Abstract
Background Transition of care on admission to the hospital and between clinical areas are risk points for medication errors. All type of medication errors can be reduced by improving communication at each transition point of care. Objectives This study examines the impact of pharmacist obtained best possible medication histories on medication errors at admission due to unintentional medication discrepancies in older patients. Setting This was a prospective, single-center study conducted in an Internal Medicine Department of a tertiary care teaching hospital in Saudi Arabia. Methods Patients ≥ 65 years with an existing drug therapy on admission were eligible. The best possible medication history taken by the pharmacist from different sources of medication information was compared to the admission medication order to identify and correct unintentional discrepancies. The discrepancies were classified according to the type of errors. An independent multidisciplinary team adjudicated the potential for harm of each type of medication error. Main outcome measure Number and proportion of unintentional medication discrepancies upon admission and associated medication errors. Secondary outcomes included clinical significance and drug classes involved in the discrepancies and risk factors for the occurrence of these discrepancies. Results A total of 375 evaluable patients were identified. Among 375 medication histories, 609 discrepancies were detected of which 226 were recorded as unintentional. 151 patients (42.4%) had ≥ 1 unintended discrepancy. Drug omission (37%) was the most frequent type of error. Nervous system (24.5%), and cardiovascular system (21.2%) were the most common drug classes involved in medication errors. Three-fifths of the UMD had the potential to cause temporary harm with initial or prolonged hospitalization. The number of medications prescribed upon admission (OR 1.32, 95% CI 1.09–1.54, p < 0.034), number of sources consulted for the best possible medication history (OR 1.53, 95% CI 1.38–1.76, p < 0.01) and the completion of medication review process within 24 h (OR 0.89, 95% CI 0.86–0.94, p < 0.03) of the admission were the 3 most significant predictors of the discrepancies. Conclusions In elderly patients, medication histories are often recorded inaccurately by physicians at the time of hospital admission, this creates the potential for medication errors starting at admission. In older adults, best possible medication histories are also useful in detecting drug related pathology or drug–drug interactions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Impacts on Practice
-
Implementation of a medication-error-reducing intervention in older adults in Saudi Arabia is feasible.
-
When combined with relevant patient’s clinical data, best possible medication history obtained at hospital admission may also be useful in the identification of drug-induced disease.
-
Working in multidisciplinary teams and using multiple medication information sources is crucial for the identification and interception of potentially harmful medication errors.
Introduction
The prevention of medication errors has become a major public health concern, especially in elderly patients. In this population, drug-related harm is accountable for significant morbidity and mortality [1]. The untoward medical occurrences with the use of the drug may lead to an increase in the emergency department visits, the risk of ADR-related hospitalization, and increase in length of hospital stay [2, 3]. The consequences of medication-related adverse events among older people include significant costs both in terms of human suffering and economic cost. By reducing medication errors, it is possible to limit and mitigate clinical as well as economic costs associated with adverse drug events [4, 5].
It has been well documented that the transition of care on admission to the hospital and between clinical areas are risk points for medication errors [6, 7]. All type of medication errors can be reduced by improving communication at each transition point of care [8]. To address this problem, medication reconciliation (MR) has been adopted as a standard practice in many developed countries. Several international patient safety organizations such as the World Health Organization (WHO) [9], the Joint Commission International (JCI) [10], and Institute for Health Care Improvement (IHI) [11] acknowledged MR as an important process to improve patient safety by identifying unintentional medication discrepancies (UMD) at transitions of care. A ‘best possible medication history’ (BPMH) is the cornerstone of the medication reconciliation process. It is appropriate to use at least 3 medication information sources, or even more to collect BPMH [9]. There are certain performance indicators of the MR process to assess the degree of implementation and the impact on errors, such as the percentage of patients reconciled within 24 h, and the number of UMD per patient.
In our previous pilot study, we found that MR interventions at hospital admission had a significant impact on reducing medication errors and subsequent potential risk of harm [12]. In fact, our previous study also showed that patients aged ≥ 65 years were at increased risk for UMD. Based on these results, we have implemented pharmacist-led MR in an internal medicine unit of our institute in January 2016, which was before the start of our study. It was implemented by using WHO’s High 5 s project website [13].
Aim of the study
To examine the effect of pharmacist-led MR on the frequency, nature of medication discrepancies and associated medication errors among elderly patients admitted to internal medicine unit of our institute. Our secondary objectives were to identify clinical significance of UMD, drug classes involved in UMD and risk factors associated with UMD.
Ethics approval
The local clinical research ethics committee evaluated the study protocol and granted ethical approval for the conduct of the study and waived the requirement for informed consent for the review of medical records (protocol # KFH-PHY-121).
Method
Study design, settings and Study population
This cross-sectional study was conducted in a 500-bed tertiary care teaching hospital in the Eastern province of Saudi Arabia during the month of December 2016 to February 2017. Patients were selected from internal medicine ward. The hospital internal medicine ward admits approximately 1100 older patients each year. Based on an in-hospital prevalence rate of UMD upon admission of 59% obtained from previous pilot study data [12], with 95% confidence interval and a precision value of 5%, a sample size of 372 participants was calculated. To account for missing or unusable data, the sample size was increased by 10%. The final required sample size was approximately 409 elderly patients. All patients ≥ 65 years of age admitted to internal medicine ward with an existing drug therapy on admission were included in our study. All patients were enrolled consecutively. Patients discharged, transferred to another unit or hospital or deceased before the pharmacist could conduct admission medication reconciliation were excluded.
Medication reconciliation process
Like typical MR process, the process at our institute also consists of four key steps. These steps are as follows: (1) Obtain and document a BPMH Within 24–48 h of patient admission in internal medicine ward, a clinical pharmacist obtains a BPMH and compile a comprehensive list of medicines the patient is currently taking through comprehensive-structured interviews by using multiple sources including a patient/patient’s attendant and/or caregiver interview, outpatient medical records, discharge medication list (if a patient was recently discharged from a hospital), outpatient prescriptions/pharmacy records and patient’s medication list. (2) Document a list of medications prescribed upon admission. (3) Reconcile and document The pharmacists then compared the BPMH with the medication(s) prescribed upon admission. Any difference between medication use at home and the admission medication order was considered to be an admission medication discrepancy. All medication discrepancies were judged for intentional, undocumented intentional, and unintentional discrepancies. A discrepancy could include an omission of medication, modification of dose, frequency and/or route of administration, use of the drug without indication, and therapeutic duplication. Discrepancies that were judged by the pharmacist as unintentional were noted in the patient’s chart. (4) Communicate information with the prescriber and make clinical decisions based on the comparison When discrepancies were found, the pharmacists discussed each case with the primary prescriber/admitting physician, or the referring physician to determine if the discrepancy was intended or unintended. Through this process, all discrepancies were brought to the attention of the prescriber and resolved after discussion with the pharmacist.
Assessment of potential harm associated with unintentional medication discrepancies
Medication errors at admission (MEA) associated with UMD were categorized by using the widely recognized “National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP) index [14]. For rating the potential harm associated with MEA, we have used modified Gleason scale [15]. The modified Gleason scale for potential harm rating also takes patient’s clinical characteristics and the high-alert medications (by the Institute for Safe Medication Practices; ISMP) [16] into account. The errors were grouped into three categories: level 1: no potential harm (NCC MERP category C), level 2: monitoring or intervention potentially required to preclude harm (NCC MERP category D), and level 3: potential harm (NCC MERP categories E and above). A multidisciplinary team of seven members (consisting of two geriatricians, one resident doctors, two clinical pharmacists, one pharmacotherapy specialist, and one academic pharmacologist) agreed upon the potential harm associated with UMD. Ratings of medication errors for their potential harms were initially rated by two study pharmacists, followed by blind, independent review by other members of the team. Inter-rater reliability of harm ratings for three categories of error groups was also analyzed. There was a substantial agreement rate between pharmacist and physician ratings (Cohen’s kappa = 0.78).
Study variables
Quantitative variables include age, number of drugs taken before admission, and the number of drugs prescribed upon admission. Gender of patient, residence status of the patient (institutionalized or non-institutionalized), mode of patient admission to internal medicine unit (transfer from emergency departments or referred from other hospital), patient status for medication interview (eligible and cooperative, eligible but confused, not eligible), time frame for MR completion (≤ 24 h, > 24–48 h, > 48 h), number of medication information sources used to obtain BPMH, duration of the MR process, and the number of UMD per patient were treated as categorical variables. The drugs were classified uniformly by using WHO Anatomical Therapeutic Classification (ATC) [17].
Statistical analysis
Results of categorical variables are presented with frequencies and percentages. The mean and standard deviation are used to report quantitative variables. A number of UMD per patient was taken as a non-parametric variable and reported by a median, and interquartile range (IQR). The comparison of quantitative variables between patients with UMD and without UMD was performed by student t test. Categorical variables were compared by Chi square test. Inter-rater reliability for assessing the potential for UMD to cause patient harm was analyzed by kappa (κ) statistic for multiple raters.
A multivariate logistic regression analysis was carried out to study the factors associated with the presence of at least one UMD. Those variables with statistical significance in the univariate logistic regression analysis were included in multivariate logistic regression analysis. The results of the regression analyses are presented as unadjusted odds ratios (OR) with their 95% confidence intervals (CI). A significance level of p < 0.05 was used for all analysis. Statistical analysis was performed using STATA version 14 (STATA Corp., Texas, USA).
Results
Characteristics of the study population
A total of 409 individuals were enrolled in the study. Thirty-four patients were excluded due to the lack of time to complete the MR process by the pharmacists before patient discharge and some patients were deceased, discharged or transferred before the pharmacist could conduct admission MR. Thus, there were 375 evaluable patients; of these, 61% (n = 229/409) were female and 39% (n = 146/409) were male. The mean (SD) age of the patients was 73 ± 7.71 (CI 95% 72.25–73.75) years. Majority of older adults (86%) were community-dwelling at the time of admission. Eighty-eight percent (n = 330/375) of the patients were admitted through the emergency room while only 12% (n = 45/375) were referred from other hospitals. Seventy percent (n = 300/375) patients were eligible for medication-history interview, of which, 120 patients were found not to be fully cooperative due to underlying medical conditions (confusion, inconsistency, altered consciousness). Characteristics of the study population and variables related to the MR process are shown in Table 1.
Medication reconciliation process
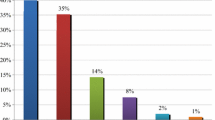
The various sources of information were consulted to obtain BPMH. On average, pharmacist checked 4.57 ± 1.57 (CI 95% 4.41–4.73) medication information source per patient to build the BPMH. As shown in Fig. 1, the outpatient medical records 70% (n = 263/375) was the most common source of information used to obtain BPMH, followed by patient’s interview 66% (n = 248/375), hospital information system (previous hospitalization and previous BPMH) 51% (n = 191/375), hospital orders 45% (n = 169/375), and other sources of information 23% (n = 86/375). In 59% (n = 221/375) of patients, more than three sources of medication information were used to complete BPMHs.
The average number of medication information sources used to build BPMH were significantly higher in the patients with UMD (4.63 ± 1.07 vs. 4.21 ± 1.97; p = 0.044) (Table 1). The average time required by the pharmacist to reconcile a complete list of pre-admission medications was 7.2 ± 3.8 min/patient (range: 4–16 min). The average time spent by a pharmacist to complete MR process (excluding the time delay caused by prescriber communication) was 42 ± 12.64 min/patient (CI 95% 40.72–43.28) (range: 28–54 min). The average number of medications reconciled by the pharmacist at the time of admission was 7.34 ± 4.22 (CI 95% 6.93–7.75) per patient. The mean number of medications prescribed at admission was 6.01 ± 3.37 (CI 5.68–6.34) while the mean number of medications taken by the patient before admission was 5.8 ± 3 (CI 95% 5.5–6.1).
Intentional and unintentional medication discrepancies
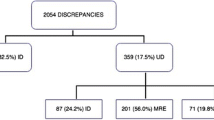
Among 375 BPMH, 609 discrepancies were detected with an average of 1.62 ± 1.54 (95% CI 1.46–1.78) discrepancies per patient. Of 609 discrepancies, 383 were undocumented intentional discrepancies and 226 were recorded as unintentional after consultation with the concerned principal prescriber. Distribution of the number of patients according to the number of UMD recorded are shown in Table 2. One hundred and fifty-nine patients (42.4%; CI 95%, 45.7–61.6%) had at least one UMD with an average of 1.42 ± 1.06 UMD per patient (median: 1, range: 0–7). Finally, the overall rate of UMD was 0.60 per patient. About 71% (161/226) of the unintended prescribing discrepancies were accepted and corrected by the physicians.
Types of errors, potential harm rating, and drug classes involved in unintended medication discrepancies
Among UMD, drug omission (n = 84; 37%) was the most frequent type of “medication error, followed by the discrepant dose (n = 37; 16%), discrepant frequency (n = 39; 17%), drug commission (n = 22; 10%), incorrect drug (n = 25; 11%), and missing dose (n = 19; 8%) (Table 3). Of 226 UMD, 94 (42%) were unlikely to have been harmful to the patient (level 1), 83 (37%) were likely required patient monitoring or intervention to prevent patient harm (level 2) and 49 (22%) had the potential to cause temporary harm with initial or prolonged hospitalization. Different examples of each type of medication errors that were found in the present study are shown in “Appendix”.
With regard to ATC of drugs involved in UMD, the four most common classes involved were nervous system [N] (n = 55; 24.5%), cardiovascular system [C] (n = 48; 21.2%), gastrointestinal system [A] (n = 38; 16.8%) and anti-infectives [J] (n = 27; 12%) (Table 4). More than one third (36.3%) of the UMD that belongs to ATC class N were not likely to be harmful. ATC Class C (cardiovascular) drugs were mostly present in levels 1 and level 2 harm with 41.6% and 47.9%, respectively. More than half of UMD that had the potential to cause temporary harm to the patient were from ATC class A (alimentary tract and metabolism) drugs. Among class J (anti-infectives for systemic use) drugs, 48% and 40% of UMD were rated as level 1 and level 2 risk of potential harm, respectively.
Predictors of unintended medication discrepancies
In univariate analyses, the following covariates were associated with the presence of any discrepancy: number of pre-admission drugs, number drugs prescribed at the time of admission, number of sources consulted to obtain BPMH and time frame in which the MR process completed after patient admission. On multivariate analysis, the number of medications prescribed upon admission (OR 1.32, 95% CI 1.09–1.54, p < 0.034), number of drug information sources consulted for BPMH (OR 1.53, 95% CI 1.38–1.76, p < 0.01) and the completion of MR process within 24 h of the admission (OR 0.89, 95% CI 0.86–0.94, p < 0.03) were the 3 most significant predictors of the discrepancy (Table 5).
Discussion
To the best of our knowledge, this is the first study to investigate UMD exclusively in an elderly patient population in the Middle East region. Accurate medication history is important to avoid a variety of prescribing pitfalls, including excessive doses, drug-disease interactions, and drug–drug interactions at hospital admission.
In the present study, 42% of patients had at least one UMD between physician admission orders and a BPMH, with an average of 0.6 UMD per patient. The average number of UMD per patient reported in the literature varies between 0.62 and 1.2 [8,9,10,11,12]. These differences in the rate of UMD are likely due to the different study designs, studied patient population, differences in a transition point, and study settings. Previous studies performed in different settings of Saudi Arabia reported the similar percentage of UMD at admission [12, 18, 19].
According to WHO Action on Patient Safety (“High 5s”) initiative goal, it is recommended to reconcile the medications within specified time frames (within 24 h of admission) in order to resolve potential problems early in the process [13]. Delaying MR outside 24 h’ time frame in most acute admissions may reduce chances of it occurring at all during patients’ hospital stay. Moreover, it will increase the likelihood of a patient being harmed due to any prescribing errors. In our study, in 81% of patients, MR process was performed within 24 h of the patient admission which is line with the recommendations of various patient safety agencies and represents a good performance index. This is also consistent with previous findings by Rappaport et al. [20] wherein 94% of patients, the MR was performed in the first 24 h of hospitalization. It should, however, also be considered that the majority of those patients, who are admitted and discharged in less than 2 days, are more likely to use their own medicines during admission and less likely to experience significant changes in long-term medication. They are also less likely to be prescribed large numbers of medicines and subsequently intervention from pharmacist [21].
The average time spent by a pharmacist to complete MR process (excluding the time delay caused by prescriber communication) was 42 ± 12.64 min/patient which is comparable to other studies: 48.6 min [22] and 92.2 ± 44.3 min/patient [23]. In geriatrics, the average time for complete MR process (chart review prior to medication history, medication history interview, and necessary interventions/documentation) was expected to be longer, due to the fact that the number of medications generally increases with age.
In the older adult population, obtaining this medication history can be challenging. The BPMH relies heavily on the completeness, accuracy and up-to-date medication information collected from various sources of medication information. In the present study, 68% of patients were able to provide their own medication history and pharmacist accessed 4.57 ± 1.57 sources per patient to build the BPMH and in 59% of patients, more than three sources of medication information were used to complete BPMHs. It is important to use more than one medication information source to collect BPMH because there is not one perfect source. Recently, Stockton et al. [24] reported that clinically relevant medication errors are common in MR process informed by electronic medication dispensing data. Similarly, Pippins et al. [25] reported that relying on family members or caregivers as sources of medication information was a risk factor for drug-related adverse events. A meta-analysis estimated that 27–54% of patients suffer at least one UMD due to medication history errors [26]. Therefore, the availability of a variety of medication information sources and the accuracy of information retrieved at the time of hospital admission is crucial.
In the present study, twenty-two percent of UMD (49/226) were judged to have the potential to cause moderate to severe discomfort or clinical deterioration. Previous studies identified a wide range of UMD, from 14.7% up to 66.2%; at different care transitions that are able to cause potential damage to patients [2, 11, 19, 22, 24]. The wide range of percentage may be due to the methodological differences concerning the definition of UMD and classification of potential harm associated with UMD. However, using the same classification of potential harm, Quélennec et al. [15] reported that 27.2% of UMD could have had a significant clinical impact. Currently, there is no gold method to assess harm associated with medication errors. However, the method we adapted from Quélennec et al. [15] adjudicated the potential harms based on patients’ medical and surgical histories, the motive for hospitalization and biological data. Using clinical information may also help in identification and prevention of potential drug-induced disease or error of commission. In fact, many of the examples of medication errors that are summarized in “Appendix” were potentially harmful when examine with patient’s clinical information.
Like our findings, previous studies have also reported that omission of a drug is the most common type of medication error at the time of hospital admission [12, 18, 24, 27]. We have found that 37% of hospitalized patients have at least one drug omitted from their regimen. While most omissions errors do not result in significant patient harm, however, in some cases patient injury and consequences of associated errors may be more devastating. For instance, our study observed the “omission of enoxaparin in a patient with radiological evidence of pulmonary embolism”. Our findings are not different from those reported by Cortejoso et al. [27] in their prospective study that 30.4% of pharmacist interventions were involved in clinically significant drug–drug or drug-disease interactions that requiring monitoring. In fact, many errors were involved in drug–drug interaction, wrong or discrepant dose, and discrepant frequency.
Failure to verify medication history at admission can result in a medication error with varying degree of harm. The degree of harm involved with medication error associated with UMD could range from “life-threatening” to “no harm”. Moreover, failure to reconcile medications may be compounded by the practice of writing “blanket” orders, such as “resume home medications,” which are highly error-prone and may cause adverse drug events. These blanket orders may also cause potential clinically significant drug–drug interactions with those medications prescribed at admission. In general, most of the drug interaction does not result in significant harm to patients, however, in our study we noticed some clinically significant drug interactions that were likely to have been harmful to the patient or require monitoring to preclude harm (“Appendix”). Moreover, new disease condition or worsening of existing disease conditions may lead to drug-disease interaction. In such cases, resumption of patient’s stable home dose or drug treatment upon hospital admission without careful evaluation could be potentially dangerous. Developing strategies to reduce prescribing errors in older adults is dependent on identifying the key causative factors that lead to these errors. One Dutch study found that prescriber (such as medical specialty e.g. orthopedics) and drug (such as dosage form e.g. inhalation devices) characteristics were the factors most strongly associated with prescribing errors [28].
We found that nearly two-thirds of UMD involved drug belongs to the ATC code N (nervous system), ATC code C (cardiovascular system) or ATC code A (alimentary tract and metabolism). Older people are often prescribed medication from these therapeutic groups. With regard to potential harm associated with the UMD, drugs from ATC code “A” and ATC code “C” were mostly recorded for level 2 and level 3 harm categories. Most of these drugs require close monitoring in elderly and an error involving them that could cause harm to patients. Perhaps, longer interruption (omissions) of these agents may increase the risk of several potential adverse outcomes in older people with comorbidities; however, in older adults, the duration of hospital admission may be difficult to predict. Some evidence suggests that MR interventions may be worthwhile, especially those involving pharmacy or targeting high-risk groups [29]. Nevertheless, a systematic evaluation of MR process is crucial in order to prioritize patients that most benefit from such process.
Multivariable analyses indicated that the odds of experiencing 1 or more medication discrepancies were more in those patients taking a greater number of medications before admission, a greater number of medications prescribed upon admission and number of medication information source used for BPMH. However, some investigators have not found associations between the occurrence of unintended discrepancies and increased number of medications upon admission [30,31,32]. The greater the number of medication information sources used to build BPMH, the greater the likelihood of subsequently finding conflicts between different sources of information. In our study, MR process that completed in “within 24 h of admission” was significantly associated with lower number of discrepancies as these patients are more likely to encounter pharmacist intervention before the significant changes in drug therapy upon admission that may result in UMD.
This study has several important limitations. It was conducted at a single academic hospital on a small sample size of elderly patients aged 65 or older admitted to the internal medicine unit. We may have underestimated the number of medication errors because we were unable to interview patients who were unconscious/severely ill or unwilling to participate and had no caregivers present. Our harm assessment is based on rating ‘potential’ harm determined by our team of experts and the actual harm of errors intercepted are unknown. We are not aware of the effect of this MR process interventions on medical outcomes because the eligible patients were not followed beyond the study.
Conclusions
High prevalence of UMD at the time of hospital admission was found in elderly patients. Nearly three-fifths of the UMD were associated with a potential harm to the patients. The number of medications taking pre and post-admission and a number of medication information sources used to collect BPMHs were identified as a risk factors for UMD in elderly patients. Utilization of a pharmacy-led medication reconciliation program decreased the number of medication errors at hospital admission. Medication reconciliation is crucial in reducing these errors in elderly patients. Future research could explore MR process in older patients at other care transition points, such as inter-hospital transfer and hospital discharge. Cost–benefit analyses from the implementation of medication reconciliation in geriatrics will need also to be investigated.
References
Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta-analysis. Qual Saf Health Care. 2006;15(1):23–31.
Alhawassi TM, Krass I, Pont LG. Antihypertensive-related adverse drug reactions among older hospitalized adults. Int J Clin Pharm. 2018;40(2):428-35.
Marcum ZA, Amuan ME, Hanlon JT, Aspinall SL, Handler SM, Ruby CM, et al. Prevalence of unplanned hospitalizations caused by adverse drug reactions in older veterans. J Am Geriatr Soc. 2012;60(1):34–41.
Sultana Janet, Cutroneo Paola, Trifirò Gianluca. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4(Suppl1):S73–7.
Formica D, Sultana J, Cutroneo PM, Lucchesi S, Angelica R, Crisafulli S, et al. The economic burden of preventable adverse drug reactions: a systematic review of observational studies. Expert Opin Drug Saf. 2018;17(7):681–95.
Weant KA, Bailey AM, Baker SN. Strategies for reducing medication errors in the emergency department. Open Access Emerg Med. 2014;6:45.
Wong JD, Bajcar JM, Wong GG, Alibhai SM, Huh JH, Cesta A, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373–9.
Munday A, Kelly B, Forrester J, Timoney A, McGovern E. Do general practitioners and community pharmacists want information on the reasons for drug therapy changes implemented by secondary care? Br J Gen Pract. 1997;47(422):563–6.
Abdellatif A, Bagian JP, Barajas ER, Cohen M, Cousins D, Denham CR, et al. Assuring medication accuracy at transitions in care. Jt Comm J Qual Patient Saf. 2007;33(7):450–3.
Joint Commission on Accreditation of Healthcare Organizations, USA. Using medication reconciliation to prevent errors. Sentin Event Alert. 2006;23(35):1–4.
Institute for Healthcare Improvement. Medication reconciliation to prevent adverse drug events 2016. http://www.ihi.org/topics/adesmedicationreconciliation/Pages/default.aspx. Accessed 24 Dec 2016.
Mazhar F, Akram S, Al-Osaimi YA, Haider N. Medication reconciliation errors in a tertiary care hospital in Saudi Arabia: admission discrepancies and risk factors. Pharm Pract (Granada). 2017;15(1):864.
Leotsakos A, Zheng H, Croteau R, Loeb JM, Sherman H, Hoffman C, et al. Standardization in patient safety: the WHO High 5s project. Int J Qual Health Care. 2014;26(2):109–16.
National Coordinating Council for Medication Error Reporting and Prevention (NCC MERP), NCC MERP Index for Categorizing Medication Errors. http://www.nccmerp.org. Accessed 1 June 2016.
Quélennec B, Beretz L, Paya D, Blicklé JF, Gourieux B, Andrès E, et al. Potential clinical impact of medication discrepancies at hospital admission. Eur J Intern Med. 2013;24(6):530–5.
Institute for Safe Medication Practices. ISMP’s list of high-alert medications. https://www.ismp.org/tools/highalertmedicationlists.asp (2003). Accessed 18 Jan 2016.
WHO Collaborating Centre for Drug Statistics Methodology. Anatomic therapeutic chemical (ATC) classification index with defined daily doses (DDDs). WHO Collaborating Centre for Drug Statistics Methodology, Oslo, Norway. http://www.whocc.no/atcddd/ (2003). Accessed 5 Jan 2017.
Abdulghani KH, Aseeri MA, Mahmoud A, Abulezz R. The impact of pharmacist-led medication reconciliation during admission at tertiary care hospital. Int J Clin Pharm. 2018;40(1):196–201.
AbuYassin BH, Aljadhey H, Al-Sultan M, Al-Rashed S, Adam M, Bates DW. Accuracy of the medication history at admission to hospital in Saudi Arabia. Saudi Pharm J. 2011;19(4):263–7.
Rappaport R, Arinzon Z, Feldman J, Lotan S, Heffez-Aizenfeld R, Berner Y. The need for medication reconciliation increases with age. Isr Med Assoc J. 2017;19(10):625–30.
Suggett E, Marriott J. Risk factors associated with the requirement for pharmaceutical intervention in the hospital setting: a systematic review of the literature. Drugs Real World Outcomes. 2016;3(3):241–63.
Cadman B, Wright D, Bale A, Barton G, Desborough J, Hammad EA, et al. Pharmacist provided medicines reconciliation within 24 hours of admission and on discharge: a randomised controlled pilot study. BMJ Open. 2017;7(3):e013647. https://doi.org/10.1136/bmjopen-2016-013647.
Meguerditchian AN, Krotneva S, Reidel K, Huang A, Tamblyn R. Medication reconciliation at admission and discharge: a time and motion study. BMC Health Serv Res. 2013;13(1):485.
Stockton KR, Wickham ME, Lai S, Badke K, Dahri K, Villanyi D, et al. Incidence of clinically relevant medication errors in the era of electronically prepopulated medication reconciliation forms: a retrospective chart review. CMAJ Open. 2017;5(2):E345. https://doi.org/10.9778/cmajo.20170023.
Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–22.
Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–5.
Cortejoso L, Dietz RA, Hofmann G, Gosch M, Sattler A. Impact of pharmacist interventions in older patients: a prospective study in a tertiary hospital in Germany. Clin Interv Aging. 2016;11:1343.
Fijn R, Van den Bemt PM, Chow M, De Blaey CJ, De Jong-Van den Berg LT, Brouwers JR. Hospital prescribing errors: epidemiological assessment of predictors. Br J Clin Pharmacol. 2002;53(3):326–31.
Mueller SK, Sponsler KC, Kripalani S, Schnipper JL. Hospital-based medication reconciliation practices: a systematic review. Arch Intern Med. 2012;172(14):1057–69.
Johnston R, Saulnier L, Gould O. Best possible medication history in the emergency department: comparing pharmacy technicians and pharmacists. Can J Hosp Pharm. 2010;63(5):359.
Coleman EA, Smith JD, Raha D, Min S-J. Posthospital medication discrepancies: prevalence and contributing factors. Arch Intern Med. 2005;165(16):1842–7.
Hias J, Van der Linden L, Spriet I, Vanbrabant P, Willems L, Tournoy J, et al. Predictors for unintentional medication reconciliation discrepancies in preadmission medication: a systematic review. Eur J Clin Pharmacol. 2017;73(11):1355–77.
Acknowledgements
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Funding
No external sources of funding were used for this study or for the writing, correction, and submission of this article.
Additional information
Faizan Mazhar was previously worked at King Fahd Military Medical Complex, Dhahran, Saudia Arabia, where the where part of the work has been carried out.
Appendix
Appendix
Examples of medication errors detected in study according to type of unintentional medication discrepancies
Type of error | Examples |
|---|---|
Drug omission | (1) The omission of antifungal prophylaxis in hematopoietic stem cell transplant patient (2) The omission of phenytoin in a patient with a recent new onset of seizure following neurosurgery (3) The omission of vancomycin coverage in a patient with confirmed MRSA infection (4) The omission of prophylaxis against Pneumocystis carinii Pneumonia in the immuno-compromised patient (5) The omission of ibuprofen in a patient recently started on allopurinol for gout (6) The omission of warfarin at admission in a patient with mitral valve replacement surgery |
Discrepant or incorrect dose | (1) The physician prescribes “warfarin 10 mg” on admission while “simvastatin 10 mg” was recorded in patient home medication list that was continued upon admission without dose adjustment. Simvastatin may enhance the anticoagulant effect of warfarin (a clinically significant interaction) (2) “Omeprazole 20 mg PO” was prescribed upon admission, while dabigatran was recorded as a home medication. Omeprazole may decrease serum concentrations of the active metabolite of Dabigatran. Monitor drug therapy is recommended (3) “Wellbutrin XL (bupropion) 300 mg OD” was prescribed upon admission, while “Wellbutrin SR (bupropion) 150 mg” was recorded as a home medication. An abrupt increase in dose may cause Insomnia and agitation (4) Controlled-release carbidopa/levodopa 50/200 (Sinemet CR) twice daily was prescribed upon admission, while standard carbidopa/levodopa (Sinemet 25/100) once daily was recorded as a home medication for restless legs syndrome. The discrepant dose was prescribed for Parkinson disease instead of restless legs syndrome (5) “Phenytoin 100 mg TID” for post-hemorrhagic stroke seizures as home medication was continued on admission, while “Invokana (canagliflozin) 100 mg” was prescribed on admission as an add-on treatment for type-2-diabetes mellitus. Phenytoin may decrease the serum concentration of canagliflozin and it is recommended to start treatment at 300 mg/day in patients receiving concomitant phenytoin |
Discrepant or wrong frequency | (1) “Terlipressin acetate 2 mg/4 h IV infusion” for bleeding oesophagal varices associated with liver cirrhosis was recorded in admission medication order, while “terlipressin acetate 2 mg/4 h IV bolus” was noted inter-hospital transfer records. Evidence for the use of terlipressin by continuous intravenous infusion is not convincing and it is currently not approved (2) Patient with a history of NYHA Class I CHF prescribed “pioglitazone 15 mg BID”, while pioglitazone 15 mg OD was recorded in patient home medication list. There was a two-fold increase in the dose of pioglitazone than recommended dose in patients with NYHA Class I or Class II CHF (3) “Isosorbide dinitrate ER 40 mg TID” was prescribed on admission, while “isosorbide dinitrate 40 mg BID” was recorded in pre-admission medications. An abrupt increase in dose due to change in tablet formulation (4) “Pregabalin 300 mg/day TID” for diabetes-associated neuropathic pain was recorded in admission, while “pregabalin 75 mg BID” was recorded as a home medication. Pregabalin dosage greater than 500 mg/day increase risk of adverse effect with no additional benefit (5) “Carvedilol ER 80 mg OD” for CHF was recorded in pre-admission medications, while “carvedilol IR 3.125 mg BID” was recorded in admission medications. The physician ordered starting dose of carvedilol for the treatment of CHF on admission, however, the patient was already on escalated-dose of carvedilol for post-MI heart rate control |
Drug commission (previously stopped drug) | (1) A patient recently started on “sertraline 50 mg” for social anxiety disorder was admitted for acute diarrhoea. The physician failed to discontinue sertraline. Sertraline tends to cause more loose stools than constipation and should likely be avoided over other alternatives that would be less likely to exacerbate diarrhoea (2) Sitagliptin was recorded in a patient admitted for suspected acute pancreatitis. The treating physician missed discontinuing sitagliptin as a possible suspect or interacting agent of patient condition (3) A patient admitted for an acute urinary tract infection with delirium was taking “oxybutynin” before admission. Oxybutynin was also recorded in admission medication order. The treating physician failed to identify oxybutynin as a precipitating risk factor for delirium (4) Patient with a history of CHF and HTN admitted for the management of acute renal colic, was taking “hydrochlorothiazide 50 mg daily” before admission. Hydrochlorothiazide 50 mg was also recorded in admission order. Based on patients’ renal function SCr 3.1 mg/dL, eGFR 26 mL/min/1.73 m2, hydrochlorothiazide should be held or substituted with another drug (5) “Moxifloxacin 400 mg IV OD” was recorded in patient’s admission order for the management of COPD exacerbation. However, past medical record showed that the patient reportedly discontinued levofloxacin due to tendinitis |
Incorrect drug | (1) The patient was taking “tiagabine” for seizure control, however, upon admission, he was prescribed “tizanidine” (2) “Metoclopramide 10 mg IV” was prescribed for acute diabetic gastric stasis. However, the patient record shows taking “risperidone 2 mg” in the past 6 months for major depression. Concomitant use of risperidone and metoclopramide more likely to cause tardive dyskinesia (3) A patient with a medical history of CHF was admitted for acute gastroenteritis with severe nausea, vomiting and hypokalemia. “Ondansetron 10 mg IV” was prescribed upon admission for the treatment of vomiting. Ondansetron is a QTc-prolonging agent. Hypokalemia is an additive risk factor for drug-induced QT prolongation, particularly in patients with CHF (4) “Atorvastatin 10 mg” substituted for “rosuvastatin” for secondary prevention stroke, however, past medical record showed that the patient recently switched to rosuvastatin due to complain of distal Muscle weakness (5) “Celecoxib 100 mg BID” was prescribed for an acute gout episode to a patient with a recent history of unstable angina. NSAIDs, in particular, COX-II inhibitors, may pose a risk of serious cardiovascular thrombotic events. This especially important in a patient with cardiovascular disease |
Rights and permissions
About this article
Cite this article
Mazhar, F., Haider, N., Ahmed Al-Osaimi, Y. et al. Prevention of medication errors at hospital admission: a single-centre experience in elderly admitted to internal medicine. Int J Clin Pharm 40, 1601–1613 (2018). https://doi.org/10.1007/s11096-018-0737-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0737-2