitoring plans, communication with patients and primary care providers, and deprescribing champions are important for successful deprescribing. Further research is needed to assess the impact of deprescribing on long-term clinical outcomes.
References
(CIHI) CI for HI. Drivers of Prescription Drug Spending in Canada—drug_spend_drivers_en.pdf (Internet) (cited 2017 Mar 12). https://www.cihi.ca/en/drug_spend_drivers_en.pdf.
Tinetti ME, Bogardus ST, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. 2004;351(27):2870–4.
Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. Review of deprescribing processes and development of an evidence-based, patient-centred deprescribing process: patient-centred deprescribing process. Br J Clin Pharmacol. 2014;78(4):738–47.
O’Sullivan D, O’Mahony D, O’Connor MN, Gallagher P, Cullinan S, O’Sullivan R, et al. The impact of a structured pharmacist intervention on the appropriateness of prescribing in older hospitalized patients. Drugs Aging. 2014;31(6):471–81.
Frank C. Deprescribing: a new word to guide medication review. CMAJ. 2014;186(6):407–8.
Scott IA, Hilmer SN, Reeve E, Potter K, Le Couteur D, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827.
Thompson W, Farrell B. Deprescribing: what is it and what does the evidence tell us? Can J Hosp Pharm. 2013;66(3):201–2.
Farrell B, Tsang C, Raman-Wilms L, Irving H, Conklin J, Pottie K. What are priorities for deprescribing in elderly patients? capturing the voice of practitioners: a modified Delphi process. PLoS. 2015;10:1–16.
Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE. What is polypharmacy? a systematic review of definitions. BMC Geriatr (Internet). 2017 Oct 10 (cited 2018 May 27);17. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5635569/.
Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis: effect of deprescribing in older adults on mortality and health. Br J Clin Pharmacol. 2016;82(3):583–623.
Vogel L. Network tackles overprescribing. CMAJ. 2016;188(15):1075.
Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386–9.
Schuling J, Gebben H, Veehof LJG, Haaijer-Ruskamp FM. Deprescribing medication in very elderly patients with multimorbidity: the view of Dutch GPs. A qualitative study. BMC Fam Pract (Internet). 2012 Dec (cited 2016 Dec 18);13(1). http://bmcfampract.biomedcentral.com/articles/10.1186/1471-2296-13-56.
Gnjidic D, Couteur DGL, Hilmer SN. Discontinuing drug treatments. BMJ. 2014;21(349):7013.
Gnjidic D, Le Couteur DG, Kouladjian L, Hilmer SN. Deprescribing trials: methods to reduce polypharmacy and the impact on prescribing and clinical outcomes. Clin Geriatr Med. 2012;28(2):237–53.
Howland RH. Deprescribing to reduce medication use: will this help your patient? J Psychosoc Nurs Ment Health Serv. 2016;54(11):21–4.
Reeve E, Wiese MD. Benefits of deprescribing on patients’ adherence to medications. Int J Clin Pharm. 2014;36(1):26–9.
Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428–37.
Anderson K, Freeman C, Rowett D, Burrows J, Scott I, Rigby D. Polypharmacy, deprescribing and shared decision-making in primary care: the role of the accredited pharmacist: the role of the accredited pharmacist. J Pharm Pract Res. 2015;45(4):446–9.
Reeve E, Thompson W, Farrell B. Deprescribing: a narrative review of the evidence and practical recommendations for recognizing opportunities and taking action. Eur J Intern Med. 2017;1(38):3–11.
Harriman K, Howard L, McCracken L. Deprescribing medication for frail elderly patients in nursing homes: a survey of Vancouver family physicians. BCMJ. 2014;56(9):436–41.
Kaur S, Mitchell G, Vitetta L, Roberts MS. Interventions that can reduce inappropriate prescribing in the elderly: a systematic review. Drugs Aging. 2009;26(12):1013–28.
Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J. 2007;9(6):430–4.
Canadian Deprescribing Network (CaDeN) (Internet). Deprescribing.org. (cited 2016 Dec 19). http://deprescribing.org/caden/.
Woodward MC. Deprescribing: achieving better health outcomes for older people through reducing medications. J Pharm Pract Res. 2003;33(4):323–8.
Johansson T, Abuzahra ME, Keller S, Mann E, Faller B, Sommerauer C, et al. Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis: impact of strategies to reduce polypharmacy. Br J Clin Pharmacol. 2016;82(2):532–48.
Alldred DP, Kennedy M-C, Hughes C, Chen TF, Miller P. Interventions to optimise prescribing for older people in care homes. In: The Cochrane Collaboration, editor. Cochrane database of systematic reviews (Internet). Chichester: Wiley; 2016 (cited 2016 Dec 19). http://doi.wiley.com/10.1002/14651858.CD009095.pub3.
Linsky A, Simon SR, Stolzmann K, Meterko M. Patient perceptions of deprescribing (PPoD): survey development and psychometric assessment. Med Care. 2016;55:306–13.
Farrell B, Pottie K, Rojas-Fernandez CH, Bjerre LM, Thompson W, Welch V. Methodology for developing deprescribing guidelines: using evidence and GRADE to guide recommendations for deprescribing. Quinn TJ, editor. PLoS ONE. 2016;11(8):e0161248.
McKean M, Pillans P, Scott IA. A medication review and deprescribing method for hospitalised older patients receiving multiple medications: deprescribing in older patients. Intern Med J. 2016;46(1):35–42.
Martin P, Tamblyn R, Ahmed S, Benedetti A, Tannenbaum C. A consumer-targeted, pharmacist-led, educational intervention to reduce inappropriate medication use in community older adults (D-PRESCRIBE trial): study protocol for a cluster randomized controlled trial. Trials (Internet). 2015 Dec (cited 2016 Dec 19);16(1). http://trialsjournal.biomedcentral.com/articles/10.1186/s13063-015-0791-1.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Authors Rachel Edey, Nicholas Edwards, Jonah Von Sychowski, Ajay Bains, Jim Spence, and Dan Martinusen do not have any conflicts of interest to disclose or financial interests to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Edey, R., Edwards, N., Von Sychowski, J. et al. Impact of deprescribing rounds on discharge prescriptions: an interventional trial. Int J Clin Pharm 41, 159–166 (2019). https://doi.org/10.1007/s11096-018-0753-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0753-2