Abstract
Background Physicians identify from 45.7 to 96.2 % of Adverse Drug Reactions (ADRs) in their patients, with under-reporting ranging from 6 to 100 %. In order to improve ADR reporting, several interventions have been evaluated in different studies, but not with regard to ADR identification. In addition, it is not known whether some patient characteristics might influence on ADR identification and reporting by physicians. Objectives (a) To assess the effectiveness of a comprehensive intervention directed to Emergency Department physicians and coordinated by a pharmacist in a tertiary care pediatric hospital on ADR identification and reporting. (b) To assess if some of the children’s characteristics might influence on ADR identification and reporting. Setting The Emergency Department of the Hospital Infantil de México “Federico Gómez”, which is a national pediatric institute of health in México. Methods A Quasi-experimental, pre-post test trial was designed. During the intervention, the pharmacist gave talks on Pharmacovigilance and on the program for electronic capture of data, took part in patient visits, left reminders, improved accessibility to ADR report format and performed feedback activities. To classify and quantify correctly identified ADRs and ADRs reported to the Institutional Pharmacovigilance Center (IPC), 1136 clinical records were reviewed. The models were adjusted for patient variables. Main outcome measures Total ADRs, ADRs correctly identified by physicians, ADRs reported to the IPC by physicians. Results Before the intervention, 97 % of ADRs were correctly identified and 6.1 % reported by physicians. During the intervention, 99.6 % were correctly identified and 41.2 % were reported, and after the intervention, 99.6 and 41.7 %, respectively. Identification during the intervention showed a sevenfold increase with regard to preintervention and was maintained post-intervention. ADR reporting during the intervention showed a 14-fold increase with regard to pre-intervention and was maintained during post-intervention. Conclusion Physicians do identify ADRs, but fail to report them. The intervention increased ADR correct identification and reporting. The effect was maintained after the intervention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impacts on practice
-
A comprehensive, pharmacist-coordinated intervention has an effect on ADR identification and reporting by physicians.
-
Patient characteristics may affect ADR reporting by physicians to Regulatory Agencies.
Introduction
During the post-marketing development stage of a drug (Phase IV of research), Adverse Drug Reactions (ADRs) may occur that were not previously identified in Phases I, II and III, and can that be attributed, among other things, to larger numbers of individuals exposed to the drug or to the lack of safety data in vulnerable populations such as the children [1, 2]. The importance of generating information in the pediatric population relates to the fact that children are more vulnerable to the development ADRs for causes such as: (a) unlicensed and off-label prescribing of drugs, such as midazolam [3]; (b) exposure to drugs during prenatal and breastfeeding periods, such as valproate [4, 5] and (c) due to special pharmacokinetic and pharmacodynamic characteristics, as in the case of penicillin G [6]. Additionally, different studies have identified factors that predispose pediatric patients to the development of ADRs, such as age, female gender, number of administered drugs, off-label use of drugs, oncologic diagnosis and receiving general anesthesia [7, 8]. This generates an impact on morbidity, mortality [9, 10] and costs [11]. On the other hand, Spontaneous Reporting is essential to safety surveillance of marketed drugs through the generation of signals [12], with physicians being the healthcare professionals that most report ADRs [13]. The initial requirement in order for physicians to generate a report is the suspicion that a sign or symptom appearing in a patient is possibly related to the administration of a drug and, in second term, once identified, to report it to the corresponding Regulatory Agencies [14]. In Mexico, healthcare professionals have an obligation to report all suspicions, adverse events and reactions, both expected and unexpected, that they have knowledge of, directly to the National Regulatory Authorities [15].
When the clinical record review is used as the gold standard, physicians have been found to identify from 70 % [16] to 76 % [17] of ADRs suffered by their adult patients, whereas in children this occurs in 45–96 % [18–20]. However, in spite of identifying an ADR, physicians not always report it to the corresponding Pharmacovigilance Center, which results in under-reporting figures ranging from 6 % to up to 100 % [21].
To improve ADR under-reporting, different interventions that show positive effect have been studied, including: educational activities (sessions and reminders); help from other healthcare professionals (pharmacists, nurses, clinicians); amendments to the report form or to the completion procedure; economic incentives to physicians; increased accessibility to the report form and feedback [22–28]. However, the impact that the above mentioned interventions can have on ADR identification in physicians has not been assessed in any of these studies. In addition, it is not known whether some patient characteristics (age, body mass index, sex, number of drugs and oncologic conditions) might influence on ADR identification and reporting by physicians.
Aim of the study
In view of all this, the main purpose of this work was to assess the effectiveness of a comprehensive intervention coordinated by a pharmacist and directed to Emergency Department physicians in a tertiary care pediatric hospital on ADR identification and reporting and, as a secondary endpoint, to assess if some of the children’s characteristics might influence on the results.
Ethical approval
The research protocol was approved by the Research Commision, Ethics and Biosafety Committees of Hospital Infantil de México “Federico Gómez” (HIMFG) under permit number HIM/2011/037.
Methods
Study population and settings
The study was conducted at the Emergency Department (ED) of the HIMFG, which is a national pediatric institute of health in México.
The study population included the head of the department, 3 staff physicians, 5 Pediatric Emergency Medicine subspecialty residents and 53 Pediatrics specialty residents (18 at their first year, 23 second year and 12 third year). In the case of Pediatrics specialty residents, they had to have remained at least 2–3 months during their rotation at the ED. The head of the department and staff physicians were 2 females and 2 males, with ages ranging from 34 to 61 years; the Pediatric Emergency Medicine subspecialty residents were 3 females and 2 males with ages ranging from 28 to 29 years, and with regard to the Pediatrics residents, they were 37 females and 16 males, aged between 24 and 27 years.
The study was coordinated by the Institutional Pharmacovigilance Center (IPC), which is responsible for analyzing and submitting the reports made by HIMFG physicians to the National Regulatory Authority. Prior to the start of the study, the IPC developed an Online-Capture Pharmacovigilance System (OCPS) [29] to substitute paper-based reports and to make the reporting process more efficient.
Study design
A quasi-experimental, pre-post test study was designed [30]. The pre-intervention stage was performed from March to August 2012 (6 months). The intervention was from September to December 2012 (4 months), and the post-intervention period was from January to June 2013 (6 months).
Intervention
In order to impact on the behavior of medical staff, the pharmacist performed the following activities:
-
1.
Group informative talk on Pharmacovigilance. With duration of 60 min, it included aspects related to the importance of Pharmacovigilance in the world and in Mexico; the experiences at the HIMFG; how to suspect the presence of an ADR, as well as the capture procedure of the different items comprising the OCPS. At the end, a real-time exercise on information-capture using the OCPS was made, which was complemented by handing out a card with precise information to avoid completion errors.
-
2.
Accompanying the physicians during clinical visits.
-
3.
Reminders to medical staff. During general visits and in the later review of clinical records, the pharmacist identified those patients in which the physician detected an ADR in the clinical record but it had not been reported to IPC. When this happened, the physician was verbally asked to submit the report, in addition to leaving a self-adhesive note in the patient chart as a reminder. When no ADR was identified by the physician, the pharmacist asked for the case to be discussed with regard to causality or not of the ADR.
-
4.
Feedback to medical staff. Every week, during clinical visits, physicians were informed on the number of identified and reported ADRs. This information was also recorded in an informative chart, which was posted on a board located in the hospitalization ward of the ED.
-
5.
Improving accessibility to the ADRs report format. In addition to the OCPS having been installed at the hospitalization area for being this a shared space, the pharmacist placed, in an accessible and visible place, a clipboard with official paper formats in order for them to be manually completed in case of not having access to the computer.
Outcome measures
These outcomes were as follows:
-
1.
Total ADRs. To identify ADRs occurring in the ED at the pre-intervention, intervention and post-intervention stages, the pharmacist included only patients who remained at the ED for longer than 24 h. For the pre- and post-intervention stages, clinical records were retrieved from the medical records department of the hospital. At the intervention stage, the pharmacist attended with the physicians to clinical visits of all admitted patients and simultaneously reviewed the clinical records. In the cases where the pharmacist had any doubt, he discussed it with a pediatrician expert in pharmacovigilance. The definition of ADR was according to the World Health Organization [31]. For the assessment of a suspected ADR, the MICROMEDEX 2.0 was used as Ref. [32] and the causality analysis was performed using Naranjo’s algorithm [33].
-
2.
ADRs correctly identified by physicians. During the three stages of the study, the patients were classified and quantified as “ADR correctly identified by physicians” in those cases where, according to the pharmacist, the patient experienced an ADR and evidence was found in the clinical record on physicians having associated some clinical manifestation with the administered medication(s). To find such association, the pharmacist intentionally searched across the clinical notes of each patient’s medical record, looking for terms and actions such as “caused by drugs”, “drug-related”, “associated with drug administration”, “adverse drug reaction”, “patient diagnoses”, or if “the physician withdrew the suspected medication” and if “physician administered any drug to treat an ADR”.
-
3.
ADRs reported to the IPC. During the pre-intervention and post-intervention periods, the cases of ADRs that were submitted by the clinicians to the IPC via the OCPS were classified and quantified as “reported ADR”, whereas during the intervention, the cases of ADRs that were submitted by the physicians to the IPC via the OCPS and/or using the official paper formats, were classified and quantified as “reported ADR”.
Statistical analysis
In order to verify the validity and reproducibility of ADRs detected by the pharmacist, at the start of the study an agreement analysis using the Kappa index was performed between the pharmacist of this study and a pediatrician expert in pharmacovigilance. Level of agreement between the expert pediatrician and the pharmacist was K = 0.91 (p < 0.05) [34]. During the pre-intervention stage, 436 clinical records of patients admitted to the ED were reviewed, whereas during the intervention, 246 were reviewed and post-intervention, 454. For descriptive purposes, cases were classified into 4 categories according to the body mass index Z-score (normal weight, obesity, overweight and underweight) [35] and 3 categories according to age (infants, children and adolescents) [36]. The ICD 10 was used for the oncologic diagnosis (C00–D48) [37]. The number of medications was defined as the total number of drugs administered to the patient in the ED. The descriptive analysis included central tendency and dispersion measures, such as number of cases (%), mean (95 % CI) and median (25–75th percentiles). In the bivariate analysis, patient characteristics at all 3 stages of the study were contrasted. For the height, weight and number of medications variables, the Kruskal–Wallis non-parametric test was selected because these variables did not show a normal distribution. For categorical variables (age group, sex, body mass index category according to Z-score and patients with oncologic diagnosis), the Chi square test was used. To measure the intervention effect on correctly identified ADRs and on reported ADRs, a multiple logistic regression analysis was used, with the stage transformed into a dichotomous variable and patient characteristics entered as covariables, whether they were significant or not in the bivariate analysis. The final model was chosen with the global adjustment tests of the model by Hosmer and Lemeshow. In all cases, a p value <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS 18 statistical package.
Results
Demographic characteristics of the children at all 3 stages showed no statistically significant differences, with the exception of age and oncologic diagnosis (Table 1).
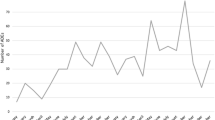
Figure 1 shows that, at the pre-intervention stage, physicians identified correctly 97 % of patients, 99.6 % at the intervention, and 99.6 % at the post-intervention stage (p < 0.05). With regard to the percentage of reports submitted by physicians to the IPC, it was 6.1 %; 41.2 and 41.7 % (p < 0.05), respectively.
Table 2 shows that, after the intervention, the ability of physicians to correctly identify patients showed a sevenfold improvement (p < 0.05). Additionally, patient characteristics (age, sex, BMI Z-score, number of medications and oncologic diagnosis) were found not to be significantly related to the ability of physicians to correctly identify patients (p ≥ 0.05).
Table 3 shows that reporting to the IPC had a 14-fold increase after the intervention (p < 0.05). Age, sex, BMI Z-score and number of medications were not related to ADRs being reported to the IPC (p ≥ 0.05). However, patients with oncologic diagnoses showed a 1.1-fold decrease in the likelihood of their ADRs being reported to the IPC (p < 0.05).
Discussion
The results of the present study indicate that an intervention that included group informative talks on Pharmacovigilance, reminders and feedback to the intervened personnel, increased availability of report forms and the company of a pharmacist in clinical patient visits, yielded a sevenfold increase in the ability of physicians to correctly identify ADRs and a 14-fold increase in ADR reporting to the IPC. Noteworthy, the effect on both variables was maintained 6 months after the intervention. Other finding not previously reported in literature was that correct identification of ADRs was not influenced by patient demographic characteristics such as age, sex, BMI Z-score, number of administered medications and having an oncologic diagnosis. However, in patients with an oncologic diagnosis, the likelihood of physicians reporting their ADRs to the IPC was decreased.
As previously mentioned in the introduction, there is evidence that the interventional activities carried out in the present work, in different combinations, have a positive effect on improving under-reporting when applied to different healthcare professionals, including physicians [22–28]. However, in this work, one more component was added, namely, the presence of the pharmacist during patient visits, the effect of which has been demonstrated in other publications [38, 39]. Of note, the elevated rate of correct identification of patients experiencing an ADR, allowed for physicians to take relevant actions upon its presence, without detriment in the reporting of ADRs to the corresponding regulatory agencies.
There are methodologies that aid healthcare professionals in ADRs identification, through computerized systems, where causal relationship is identified using algorithms, supported exclusively by clinical records and/or by laboratory and imaging tests, which are considered a practical tool due to their higher rates of ADRs detection compared with spontaneous reporting, as well as lower implementation costs and because, additionally, they are less resource-consuming than manual review of clinical records and intensive surveillance [40–43]. However, one limitation of these methodologies is that, in our country, a large number of hospitals lack electronic records that include medical history, medical and nursing notes, as well as laboratory and imaging tests results. Nevertheless, clinical records review has been reported [18] to be able to be considered the gold standard to obtain data on ADR incidence, but a drawback of medical records review is that it is time- and human resource-consuming and, therefore, it ends up being more expensive, with an additional determinant: if it is applied in medical units where clinical records have several deficiencies, the results may be not fully reliable.
The results of ADR identification at all 3 stages of the present study are similar to those found in an adult intensive care unit, where physicians wrote down in the clinical record 70 % of the ADRs that were indentified in their patients [16]. In the case of children, physicians were shown to identify only 50 % of ADRs experienced by their patients [19], while Oehme AK et al. [18] found in 1999 that 45.7 % of ADRs were identified in clinical records, with this rate increasing to 96.2 % for the year 2008, which was arrtibuted to increased awareness in medical personnel. In Mexico, in a study conducted in hospitalized adults, physicians identified and wrote down on clinical records 76 % of ADRs [17] and no publications on children were found.
It should be noted that, in the present work, additional cases found only by the pharmacist made identification more efficient, a finding consistent with another study that included pharmacists as medical record reviewers, which detected higher rates of adverse drug events than other healthcare professionals [44]. In Canada and the United States, the contribution of pharmacists to Pharmacovigilance is essential, since they report 88 and 68 % of ADRs, respectively, compared with other healthcare professionals [45]. Furthermore, it should be noted that the pharmacist’s participation is not restricted to reporting, as shown by a clinical pharmacist-implemented educational program directed to clinicians and nurses from a hospital where knowledge, attitude and perception on ADRs and on the Spontaneous Reporting process were increased [46].
Study limitations
The limitations of the study that should be considered when interpreting the results include: (a) ADR identification was considered correct when physicians wrote it down on the clinical record, which does not exclude the possibility of physicians having identified it, but failing to write it down on the clinical record; (b) The findings here encountered cannot be generalized to other departments of the same hospital; (c) The design did not consider a possible effect of ADRs being influenced by vaccination campaigns or problems in the production of some batch of medications, which would require a randomized design; (d) The intervention did not consider causes originating under-reporting by the ED; (e) Other factors that might have influenced on higher identification rates, such as ADRs characteristics, were not investigated; for example, when it comes to a rash, neutropenia or neutropenic colitis; and (f) With regard to the finding that patients with oncologic diagnoses were more likely to have their ADRs not being reported to the IPC, this could probably be explained by the fact that some physicians think that ADRs that are expected to occur frequently do not require to be reported to the IPC; a specific study would be needed to verify this possibility.
Conclusion
The present study demonstrates that HIMFG physicians do identify ADRs occurring in children admitted to the ED but they report only 6.1 % of ADRs identified in their patients. According to these results, it is necessary to implement strategies in order to substantially improve ADRs reporting, such as strategies that include group informative talks on Pharmacovigilance, presence of a pharmacist in ED activities, reminders to intervened personnel, feedback to intervened personnel and increased availability of ADR report format.
References
Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002;287(17):2215–20.
Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA. 1999;281(9):824–9.
Lindell-Osuagwu L, Korhonen MJ, Saano S, Helin-Tanninen M, Naaranlahti T, Kokki H. Off-label and unlicensed drug prescribing in three paediatric wards in Finland and review of the international literature. J Clin Pharm Ther. 2009;34(3):277–87.
Meador KJ, Baker GA, Browning N, Clayton-Smith J, Combs-Cantrell DT, Cohen M, et al. Effects of breastfeeding in children of women taking antiepileptic drugs. Neurology. 2010;75(22):1954–60.
Meador KJ, Baker GA, Browning N, Cohen MJ, Bromley RL, Clayton-Smith J, et al. Fetal antiepileptic drug exposure and cognitive outcomes at age 6 years (NEAD study): a prospective observational study. Lancet Neurol. 2013;12(3):244–52.
Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–67.
Thiesen S, Conroy EJ, Bellis JR, Bracken LE, Mannix HL, Bird KA, et al. Incidence, characteristics and risk factors of adverse drug reactions in hospitalized children—a prospective observational cohort study of 6601 admissions. BMC Med. 2013;11:237.
Rashed AN, Wong IC, Cranswick N, Tomlin S, Rascher W, Neubert A. Risk factors associated with adverse drug reactions in hospitalised children: international multicentre study. Eur J Clin Pharmacol. 2012;68(5):801–10.
Finkelstein Y, Soon GS, Acuna P, George M, Pope E, Ito S, et al. Recurrence and outcomes of Stevens-Johnson syndrome and toxic epidermal necrolysis in children. Pediatrics. 2011;128(4):723–8.
Gallagher RM, Mason JR, Bird KA, Kirkham JJ, Peak M, Williamson PR, et al. Adverse drug reactions causing admission to a paediatric hospital. PLoS ONE. 2012;7(12):e50127.
Oshikoya KA, Chukwura H, Njokanma OF, Senbanjo IO, Ojo I. Incidence and cost estimate of treating pediatric adverse drug reactions in Lagos, Nigeria. Sao Paulo Med J. 2011;129(3):153–64.
Fujimoto M, Higuchi T, Hosomi K, Takada M. Association between statin use and cancer: data mining of a spontaneous reporting database and a claims database. Int J Med Sci. 2015;12(3):223–33.
Star K, Norén GN, Nordin K, Edwards IR. Suspected adverse drug reactions reported for children worldwide: an exploratory study using VigiBase. Drug Saf. 2011;34(5):415–28.
European Medicines Agency. Good Pharmacovigilance Practices. http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000345.jsp&mid=WC0b01ac058058f32c#section4. Accessed 17 Sep 2015.
Diario Oficial de la Federación. Norma Oficial Mexicana NOM-220-SSA1-2012, Instalación y operación de la Farmacovigilancia. http://dof.gob.mx/nota_detalle.php?codigo=5284236&fecha=07/01/2013. Accessed 17 Sep 2015.
Park S, In Y, Suh GY, Sohn K, Kim E. Evaluation of adverse drug reactions in medical intensive care units. Eur J Clin Pharmacol. 2013;69:119–31.
Becerril-Ángeles M, Aranda-Jan A, Moreno-Quiróz J. Survey of adverse reactions to drugs in hospitalized patients. Rev Alerg Mex. 2011;58:179–84.
Oehme AK, Rashed AN, Hefele B, Wong IC, Rascher W, Neubert A. Adverse drug reactions in hospitalised children in Germany are decreasing: results of a nine year cohort-based comparison. PLoS ONE. 2012;7:e44349.
Neubert A, Dormann H, Weiss J, Egger T, Criegee-Rieck M, Rascher W, et al. The impact of unlicensed and off-label drug use on adverse drug reactions in paediatric patients. Drug Saf. 2004;27:1059–67.
Haffner S, von Laue N, Wirth S, Thürmann PA. Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf. 2005;28:453–64.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Gonzalez-Gonzalez C, Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Strategies to improve adverse drug reaction reporting: a critical and systematic review. Drug Saf. 2013;36(5):317–28.
Ribeiro-Vaz I, Santos C, da Costa-Pereira A, Cruz-Correia R. Promoting spontaneous adverse drug reaction reporting in hospitals using a hyperlink to the online reporting form: an ecological study in Portugal. Drug Saf. 2012;35(5):387–94.
Goldstein LH, Berlin M, Saliba W, Elias M, Berkovitch M. Founding an adverse drug reaction (ADR) network: a method for improving doctors spontaneous ADR reporting in a general hospital. J Clin Pharmacol. 2013;53(11):1220–5.
Biagi C, Montanaro N, Buccellato E, Roberto G, Vaccheri A, Motola D. Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia-Romagna region). Eur J Clin Pharmacol. 2013;69(2):237–44.
Johansson ML, Hägg S, Wallerstedt SM. Impact of information letters on the reporting rate of adverse drug reactions and the quality of the reports: a randomized controlled study. BMC Clin Pharmacol. 2011;11:14.
Herdeiro MT, Ribeiro-Vaz I, Ferreira M, Polónia J, Falcão A, Figueiras A. Workshop- and telephone-based interventions to improve adverse drug reaction reporting: a cluster-randomized trial in Portugal. Drug Saf. 2012;35(8):655–65.
Lopez-Gonzalez E, Herdeiro MT, Piñeiro-Lamas M, Figueiras A, GREPHEPI Group. Effect of an educational intervention to improve adverse drug reaction reporting in physicians: a cluster randomized controlled trial. Drug Saf. 2015;38(2):189–96.
Jasso Gutiérrez L, Ovando Hernández R, Castellanos Solís EC, Escorza Peña J, Santos Preciado JI. Diseño e implantación de un programa electrónico de Farmacovigilancia con captura en línea en el Hospital Infantil de México Federico Gómez. Bol Med Hosp Infant Mex. 2009;66:51–9.
Cook TD, Campbell DT. Quasi-experimentation. Design and analysis issues for field settings. Chicago: Rand McNally College Publising Company; 1979.
Uppsala Monitoring Centre. Glossary of terms used in Pharmacovigilance. http://www.who-umc.org/graphics/27400.pdf. Accessed 17 Sep 2015.
Truven Health Analytics. Micromedex 2.0. http://www.micromedex.com/. Accessed 17 Sep 2015.
Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Martínez González MA. Bioestadística Amigable. 2nd ed. España: Diaz de Santos; 2006.
Evaluación Nutricional. http://www.fmed.uba.ar/depto/nutrievaluacion/TABLAS%20Y%20GRAFICOS%20EVAL%20NUTRICIONAL%202012.pdf. Accessed 17 Sep 2015.
European Medicines Agency. ICH Topic E 11. Clinical Investigation of Medicinal Products in the Paediatric Population. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002926.pdf. Accessed 17 Sep 2015.
International Statistical Classification of Diseases and Related Health Problems 10th Revision. http://apps.who.int/classifications/icd10/browse/2015/en. Accessed 17 Sep 2015.
Langebrake C, Hilgarth H. Clinical pharmacists’ interventions in a German university hospital. Pharm World Sci. 2010;32(2):194–9.
Leape LL, Cullen DJ, Clapp MD, Burdick E, Demonaco HJ, Erickson JI, Bates DW. Pharmacist participation on physician rounds and adverse drug events in the intensive care unit. JAMA. 1999;282(3):267–70.
Forster AJ, Jennings A, Chow C, Leeder C, van Walraven C. A systematic review to evaluate the accuracy of electronic adverse drug event detection. J Am Med Inform Assoc. 2012;19(1):31–8.
Neubert A, Dormann H, Weiss J, Criegee-Rieck M, Ackermann A, Levy M, et al. Are computerised monitoring systems of value to improve pharmacovigilance in paediatric patients? Eur J Clin Pharmacol. 2006;62(11):959–65.
Haffner S, von Laue N, Wirth S, Thürmann PA. Detecting adverse drug reactions on paediatric wards: intensified surveillance versus computerised screening of laboratory values. Drug Saf. 2005;28(5):453–64.
Weiss J, Krebs S, Hoffmann C, Werner U, Neubert A, Brune K, et al. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed detection. Pediatrics. 2002;110(2 Pt 1):254–7.
Phansalkar S, Hoffman JM, Nebeker JR, Hurdle JF. Pharmacists versus nonpharmacists in adverse drug event detection: a meta-analysis and systematic review. Am J Health Syst Pharm. 2007;64(8):842–9.
van Grootheest AC, de Jong-van den Berg LT. The role of hospital and community pharmacists in pharmacovigilance. Res Soc Adm Pharm. 2005;1(1):126–33.
Khalili H, Mohebbi N, Hendoiee N, Keshtkar AA, Dashti-Khavidaki S. Improvement of knowledge, attitude and perception of healthcare workers about ADR, a pre- and post-clinical pharmacists’ interventional study. BMJ Open. 2012;2:e000367.
Funding
None.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ríos, O.M., Gutiérrez, L.J., Talavera, J.O. et al. A comprehensive intervention for adverse drug reactions identification and reporting in a Pediatric Emergency Department. Int J Clin Pharm 38, 80–87 (2016). https://doi.org/10.1007/s11096-015-0209-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-015-0209-x