Abstract
Background Polypharmacy is prevalent among long-term care residents in Canada, with 48.4% receiving ten or more different medications and 40.7% chronically prescribed potentially inappropriate medications. Objective We implemented a pharmacist-administered deprescribing program in a long-term care facility to determine if the number of medications taken per resident could be reduced. Setting: A long-term care facility in Newfoundland and Labrador, Canada from February 2017 to February 2018. Method: Residents were randomized to receive either a deprescribing-focused medication review by a pharmacist or usual care. Main outcome measure Change in the number of medications at 3 and 6 months. Results Forty-five residents enrolled in the study (n = 22 intervention, n = 23 control). Seventy-eight deprescribing recommendations were made, and 85.1% were successfully implemented. The average number of medications taken by residents in the intervention group was 2.68 less than the control group (p < 0.02; 95% CI − 4.284, − 1.071) at 3 months and 2.88 less (p = 0.02, 95% CI − 4.543, − 1.112) at 6 months. In 14.9% of cases, a medication had to be restarted after deprescribing was attempted because symptoms returned. Conclusion: A pharmacist-led deprescribing intervention can reduce the number of unnecessary and potentially harmful medications taken by LTC residents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts on practice
-
Residents in long-term care facilities in Canada are taking some unnecessary and potentially harmful medications that can successfully be deprescribed.
-
Pharmacists can lead deprescribing initiatives in long-term care facility by initiating deprescribing-focused medication reviews and developing plans for implementation.
-
A deprescribing plan can be successfully implemented when developed in consultation with residents and their physician, nursing staff and family, where appropriate.
Introduction
Physiologic and pharmacokinetic changes in older adults increase their risk for drug toxicity, adverse reactions and drug interactions [1]. Certain medications are recognized to be harmful in the elderly and evidence-based guidelines [2, 3] do not support their routine use. Often these medications are started when patients are younger but are not discontinued or reassessed for more appropriate, safer alternatives over time as the patient’s health status changes. Additionally, there is a tendency for prescribers to add medications to treat medical issues rather than switch or discontinue therapy that is not working optimally, especially if the medication was originally initiated by another physician [4]. This resultant polypharmacy is associated with increased risk of adverse health outcomes, including preventable emergency room visits, hospitalizations, and mortality [5, 6].
Polypharmacy is prevalent among LTC residents in Canada, with 48.4% of residents receiving 10 or more different medications [1]. Of greater concern is the use of potentially inappropriate medication (PIM), which contribute to falls, cognitive impairment, hospitalizations and mortality. Among LTC residents, 69.8% received at least one PIM, while 40.7% are chronically prescribed at least one medication from the Beers list of PIMs [1]. Evidence-based algorithms and clinical tools are available to assist health providers in evaluating medication therapies and guiding the process of safe deprescribing, which is the planned tapering, stopping, discontinuing, or withdrawing drugs for the purpose of maintaining or improving health status [2, 3, 7,8,9,10,11,12,13,14,15,16]. However, integrating the act of deprescribing into routine prescribing and medication reordering activities is a challenge in practice. Competing priorities, time constraints, lack of focus on deprescribing specifically at the time of medication renewals or ownership of the deprescribing process may be barriers to a sustainable deprescribing program in LTC facilities. Most evidence for deprescribing has targeted specific drug classes, rather than assessing overall appropriateness of medications for the specific individual [17,18,19,20]. Integrating a deprescribing focus into medication review activities by pharmacists in LTC may help sustain deprescribing assessments in practice.
Randomized-controlled trials (RCT) have investigated deprescribing interventions in frail older people carried out by physicians [6, 8]. This study aims to assess the effectiveness of a collaborative pharmacist-led deprescribing program in LTC.
Aim of the study
The aim of this study was to develop and implement a pharmacist-administered deprescribing program and assess the impact on reducing the number of medications used by LTC residents.
Ethics approval
This study received ethics approval from The Health Research Ethics Authority of Newfoundland & Labrador (HREB 20171187) and is registered with ClinicalTrials.gov (NCT 03097753).
Method
Study design
Residents of a LTC facility were randomized to receive a deprescribing intervention or usual care in a 1:1 ratio in an open trial with a parallel design.
Setting
The LTC facility, located in St. John’s, Newfoundland and Labrador, Canada, is home to approximately 210 residents. This pilot included residents from one floor of the LTC facility which consists of three units, each with 22 residents. Each unit has its own attending physician, all of whom agreed to participate in this project.
Outcomes
The primary outcome of this study was the change in the number of prescribed regular and as-needed (PRN) medications at 3 months and 6 months. Secondary outcomes included changes in patient outcomes such as survival and quality of life.
Recruitment
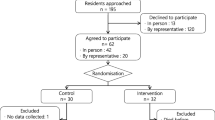
Enrollment took place from February to August 2017. Residents were informed about the study by their nurse. The research assistant (RA) then contacted the resident (or substitute decision-maker) to obtain consent.
Inclusion and exclusion criteria
Residents were eligible to participate if they were 65 years of age or older and resided on the aforementioned floor at the LTC facility. Residents were excluded if they did not take any regular scheduled medications, were palliative, or if the resident/family/care team declined participation.
Control group
Participants were assigned to the control or intervention group using a computer-generated random number sequence. Participants in the control group continued to receive usual care; medications were reviewed and reordered by the physician on a quarterly basis and the pharmacist completed an annual medication review to assess for drug interactions, dose adjustments, lab monitoring and any modifications to therapy required (i.e. not specifically deprescribing-focused), in addition to pharmacist consultation services as required.
Intervention
As the study took place over a time period when senior pharmacy students were completing their final clinical training, the intervention was performed by pharmacy students under the supervision of pharmacists. Participants in the intervention group received an in-depth medication review which focused on identifying medications that were no longer required or potentially harmful as opportunities for deprescribing. All recommendations made by students were approved by pharmacists prior to discussing with the medical team and resident.
Using the medication administration record and medical chart a list of all medications, including the dose and frequency, was generated for each participant. A medication-focused clinical history was compiled from the medical chart, including medical history, progress notes, laboratory and diagnostic test results, and by speaking with the participant and family, ward nurse and attending physician. An indication for each medication was determined based on information in the medical chart and through discussion with the physician. Relevant comorbidities, contraindications and possible side effects were documented. Participants were asked whether they still experienced symptoms that were intended targets of specific treatments. Symptom frequency and severity were recorded for any symptoms reported.
A process for deprescribing was developed based on similar studies [8, 15]. Medications were assessed for ongoing need and appropriateness according to the process algorithm depicted in Fig. 1. Appropriateness was assessed according to evidence-based criteria for medication use in the elderly, and those with an unfavorable risk/benefit ratio were recommended for deprescribing [2, 3]. A step-wise approach was taken to making deprescribing recommendations, with medications causing active harm to the participant identified as highest priority (i.e. contraindicated, toxic with no clear indication, or causing severe adverse effects). Medications unlikely to be of benefit or to cause adverse withdrawal effects were addressed next (e.g. multivitamins in those with adequate nutritional intake, aspirin or statins for primary prevention in older adults), followed by medications with a high potential for adverse withdrawal reaction (e.g. benzodiazepines, antihypertensives). Finally, lower risk medications used for symptom relief were considered for deprescribing if symptoms were controlled. Deprescribing recommendations could include discontinuing a medication, reducing the dosage, or switching to a more appropriate medication considering the participant’s risk factors and comorbidities. Recommendations also included tapering schedules for medications if an adverse withdrawal reaction or disease recurrence was likely. The pharmacist compiled their assessment into a comprehensive, individualized deprescribing plan for each resident, which specified the cessation order, dose tapering schedule, and monitoring plan. This plan was discussed with the resident, their family (where appropriate) and nursing care team and documented on the resident’s chart. The plan was discussed with the physician when they conducted rounds at the facility. When finalized, the deprescribing plan was documented in the resident’s chart and implemented over weeks to months, as appropriate. Medications were normally discontinued one at a time; however, the protocol allowed for up to three medications to be withdrawn simultaneously, provided the medications were unlikely to cause adverse withdrawal effects. The pharmacist/pharmacy student counselled the team (physician, resident, family members, and nursing staff) about potential withdrawal or rebound symptoms before deprescribing was attempted, and if the team was not in support of a medication being stopped, cessation was not attempted. The pharmacist/students reviewed participants weekly to oversee and monitor the deprescribing process and were available for support at the LTC facility Monday–Friday. The deprescribing plan could be halted or temporarily interrupted if the participant experienced discomfort or it was felt in the participant’s interest to do so. Medications could be added to alleviate withdrawal or symptom recurrence if necessary, or the deprescribed medication may be restarted.
Prior to the launch of the study, nursing and support staff of the LTC facility participated in an education session about deprescribing and polypharmacy in older adults provided by pharmacy students. This session presented evidence and facilitated brainstorming amongst the staff about non-pharmacological strategies to manage behaviours and/or withdrawal symptoms when medications were being deprescribed, and foreseeable challenges were discussed.
Data collection
To assess the primary outcome, the number of prescribed regular and PRN medications was determined by reviewing the resident medication administration record at baseline, 3 months and 6 months post-intervention.
Resident Assessment Instrument (RAI) scales for cognitive performance, depression, pain, social engagement, health status, and activities of daily living were used to assess secondary outcomes. RAI scores are measured as part of the Resident Assessment Instrument Minimum Data Set 2.0 (RAI-MDS 2.0) and routinely collected by LTC staff on a quarterly basis. RAI scores before the intervention were compared to the scores at 3 and 6 months.
Statistical methods
We provided descriptive statistics of means and range to describe baseline characteristics of study participants and used a linear regression model to estimate the difference between control and intervention groups in medication use change at 3- and 6-month follow-ups, together with confidence intervals and p-values. Changes in RAI-MDS 2.0 scores were measured using Repeated Measures ANOVA.
Following guidelines for determining sample size for pilot trials (which suggest a flat rule of at least 30 subjects or greater and a minimum of 12 subjects per treatment arm [21]), our sample size of 45 was within the range recommended by the literature.
Results
Sixty-six residents were eligible to be enrolled; 45 consented to participate (n = 22 intervention, n = 23 control, Fig. 2). Participant demographics are described in Table 1. Over the course of the study seven participants died (n = 4 intervention, n = 3 control); however, no deaths were attributed to the intervention. There was no negative impact on quality of life as reflected by changes in any of the RAI scores from baseline to end of study in either group (data not shown; available upon request).
The intervention group experienced a significant reduction in mean number of medications taken per resident at 3 and 6 months. The mean number of medications in the intervention group was 2.68 less than the control group (p < 0.02; 95% CI − 4.284, − 1.071, Fig. 3) at 3 months and 2.88 less (p = 0.02, 95% CI − 4.543, − 1.112, Fig. 3) at 6 months. Changes in medications included both regularly scheduled and PRN medications. The number of medications successfully deprescribed per resident in the intervention group ranged from 0 to 10.
Deprescribing recommendations included dose reduction, discontinuing medication, or switching to a safer agent. A total of 78 deprescribing recommendations were made; 67 recommendations (85.9%) were accepted and 57 (85.1%) were successfully implemented. Deprescribed medications are outlined in Table 2. Most recommendations reflected a lack of ongoing indication (51, 60%) or dosage was too high (10, 11.8%). Reasons for recommendations not being implemented included concern of worsening symptoms/disease, reluctance to discontinue medication prescribed by a specialist, and patient preference to remain on therapy. In 14.9% of cases, medications were restarted after deprescribing was attempted.
Discussion
Our intervention resulted in 78 recommendations made for 22 residents, indicating there is substantial opportunity to deprescribe medications for LTC residents. Most commonly, deprescribing was recommended because the original indication no longer existed, or the dosage was too high. This highlights the importance of regular medication reassessments as residents’ clinical status and medication needs change over time. Residents saw a mean reduction of 2.78 medications without adversely impacting quality of life, suggesting that medications can be safely withdrawn when a collaborative deprescribing plan is implemented.
This pilot study demonstrates how a pharmacist-led, collaborative deprescribing intervention can reduce medication use in LTC. These findings add to existing research supporting the impact that pharmacist-led deprescribing initiatives can have in reducing PIMs in LTC residents [8, 13, 15, 22], providing insight into a Canadian population. A recent meta-analysis of 41 randomized clinical studies showed that deprescribing interventions significantly reduced the number of residents with PIMs, as well as falls and all-cause mortality. They concluded that compared to other deprescribing interventions, medication review-directed deprescribing had significant benefits on older residents in nursing homes [23].
Our deprescribing assessment considered all medications with an aim to reduce any that were no longer indicated or could cause harm. Other studies have focused on deprescribing specific medication classes in the elderly. The DEFEAT-polypharmacy trial targeted anticholinergic and sedative medications through a pharmacist-led intervention [22]. This study showed similar rates of recommendations and acceptance as well as a similar reduction in medications. Due to their larger sample size, they also found a significant reduction in depression scores and frailty scores at 6 months after deprescribing. Our study was underpowered to detect changes in quality of life scores; however, no concerning trends in RAI-MDS scores were observed. This is consistent with other studies which demonstrate no worsening of function when PIMs are carefully withdrawn from elderly patients [6, 8, 20, 22].
There was a low baseline prevalence of antipsychotic and sedative use in our study, though we did successfully deprescribe these medications in five participants in the intervention group. Targeting medications such as anticholinergics, sedatives, antipsychotic and opioids, which contribute to falls and cognitive impairment is a priority in LTC; however, our comprehensive medication assessment approach identified these as well as additional opportunities to reduce PIMs by taking a holistic approach instead of targeting specific drug classes. Antihypertensives were among the most commonly identified medications for deprescribing in this study. Normally, blood pressure is not routinely monitored in LTC unless there is a concern such as headache or falls. However, by reassessing blood pressure as part of the deprescribing assessment, many residents were found to have hypotension and some reported symptoms of dizziness, falls, or low energy that could be antihypertensive-induced. We also identified examples of “deprescribing cascades” through our comprehensive medication reassessment approach. For example, discontinuing calcium supplements in residents with low fracture risk who were immobile or bedridden often led to improved bowel function and permitted subsequent deprescribing of laxatives and stool softeners as well. The holistic medication review approach may explain why we observed a larger reduction in medication use than some other studies.
The mean number of medications was decreased significantly in the intervention group at 3 months and there was a further decrease in medications at 6 months. This is likely due to the staged deprescribing approach and signifies that the residents who discontinued medications tended to stay off them. We expected there might be a temporary increase in PRN medications in the short term to manage rebound symptoms from deprescribing long-term medications (eg. PRN antacid or H2 antagonist use to manage rebound hyperacidity following discontinuing PPI); however, this was not observed and may be attributed to the staff education to promote non-pharmacologic strategies in support of deprescribing plans and a teamwork approach to providing care.
Physicians were highly accepting of the deprescribing recommendations in this study (85.9% acceptance rate). Reasons for not accepting recommendations were consistent with those cited in literature, including off-label use of a medication, concerns about worsening conditions, patient frailty, patient preference to maintain therapy, specialist prescribing therapy, and a previous unsuccessful trial of deprescribing [22, 24]. Sometimes the decision to deprescribe was complex, considering preferences of patients and prescribers and/or the lack of evidence from practice guidelines, in which case we followed a collaborative consensus-based approach.
This study emphasized collaboration and teamwork. The intervention involved a pharmacist-led medication assessment; however, the plan was finalized through consensus with the physician, nursing staff, resident and caregivers as appropriate. This collaborative approach reduced some barriers cited in the literature, including lack of physician time, support or confidence to make deprescribing decisions; lack of awareness of deprescribing opportunities; fear of consequences of deprescribing; and ineffective communication between team members [4, 23]. Furthermore, designating one team member responsible for initiating the deprescribing assessment may support sustainability of a deprescribing program. Understanding the perspectives of residents and their families regarding how best to integrate them into the decision-making process will be important going forward.
The major limitation of this study is our small sample size, which reduced our power to detect differences in quality of life and mortality. Another limitation is the use of RAI-MDS data as a measure of quality of life. RAI-MDS is a useful tool for quality improvement programs and initiatives but evidence for the reliability and validity of these scores remains inconclusive [25]. As these scores were routinely collected by staff prior to this project, we looked for changes in the RAI-MDS scores as a surrogate measure to ensure the intervention was not causing harm. However, change in RAI-MDS scores is not a robust measure and caution should be used when interpreting change.
As the physicians and nursing staff caring for residents in each group were the same and the study was not blinded there could have been a carryover effect from participating in the intervention; however, if anything this would underestimate the effect of the intervention. There were several strengths of this study. The RA who conducted data collection and analysis was blinded to participant allocation to mitigate bias resulting from the open study design. We had no losses to follow up, and introduced a new process within the LTC facility, using resources and processes that were already in place. Although this was a pilot, the RCT design detected a significant difference between groups despite the small sample size.
We demonstrated that a pharmacist-led deprescribing program is effective at decreasing the number of PIMs taken by residents, however, scaling up this program would necessitate more resources to make this standard of care. We relied on pharmacy students to provide medication reviews, as the pharmacist time allocation for clinical services to the LTC facility was insufficient to complete the study in a timely fashion. The significant advantages of a pharmacist-led intervention as compared to a targeted drug class approach including identifying greater deprescribing opportunities using this holistic approach and improved sustainability by granting accountability to a single team member for seeking deprescribing opportunities. An economic evaluation could inform whether increasing pharmacist time to expand this model of care for all LTC residents on a regular basis is worthwhile.
Conclusion
A pharmacist-led deprescribing intervention can reduce the number of unnecessary and potentially harmful medications taken by LTC residents. Further research is warranted to assess the cost-effectiveness of a pharmacist-led deprescribing program in LTC facilities.
References
Canadian Institute for Health Information. Drug use among seniors in Canada, 2016. Ottawa (ON); 2018.
American Geriatrics Society. Updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227–46.
O’Mahony D, Gallagher P, Ryan C, Byrne S, Hamilton H, Barry P, et al. STOPP & START criteria: a new approach to detecting potentially inappropriate prescribing in old age. Eur Geriatr Med. 2010;1(1):45–51.
Anderson K, Stowasser D, Freeman C, Scott I. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ open. 2014;4(12):e006544.
Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13(1):57–655.
Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583–623.
Thompson W, Farrell B. Deprescribing: what is it and what does the evidence tell us? Can J Hosp Pharm. 2013;66(3):201–2.
Potter K, Flicker L, Page A, Etherton-Beer C. Deprescribing in frail older people: a randomised controlled trial. PLoS ONE. 2016;11(3):e0149984.
Farrell B, Pottie K, Thompson W, Boghossian T, Pizzola L, Rashid FJ, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(5):354–64.
Pottie K, Thompson W, Davies S, Grenier J, Sadowski CA, Welch V, et al. Deprescribing benzodiazepine receptor agonists: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(5):339–51.
Farrell B, Black C, Thompson W, McCarthy L, Rojas-Fernandez C, Lochnan H, et al. Deprescribing antihyperglycemic agents in older persons: evidence-based clinical practice guideline. Can Fam Physician. 2017;63(11):832–43.
Bjerre LM, Farrell B, Hogel M, Graham L, Lemay G, McCarthy L, et al. Deprescribing antipsychotics for behavioural and psychological symptoms of dementia and insomnia: evidence-based clinical practice guideline. Can Fam Physician. 2018;64(1):17–27.
Hardy JE, Hilmer SN. Deprescribing in the last year of life. J Pharm Pract Res. 2011;41(2):146–51.
Garfinkel D, Zur-Gil S, Ben-Israel J. The war against polypharmacy: a new cost-effective geriatric-palliative approach for improving drug therapy in disabled elderly people. Isr Med Assoc J. 2007;9(6):430–4.
Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults. Arch Intern Med. 2010;170(18):1648–54.
Scott IA, Hilmer SN, Reeve E, Potter K, Couteur DL, Rigby D, et al. Reducing inappropriate polypharmacy: the process of deprescribing. Jama Intern Med. 2015;175(5):827–34.
Zarowitz BJ, Stebelsky LA, Muma BK, Romain TM, Peterson EL. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25(11):1636–45.
Tamai IY, Rubenstein LZ, Josephson KR, Yamauchi JA, Morand BR, Lukosevicius I. Impact of computerized drug profiles and a consulting pharmacist on outpatient prescribing patterns: a clinical trial. Drug Intel Clin Phar. 1987;21(11):890–5.
Britton ML, Lurvey PL. Impact of medication profile review on prescribing in a general medicine clinic. Am J Health-Syst Pharm. 1991;48(2):265–70.
Blakey SA, Hixson-Wallace JA. Clinical and economic effects of pharmacy services in a geriatric ambulatory clinic. Pharmacotherapy. 2000;20(10):1198–203.
Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2015;25(3):1057–73.
Ailabouni N, Mangin D, Nishtala PS. DEFEAT-polypharmacy: deprescribing anticholinergic and sedative medicines feasibility trial in residential aged care facilities. Int J Clin Pharm-Net. 2019;41(1):167–78.
Kua C-H, Mak VSL, Lee SWH. Health outcomes of deprescribing interventions among older residents in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc. 2019;20(3):362–372.e11.
Ailabouni NJ, Nishtala PS, Mangin D, Tordoff JM. Challenges and enablers of deprescribing: a general practitioner perspective. PLoS ONE. 2016;11(4):e0151066.
Hutchinson AM, Milke DL, Maisey S, Johnson C, Squires JE, Teare G, et al. The resident assessment instrument-minimum data set 20 quality indicators: a systematic review. BMC Health Serv Res. 2010;10(1):166.
Acknowledgements
Monica Vaters and Allison Power of St. Patrick’s Nursing Home. Katrina Legge of Lawton’s Nursing Home Services. David Snook, Kayla LaCosta, Meiling Liu, Hubert Ajiboye, Kelsey Maidment, and Jillian McKinnis pharmacy students. Dr. Hai Van Nguyen for statistical analysis support.
Funding
This research was funded by Canadian Institutes of Health Research – NL SUPPORT and Eastern Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Balsom, C., Pittman, N., King, R. et al. Impact of a pharmacist-administered deprescribing intervention on nursing home residents: a randomized controlled trial. Int J Clin Pharm 42, 1153–1167 (2020). https://doi.org/10.1007/s11096-020-01073-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-020-01073-6