Abstract
Background Care transitions are risk points for medication discrepancies, especially in the elderly. Objective This study was undertaken to assess prevalence and describe medication reconciliation errors during admission in elderly patients and to analyze associated risk factors. We also evaluate the effect of these errors on the length of hospital stay. Setting General surgery, orthopedics, internal medicines and infectious diseases departments of a 1070-bed Spanish teaching hospital. Method This is a prospective observational study. Patients >65 years and taking ≥5 medications were randomly selected from those admitted to hospital. The pharmacist obtained the best possible medication history based on medical records, medical notes from patients’ previous admissions to hospital, “brown bag” review, community care prescriptions, and comprehensive patient interviews. It was compared to current inpatient prescription to detect unintentional discrepancies (discrepancy with no apparent clinical explanation), which were reported to the physician. When the physician accepted the discrepancy by changing the medication order, it was recorded as a medication reconciliation error and classified by type of error. Several variables were analyzed as possible risk/protective factors. Main outcome measure Is prevalence of medication reconciliation errors at admission. Results Reconciliation was performed on 206 patients. Medication reconciliation errors occurred in 49.5 % (102/206) of patients. 1996 medications were recorded, and 359 had unintentional discrepancies (56.0 % (201/359) medication reconciliation errors). The most common was omission (65.1 %). Identified risk factors were as follows: physician experience, number of pre-admission prescribed medications, and previous surgeries. Computerized order entry system was a protective factor. Conclusion Medication reconciliation errors occur in almost half of the elderly patients at admission, especially omissions. Risk factors were a larger number of previous medications, less physician years of experience, and more previous surgeries. Having a computerized order entry system in the hospital protected against some errors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impacts on practice
-
Admission to hospital of the elderly who take multiple medications is a critical point for patient safety.
-
At admission of elderly to hospital, special attention must be paid to patients with a high number of pre-admission prescribed medications, and a high number of previous surgeries.
-
The experience of the physician is a critical factor in obtaining a complete medication history of elderly admitted to hospital.
Introduction
One of the major causes of medication errors and other adverse drug events is the lack of communication while patients are transferred across care settings [1]. Transitions during hospital stay (admissions, transfers, and discharges) are potential risk points for acquiring inaccurate information about patients’ medications. Admission medication history is generally used to establish medication regimen during hospitalization. If inaccurate information is used, errors in medication initiation, inadvertent discontinuation, inappropriate route or dose may occur.
Medication reconciliation (MR) is the process of comparing a patient’s medication orders to all of the medications that the patient has been taking. It should be done at every transition of care in which new medications are ordered or existing orders are rewritten [2]. It should be done to provide the appropriate medication to the patient and avoid medication errors such as omissions, duplications, wrong medication initiation, or dosing errors. MR is endorsed by patient safety organizations around the world [3]. Based on evidence for its positive impact, MR has been recently designated as a required organizational practice by hospital accreditation authorities in Canada, the USA, and the UK. Most of MR studies report discrepancy reduction as their primary outcome [4]. However, the actual effect of MR on reducing clinically significant discrepancies in the inpatient setting remains unclear [5].
Major barriers in completing an accurate and complete medication history include factors relating to the patient, the system, and the health care professionals [6]. The degree to which patients can directly provide up-to-date medication histories, the patients’ impaired cognitive status, poor health literacy, and poor language proficiency make it more complicated for them to report all medications they are taking at the time of admission [7]. The availability of electronic medication data sources, computer applications integrated into the computerized physician order entry (CPOE) system, and electronic medical records may facilitate obtaining a more accurate and comprehensive pre-admission medication list. This would make MR process easier [8]. Most studies involve pharmacists in MR because they are skilled interviewers for medication review.
This requires a substantial use of human resources beyond usual care of patients [9]. Given the limited resources, targeting interventions to subsets of high-risk patients has been suggested [4].
Aim of the study
The purpose of this study was to assess the prevalence of medication reconciliation errors (MREs) at hospital admission in elderly patients and analyze the associated risk factors and medications involved. As a secondary objective, the possible impact of MREs on the length of hospital stay has also been evaluated.
Ethics approval
The study was approved by the Hospital Research Ethics Committee. Informed consent was obtained before the patients joined the study.
Method
A prospective observational study was conducted at a 1070 bed tertiary care teaching hospital. This study took place between December 1, 2011 and July 31, 2012. MR data were collected from Monday through Friday by a hospital pharmacist specialist with 4 years of experience who had previously worked on a MR project in the emergency department. Elderly patients over 65 years old who had been taking five or more medications prior to admission were included.
The study excluded patients who were discharged from the hospital within 48 h and patients who were unable to communicate and did not have a caregiver who could be interviewed.
Patients were randomly selected using a computer-generated random number list from among those patients admitted to internal medicine, infectious diseases, orthopedic surgery, and general surgery units.
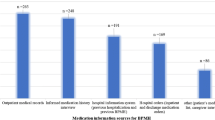
A pharmacist obtained the best possible medication history (BPMH) through several sources: paper-based hospital medical records, the medication list collected at the emergency department, previous reports from other health care settings, and electronic medical records and prescriptions which are used in community care. The BPMH was reviewed by conducting a standardized interview with the patient and/or their caregiver and checking his “brown bag” (medicine containers available at the time of MR process) within the first 24 h after admission. There were no electronic communications between different levels of care (hospital and community care) or different care providers (public or private) because there were not integrated delivery systems, which supposed a technical barrier associated with informational transitions.
The pharmacist used a standardized list of questions to ensure consistency during the interview. He focused on the dosage, brand, frequency, time, and route of administration for all medications taken by the patients prior to admission. Clinical status was consulted on the medical history and documented before the interview in order to know whether patients were able to understand the questions. When interviews with the patients were not possible, their caregivers were interviewed using the same structure. Patients were also asked about their history of adverse drug reactions and allergies. The data collection forms were piloted by a study carried out at emergency department [10].
After the BPMH was verified, the pharmacist compared it with the active inpatient medications ordered by the physician to look for any medication discrepancies. Physicians were not aware about the study.
For the purpose of this study, a consensus document on terminology, classification, and evaluation of MR programs was followed [11]. Medication discrepancies were defined as any change in patients’ chronic pre-admission medications. Those medications prescribed in response to a patient’s change in his clinical status or due to mere formulary substitutions were considered intentional discrepancies (ID). However, when the pharmacist did not find any clinical explanation regarding the discrepancy, it was classified as an unintentional discrepancy (UD). The prescribing physician was then contacted through the CPOE system to clarify UD. All wards had the possibility of using CPOE. If high-alert medications were involved, the doctor was consulted by phone or in person. High-alert medications are medications which bear a heightened risk of causing significant harm to the patient when they are used in error, according to the Institute for Safe Medication Practices (ISMP) [12]. In some cases, the doctor explained the discrepancy and documented the reason in the medical record. In these cases, UD resulted in ID. When the physician decided a prescription order change, those cases were considered MREs. Finally, there were instances where the pharmacist received no final information about the reported UD, as they were not clarified by the physician. Those discrepancies were considered unresolved discrepancies (URD).
MREs were classified according to two different criteria: drug class (following the Anatomical Therapeutic Chemical Classification System) [13] and type of error. Regarding the latter, MREs were classified into seven different types: omission of a pre-admission prescribed medication, mistaken addition of a medication (commission error), different dose, different route, different frequency, different medication, and incomplete prescription. The severity of every error was assessed according to the National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) categories [14]. MREs categorized into groups E, F, G, H, I were considered harmful. Group E, the error may have contributed to or resulted in temporary harm to the patient and required intervention. Group F, the error may have contributed to or resulted in temporary harm to the patient and required initial or prolonged hospitalization. Group G, the error may have contributed to or resulted in permanent patient harm. Group H, the error required intervention necessary to sustain life, and group I, the error may have contributed to or resulted in the patient’s death.
Regarding data collection, a standardized form was designed. This form contained variables relating to the patients’ demographic and clinical information, medication history, active inpatient medications orders, the BPMH, and the discrepancies detected. Variables related to the patient included age, gender, reason for hospital admission, number and type of chronic diseases, number of previous surgeries, drug allergies, patients’ place of residence (their house or a health care facility, such as a nursing home), and length of hospital stay. Other information was also registered in the form: the admitting physician’s experience (specialists or resident physicians), the patients’ location in the hospital (medical or surgical ward), the time required to complete the BPMH, the type of admission (planned or unplanned), the day of admission (working day and non-working day), and the presence or absence of high-alert medications. MR-related variables were the number and type of medication being taken by the patient prior to admission and the number and type of UD, URD, and MRE. Herbal products and natural supplements were not included in our analysis because they were not going to be taken during hospitalization.
Specialist physicians are the doctors who are responsible for supervising, teaching, and training residents and medical students. They practice medicine in the specialty learned during residency and are ultimately responsible for all aspects of patient care. Residents are doctors who are participating in a graduate medical education program and training in a specialized area of medicine.
A descriptive analysis was conducted in relation to the demographic and treatment characteristics. Categorical variables were described and analyzed by frequency. They were compared to each other using χ 2 or Fisher’s exact test. Continuous variables were compared using Student’s t test and nonparametric tests (Mann–Whitney U) for non-normal distributions. Odds ratios (OR) and 95 % confidence intervals (CI) were calculated in a univariate analysis.
In addition, a multivariate analysis was performed using a forward stepwise logistic regression using likelihood ratio test, with p values at 0.10 as the threshold for entering or removing variables. The logistic regression model was drawn up from variables identified by the univariate analysis. Logistic regression was carried out to assess the independent relationship between potential risk factors and the outcome variable (MRE vs. no MRE). The goodness of fit of the final model was assessed using the Hosmer–Lemeshow test. Statistical analysis was carried out using Statistical Package for Social Science (SPSS Inc., Chicago, IL) Version 15.0; a p value < 0.05 was considered as statistically significant.
Results
Overall, 233 patients were included in the study. 22 patients were excluded due to their inability to communicate and the absence of a caregiver. During the study, there were five withdrawals due to a length of hospital stay of less than 48 h (n = 4) or death (n = 1). Therefore, medication history interviews and reconciliation were only performed on 206 patients.
Baseline demographic and clinical characteristics of the population are summarized in Table 1.
The mean number of medications per patient before admission was 8.4 ± 3.0 (range 5–21), and the mean number of medications prescribed by physicians in the hospital was 9.7 ± 3.0 (range 1–20), consistent with the numerous diseases associated with the patients.
There were 1996 medications pertaining to the patients’ BPMHs. 1686 of them had discrepancies. A total of 2054 discrepancies were detected (some medications had more than one discrepancy), with a mean of 8.6 ± 4.0 per patient. Once discussed with the physician, the results of the MR process are shown in Fig. 1.
The majority of discrepancies were documented in medical records as intentional (ID) by the physician. They were meanly due to the inclusion of a medication that the patient had not used prior to admission (56.7 %) or the suspension of medication in response to the patient’s clinical status (27.2 %).
One hundred forty-five patients (70.4 %) had at least one UD, with 1.7 ± 1.7 discrepancies per patient (range 1–10). Almost one-quarter of the UD were due to intentional changes that had not been documented in the medical notes. More than half of UD were MRE. They occurred in 49.5 % (IC 85 %: 42.5–56.5 %) of patients, mean of 1.0 ± 1.4 errors per patient. MREs affected 9.8 % of prescribed medications.
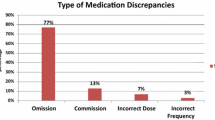
The most frequent MREs were the omission of a drug (65.1 %), followed by different dose (14.4 %), wrong drug (9.5 %), incomplete prescription (6.0 %), and wrong frequency (5.0 %). The most common medication groups involved in MREs were cardiovascular (34.3 %), central nervous system (22.9 %), gastrointestinal (14.9 %), and sensory organs agents (10.9 %). Drugs most often involved were simvastatin (7.1 %), lorazepam (4.1 %), allopurinol (3.1 %), and carvedilol (3.1 %). High-alert medications as defined by ISMP were involved in 3.1 % of MREs.
Only 13 (6.5 %) MREs resulted in or contributed to harm to the patient. The rest of them were categorized as A (0.5 %), B (28.9 %), C (52.2 %), and D (11.9 %).
According to the univariate analysis, the number of pre-admission prescribed medications, the number of previous surgeries, and the number of comorbidities could predispose a patient to present MREs (Table 2).
Variables which independently predicted a higher number of MREs in the multivariate logistic model were the number of pre-admission prescribed medications, the number of previous surgeries, and the admitting physician experience. Specialist physicians committed fewer MREs than resident physicians. Moreover, the CPOE system was a protective factor (Table 3).
There was not a significant difference in the number of MREs medical and surgical services had. Regarding the length of hospital stay, we found no statistically significant difference between groups (MRE vs. no MRE) either.
Discussion
Our study showed high prevalence of MREs in elderly patients. Almost half of them were affected. Despite the different definitions and methods used, this result is consistent with other studies. Most studies on this issue have corroborated that at least half of patients have had one or more MREs during the reconciliation process [15–18].
However, a recent systematic review by García Ramos and Santolaya Perrin [19] of studies of MREs at admission showed a large difference in MRE prevalence. Other authors have suggested lower percentages; some of them used a retrospective medical chart review design without patient interviews [20, 21]. This methodology could have led to a lower frequency of MREs due to the fact that some medications could not be reported in the medical chart. They could have been accessible through other sources, or obtained during patient interviews. Bukley et al. found higher average errors per patient: 3.5 ± 2.3 (mean ± SD), but they did not explain which kind of patients were those who had more errors [22].
On the other hand, in our study the whole MR process was performed by an experienced pharmacist, which could lead to an improved MRE detection. Reeder et al. [23] reported that pharmacist-obtained medication histories had fewer discrepancies than physician-obtained medication histories. Moreover, low rates of error have been shown when the patient interview was performed by a nurse [24].
Regarding the number of errors per patient, the mean of errors detected was nearly one, similar to previously reported in other studies with aged populations [15]. However, Buckley et al. obtained more MREs per patient [22].
Omission errors were the most frequent ones, which is consistent with previous studies that have also shown that up to 61 % of hospitalized patients had at least one drug omitted from their treatment [15–17]. Other studies indicate percentages between 72 and 82 % [18, 25]. Causes contributing to omissions are the lack of information about outpatient medication lists and incomplete anamnesis and complexity of medication regimens. Lack of information about outpatient medication lists an incomplete anamnesis suggest the need for quality improvement activities that identify gaps in continuity of communication. Incorporating health information exchange to current practice [26] as well as teaching on medication history taking [27] could reduce these kinds of MREs. Finally, studies with the highest percentage of omission errors were performed in palliative centers where patients had an average of 19 medications. This fact could explain the high percentage of omissions and suggests that they are more common as the number of medications increases.
Our findings relating to the most commonly involved drugs in MREs are consistent with those reported in the literature [28]. This is not surprising considering that cardiovascular and central nervous system drugs are the most commonly prescribed in aged populations in the community [29].
Gleason et al. [30] concluded that advanced age and a large number of medications were the only risk factors for MREs at hospital admission. In our study, the number of medications was a risk factor also. We found that polypharmacy increases the risk of error. We also found that the risk of suffering MREs increased by 20 % for each additional drug, and patients with MREs took significantly more medications than those without MREs (9.2 vs. 7.6; p < 0.01). This last finding is also reported by Gleason study (8.2 vs. 6.6). It is of particular concern because polypharmacy is prevalent in elderly patients. It affects more than 60 % of this population at admission [31] due to the progressive aging and greater comorbidity. On the contrary, patient age did not increase the risk of MREs, neither when considered as a continuous variable, nor when categorized into two groups (65–80 and older than 80 years old). It must be noted that we only included elderly patients in our study.
We also concluded that admitting physician's experience and the number of previous surgeries were risk factors. Regarding physician experience, Pippins et al. noted that resident physicians were responsible for more prescriptions with UD than specialist physicians. In their opinion, this suggested that the quality of anamnesis improves with professional experience and that resident physicians spend less time than they should recording medications [32].
On the other hand, we found that the use of CPOE systems was a protective factor as it could improve reconciliation process. These systems have been shown to reduce medication errors, according to the results published by several authors [32, 33]. This is due to the fact that medical orders are always legible and structured, and names of the drugs and dosage have been standardized. However, few researchers have focused on the existence or types of medication errors caused by CPOE such as ignored alerts, orders in the wrong medical records or computer crashes [34]. Regarding the MR process, we detected that the hospital's CPOE system reduced MREs significantly. This result contrasts with the one reported by Lee et al. [35], which states that the existence of discrepancies is not conditioned by the type of prescription (CPOE system, written or mixed). In our study, the CPOE system was linked to the use of other computerized sources of information, which helped to obtain more reliable information about patient treatment. Many hospitals have begun to develop and evaluate systems as well as applications that link electronic medical records (EMR) and CPOE systems in order to facilitate effective MR. Other tasks like documentation of medication changes could also be optimized electronically in order to maintain EMR updated [36]. In this sense, Kwan et al. found that frequent changes in medication regimens were a risk factor for MREs [8]. This finding highlights the importance of maintaining updated records of patients’ medication.
The number of surgical interventions that the patient had undergone prior to hospitalization is associated with an increased risk of MRE in multivariate analysis. Perhaps, it is because these patients have a greater number of more complex diseases and treatments, something that we have not analyzed independently.
Likewise, other authors found that a patient having more than five outpatient visits during the previous year is significantly more likely to have MREs [37]. Patients’ lack of knowledge of their medications and lack of family support may also obstruct the transmission of medical treatment information [7].
Our study supports pharmacist’s role in the process of MR. According to Reeder et al., this is the professional with specific training in pharmacotherapy, suitable for obtaining the BPMH [21]. When the MR is led by pharmacists, it has proven to be one of the most cost-effective strategies to improve patient’ safety [38].
We found that most MREs had no clinical significance, concurs with the data shown by the systematic review of Kwan et al. [9].
This study adds to the current body of knowledge in MR that there are no differences in rates of reconciliation errors between patients admitted to medical or surgical services. Pharmacist intervention in MREs does not affect the length of stay. The computerized physician order entry is a protective factor to prevent reconciliation errors.
One of the future paths of development that we consider important is the creation of a system based on Information Technology to centralize the medication reconciliation. Also, it is vital the analysis of what happens after the hospital discharge in order to know the risk factors to MREs and to determine policies to avoid them.
As a limitation, we had a high rate of discrepancies which were not resolved, and they could be MREs, so it would be interesting to improve communication with the physician in the future in order to solve all discrepancies. Also, physicians did not use the CPOE system to clarify discrepancies. Another limitation of our study—and MR processes in general—is that there is no gold standard for obtaining consistent medication histories from patients. Finally, our study involved a small sample of patients from a single community hospital. Accuracy and reliability of the MR process could be affected due to the fact that was carried out by only one pharmacist. In order to minimize this limitation, in all cases, a clinical interview was done and also standardized methods were used to gather all the information related to the patient treatment.
Conclusion
Our findings suggest that admission to hospital is a critical point for patient safety because MREs occur at this point in almost half of elderly patients, mainly omissions. Predicting factors were the number of pre-admission prescribed medications, the number of previous surgeries, and the admitting physician experience. Our findings stress the importance of a complete as well as an accurate and current medication history at admission, especially in elderly patients who take multiple medications. It is also important for resident physicians to better learn how to record medications in order to obtain the BPMH. Promoting the use of CPOE system could decrease MREs.
References
Rozich J, Resar R. Medication safety: one organization’s approach to the challenge. Qual Manag Health Care. 2001;8:27–34.
Using Medication Reconciliation to prevent error. Joint Commission Sentinel Alert, Issue 35 (25 Jan 2006). http://www.jointcommission.org/sentinel_event_alert_issue_35_using_medication_reconciliation_to_prevent_errors/. Accessed 13 Nov 2015.
World Health organization: High 5s: Action on patient safety. standard operating protocol fact sheet: medication reconciliation. 2006. http://www.who.int/patientsafety/implementation/solutions/high5s/ps_medication_reconcili ation_fs_2010_en.pdf. Accessed 15 April 2015.
Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172:1057–69.
Redmond P, Grimes T, McDonnell R, Boland F, Hughes C, Fahey T. Tackling transitions in patient care: the process of medication reconciliation. Fam Pract. 2013;30(5):483–4.
Kennelty KA, Chewning B, Wise M, Kind A, Roberts T. Kreling D Barriers and facilitators of medication reconciliation processes for recently discharged patients from community pharmacists’ perspectives. Res Social Adm Pharm. 2015;11(4):517–30.
Cumbler E, Wald H, Kutner J. Lack of patient knowledge regarding hospital medications. J Hosp Med. 2010;5(2):83–6.
Kaboli PJ, Fernandes O. Medication reconciliation: moving forward. Arch Intern Med. 2012;172(14):1069–70.
Kwan JL, Lo L, Sampson M, Shojania KG. Medication reconciliation during transitions of care as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158:397–403.
Rodríguez Vargas Silveira B, Delgado E, Bermejo Vicedo T. Estudio prospectivo de conciliación de la medicación al ingreso hospitalario. Aten Farm. 2011;13(5):272–8.
Roure Nuez C, Aznar Saliente T, Delgado Sánchez O, Fuster L, Villar I. Consensus document on terminology and classification of programs medication reconciliation. Mayo ed. Barcelona: Spanish Society of Hospital Pharmacy; 2009.
Institute for Safe Medication Practices: ISMP’s List of High-Alert Medications in acute care sites. http://www.ismp.org/tools/highalertmedications.pdf. Accessed 20 May 2015.
World Health Organization: ATC/DDD index. http://www.whocc.no/atc_ddd_index/. Accessed 25 April 2013.
National Coordinating Council for Medication Error Reporting and Prevention: Medication Error Index, 1996. [update 20 Feb 2001]. http://www.nccmerp.org/types-medication-errors. Accessed 20 May 2013.
Delgado O, Nicolás J, Martínez I, Serrano Fabiá A, Anoz Jiménez L, Fernández Cortés F. Reconciliation errors at admission and departure in old and polymedicated patients. Prospective, multicenter randomized study. Med Clin. 2009;133:741–4.
Vasileff HM, Whitten LE, Pink JA, Goldsworthy SJ, Angley MT. The effect on medication errors of pharmacists charting medication in an emergency department. Pharm World Sci. 2009;31:373–9.
van den Bemt PM, van der Schrieck-de Loos EM, van der Linden C, Theeuwes AM, Pol AG. Dutch CBO WHO High 5 s Study Group. Effect of medication reconciliation on unintentional medication discrepancies in acute hospital admissions of elderly adults: a multicenter study. J Am Geriatr Soc. 2013;61(8):1262–8.
Rentero L, Iniesta C, Urbieta E, Madrigal M, Pérez MD. Causas y factores asociados a los errores de conciliación en servicios médicos y quirúrgicos. Farm Hosp. 2014;38(5):398–404.
García Ramos SE. Santolaya Perrin R Medication reconciliation at hospital admission. Aten Farm. 2012;14:7–17.
Unroe KT, Pfeiffenberger T, Riegelhaupt S, et al. Inpatient medication reconciliation at admission and discharge: a retrospective cohort study of age and other risk factors for medication discrepancies. Am J Geriatr Pharmacother. 2010;8:115–26.
Manias E, Gerdtz MF, Weiland TJ, Collins M. Medication use across transition points from the emergency department: identifying factors associated with medication discrepancies. Ann Pharmacother. 2009;43:1755–64.
Buckley MS, Harinstein LM, Clark KB, Smithburger PL, Eckhardt DJ, Alexander E, et al. Impact of a clinical pharmacy admission medication reconciliation program on medication errors in “high-risk” patients. Ann Pharmacother. 2013;47:1599–610.
Reeder TA, Mutnick A. Pharmacist- versus physician-obtained medication histories. Am J Health Syst Pharm. 2008;65:857–60.
Zoni AC, Durán-García ME, Jiménez-Muñoz AB, Salomón Pérez R, Martin P, Herranz Alonso A. The impact of medication reconciliation program at admission in an internal medicine department. Eur J Intern Med. 2012;23:696–700.
Kemp LO, Narula P, McPherson ML, Zuckerman I. Medication reconciliation in hospice: a pilot study. Am J Hosp Palliat Care. 2009;26:193–9.
Kern LM, Wilcox A, Shapiro J, Dhopeshwarkar RV, Kaushal R. Which components of health information technology will drive financial value? Am J Manag Care. 2012;18:438–45.
Chan AH, Garratt E, Lawrence B, Turnbull N, Pratapsingh P. Black PN Effect of education on the recording of medicines on admission to hospital. J Gen Intern Med. 2010;25:537–42.
Andreoli L, Alexandra JF, Tesmoingt C, Eerdekens C, Macrez A, Papo T, et al. Medication reconciliation: a prospective study in an internal medicine unit. Drugs Aging. 2014;31(5):387–93.
Garrido-Garrido EM, García-Garrido I, García-López-Durán JC, García-Jiménez F, Ortega-López I, Bueno-Cavanillas A. Study of polymedicated patients over 65 years-old in an urban primary care centre. Rev Calid Asist. 2011;26:90–6.
Gleason KM, McDaniel MR, Feinglass J, Baker DW, Lindquist L, Liss D, et al. Results of the medications at transitions and clinical handoffs (MATCH) study: an analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med. 2010;25:441–7.
Buurman BM, Hoogerduijn JG, de Haan RJ, Abu-Hanna A, Lagaay AM, Verhaar HJ, et al. Geriatric conditions in acutely hospitalized older patients: prevalence and one-year survival and functional decline. PLoS One. 2011;6(11):e26951.
Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23:1414–22.
Shulman R, Singer M, Goldstone J, Bellingan G. Medication errors: a prospective cohort study of hand-written and computerised physician order entry in the intensive care unit. Crit Care. 2005;9:516–21.
Koppel R, Metlay JP, Cohen A, Abaluck B, Localio AR, Kimmel SE, et al. Role of computerized physician order entry systems in facilitating medication errors. JAMA. 2005;293(10):1197–203.
Lee JY, Leblanc K, Fernandes OA, Huh JH, Wong GG, Hamandi B, et al. Medication reconciliation during internal hospital transfer and impact of computerized prescriber order entry. Ann Pharmacother. 2010;44:1887–95.
Lesselroth BJ, Felder RS, Adams SM, Cauthers PD, Dorr DA, Wong GJ, et al. Design and implementation of a medication reconciliation kiosk: the Automated Patient History Intake Device (APHID). J Am Med Inform Assoc. 2009;16(3):300–4.
Osorio SN, Abramson E, Pfoh ER, Edwards A, Schottel H, Kaushal R. Risk factors for unexplained medication discrepancies during transitions in care. Fam Med. 2014;46(8):587–96.
Mekonnen AB, McLachlan AJ, Brien JA. Effectiveness of pharmacist-led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta-analysis. BMJ Open. 2016;6(2):e010003.
Acknowledgments
We would like to thank Justino Rodríguez Vargas, patients, caregivers, physicians, nurses, and pharmacists of the Ramon y Cajal University Health Centre for their collaboration to this study.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Rodríguez Vargas, B., Delgado Silveira, E., Iglesias Peinado, I. et al. Prevalence and risk factors for medication reconciliation errors during hospital admission in elderly patients. Int J Clin Pharm 38, 1164–1171 (2016). https://doi.org/10.1007/s11096-016-0348-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-016-0348-8