Abstract
Background Medication reconciliation has been mandated by the Irish government at transfer of care. Research is needed to determine the contribution of clinical pharmacists to the process. Objective To describe the contribution of emergency department based clinical pharmacists to admission medication reconciliation in Ireland. Main Outcome Measure Frequency of clinical pharmacist’s activities. Setting Two public university teaching hospitals. Methodology Adults admitted via the accident and emergency department, from a non-acute setting, reporting the use of at least three regular prescription medications, were eligible for inclusion. Medication reconciliation was provided by clinical pharmacists to randomly-selected patients within 24-hours of admission. This process includes collecting a gold-standard pre-admission medication list, checking this against the admission prescription and communicating any changes. A discrepancy was defined as any difference between the gold-standard pre-admission medication list and the admission prescription. Discrepancies were communicated to the clinician in the patient’s healthcare record. Potentially harmful discrepancies were also communicated verbally. Pharmacist activities and unintentional discrepancies, both resolved and unresolved at 48-hours were measured. Unresolved discrepancies were confirmed verbally by the team as intentional or unintentional. A reliable and validated tool was used to assess clinical significance by medical consultants, clinical pharmacists, community pharmacists and general practitioners. Results In total, 134 patients, involving 1,556 medications, were included in the survey. Over 97 % of patients (involving 59 % of medications) experienced a medication change on admission. Over 90 % of patients (involving 29 % of medications) warranted clinical pharmacy input to determine whether such changes were intentional or unintentional. There were 447 interventions by the clinical pharmacist regarding apparently unintentional discrepancies, a mean of 3.3 per patient. In total, 227 (50 %) interventions were accepted and discrepancies resolved. At 48-hours under half (46 %) of patients remained affected by an unintentional unresolved discrepancy (60 % related to omissions). Verbally communicated discrepancies were more likely to be resolved than those not communicated verbally (Chi-square (1) = 30.029 p < 0.05). Under half of unintentional unresolved discrepancies (46 %) had the potential to cause minor harm compared to 70 % of the resolved unintentional discrepancies. None had the potential to result in severe harm. Conclusion Clinical pharmacists contribute positively to admission medication reconciliation and should be engaged to deliver this service in Ireland.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Impacts on practice
-
The majority of adult patients admitted to acute hospital care in Ireland need to have their medication reconciled.
-
Clinical pharmacists contribute positively to patient care by preventing unintentional non-reconciliations which may lead to harm.
-
Aligning clinical pharmacist services with the admission process facilitates timely medication reconciliation (within 24 h of patient admission).
-
This study describes a model for acute hospitals wishing to deliver admission medication reconciliation services.
Introduction
Pharmacists have a responsibility to optimise patient safety during the medication use process. The vulnerability of patients due to medication mismanagement at transfer of care is well acknowledged [1–5]. Transfer of care occurs when the main duty of care changes from one clinician to another, for example when a patient is admitted to or discharged from hospital. A systematic review identified that one-third of patients experienced a medication history error on admission and over half of these errors were clinically significant [6]. There is evidence that this problem also exists in the Irish setting: on discharge, medication non-reconciliation was identified in 50 % of patients [7]. Non-reconciliations originating at admission frequently occurred due to the omission of a pre-admission medication but also occurred due to lapses in communication or documentation about changes made to a patient’s longstanding pre-admission medication. A challenge to medication reconciliation is identifying which medication the patient was actually using before they were admitted to hospital, as opposed to those medications prescribed or dispensed for the patient. This list, known as the gold standard pre-admission medication list (GSPAML), takes time and effort to collate [8].
Medication reconciliation is widely recognized and mandated nationally and internationally as a tool for the prevention of medication misuse and consequent patient harm at points of transfer of care [9–11]. In the UK, comprehensive guidance clearly describes the admission medication reconciliation process. A series of three steps are outlined; namely, collecting, checking and communicating [12]. Each of these steps is comprised of several tasks. Some of these can be time-consuming and resource intensive and all are yet to be formally standardized in the Irish setting. In the UK, it is also recommended that the role of providing admission medication reconciliation be assigned to the pharmacy profession [11]. This recommendation is supported by evidence of the competency of the clinical pharmacist to elicit the most accurate medication history on admission compared to other professionals (nurses, doctors, pharmaceutical technicians) who also routinely perform this function [13–15]; and the cost effectiveness of employing clinical pharmacists for this purpose [16]. There is also evidence that improved patient outcomes result when medication reconciliation forms part of a standardized inpatient clinical pharmacy service. A randomized controlled trial in Sweden showed a reduction of 49 and 80 % respectively in emergency department visits and drug-related re-admission rates in the group of patients who had received a comprehensive clinical pharmacist service compared to those who had not [17]. A pharmacist led integrated medicines management service, including medication reconciliation at admission and discharge and inpatient monitoring and counseling, resulted in a significant improvement in the quality of prescribing and reduction in both length of stay and readmission rates [18, 19]. In the Netherlands, undertaking patient counselling at discharge in a group of patients who had received medication reconciliation on admission and discharge increased the scope for pharmacist intervention [20].
In Ireland, the Commission on Patient Safety and Quality Assurance recommended as a priority that medication reconciliation be provided to all patients at all transfer of care stages and the national “Acute Medicines Programme” recommends that pharmacists should provide this service at admission to hospital [10, 21].
Definitions
Gold-standard pre-admission medication list (GSPAML)
The most accurate list of medication the patient was actually taking or using prior to admission, including the name, dose, frequency and route of administration of each medication. Over-the-counter, herbal and “as-required” medicines were included. This was constructed according to the study protocol using as many potential sources as were available including but not limited to patient or carer interview, patient’s own drugs or own list of drugs, community pharmacist and general practitioner records. The construction of the GSPAML is described in detail in a recent publication [8].
Discrepancy
Any difference between the GSPAML and the admission medication prescription [2]. This included intentional and unintentional differences.
Endorsement
The clarification by the clinical pharmacist of an ambiguous or incomplete prescription through the provision of additional written information to facilitate continuity of supply or administration. For example, the clarification of inhaler formulation when there are two devices available.
Intervention
An action taken to resolve an apparently unintentional medication reconciliation discrepancy; including endorsement as defined above; written and/or verbal communications to the prescriber detailing the discrepancy. For example, recognising a medication omission and suggesting addition to the admission medication prescription.
Unresolved unintentional discrepancy
A medication reconciliation discrepancy subject to a clinical pharmacist’s intervention which was unresolved at 48 h into the patients admission and confirmed verbally by the physician at that time as not intentional.
Resolved unintentional discrepancy
A medication reconciliation discrepancy subject to a clinical pharmacist’s intervention which was resolved at 48 h into the patients admission by the prescribing of the medication as per GSPAML.
Omission
The absence of a medication from the admission medication prescription that the patient had been using prior to admission and which should have continued on admission (i.e. the medication was part of the GSPAML).
Commission
The inclusion of a medication on the admission medication prescription which the patient had not been using prior to admission (i.e. the medication was not part of the GSPAML).
Aim
The aim of this paper is to describe the contribution of the accident and emergency (A&E) based clinical pharmacist to medication reconciliation for adult patients on admission to acute hospital in Ireland and identify ways to further improve the process.
Method
This was a prospective observational study undertaken in two acute teaching hospitals of Trinity College Dublin: Naas General Hospital (NGH) is a 243-bed general hospital serving a predominantly rural community; the Adelaide and Meath Hospital, incorporating the National Childrens Hospital (AMNCH) is a 500-bed tertiary referral centre serving a predominately urban population. The annual volume of inpatient discharges are approximately 10,000 and 25,000 from each respective site [in-house data]. Adults over the age of 18 years were eligible for inclusion in the study if they were admitted via A&E from a non-acute setting and reported the use of at least three regular medications prior to admission [22]. The following exclusion criteria were employed: absence of the patient from the ward at the time of data collection and unavailability of an interpreter to interview non-English speaking patients. Patients were randomly selected from a list of new admissions each morning during the study period. Data were collected at each study site by clinical pharmacists involved in the delivery of admission medication reconciliation. Ethics Committee approval for the study to proceed was obtained from the relevant committee at each study site. In NGH, the committee required that verbal patient consent be obtained, following completion of the medication history interview, for use of the patient’s data in the study, whilst this was not required by the AMNCH committee. Patients in NGH who could not provide verbal consent were therefore excluded. Data were collected between February and April 2009. In order to minimise reactive bias, medical, surgical, nursing and pharmacy staff were unaware of the exact nature of the study or the data collection period [23]. The data collection process, undertaken by the clinical pharmacists, is presented in Fig. 1.
The following lists the outcome measures:
-
1.
Frequency of clinical pharmacist’s activities (analysis was performed using two units of measure: per patient and per medication)
-
2.
Frequency and nature of unresolved unintentional discrepancies at 48 h post admission
-
3.
Potential for harm averted by clinical pharmacist input and consequent to unintentional unresolved discrepancies at 48 h post admission.
Measurement of inter-rater agreement regarding the medications to be included on the gold-standard pre-admission medication list (GSPAML) was undertaken across the two study sites during the pilot phase of data collection.
Assessment of clinical significance
Two random samples of inpatient episodes were selected; the first included only patients affected by an unresolved unintentional discrepancy and the second included those where all initial discrepancies were completely resolved at 48 h. The potential for patient harm was assessed using a reliable and validated tool, which employs a visual analog scale (0–10: 0 represents no harm;10 represents death) [24]. Six assessors individually assessed and scored each case, and a mean score was then calculated. Assessors were all practising clinicians and included medical consultants, hospital clinical pharmacists, general practitioners and community pharmacists. The mean score was categorised as minor harm (<3), moderate harm (3–7) or severe harm (>7).
Data were inputted and analysed using SPSS (version 16). Descriptive statistics were used to represent process and patient outcome measures. Associations between categorical variables were examined using the Chi-square test. An a priori level of significance of 0.05 was chosen.
Results
Study populations
A total of 134 patients were recruited to the study and data were collected for 1556 medications. The majority of patients received care from a medical rather than surgical consultant, were self referred to A&E and just over half were male (Tables 1, 2).
Inter-rater agreement
There was agreement in medication name, dose, frequency, formulation and route for 99 out of the 110 medications assessed (90 %). Based on this, the kappa co-efficient (κ = 0.52) indicated moderate inter-rater agreement.
Clinical pharmacist activity
Identifying discrepancies between GSPAML and admission medication
The medications prescribed on admission for four of the 134 patients surveyed were identical to their GSPAML, whilst for the remainder (97 %) at least one initial discrepancy was identified. The range of initial discrepancies was 0–24.
Determining whether the discrepancy was intentional or unintentional
For the majority of patients (81.5 %), the prescriber documented in the healthcare record that at least one medication change was intentional. Documentation supporting a discrepancy was more likely when the change in therapy related to a medication indicated for the management of the presenting complaint (Chi-square (1) = 3.193 p < 0.05) than for medications used to manage other conditions. Documentation justifying all initial discrepancies was made in the healthcare record for five (3.8 %) patients of the 134 surveyed.
The majority of patients (n = 115; 85.8 %) experienced at least one change to their GSPAML which could not be rationalized by the clinical pharmacist. For nine patients (6.7 %) involving eleven medications (0.7 %) the changes were documented by the team but were not regarded as rational and so necessitated clinical pharmacist intervention. Most related to medication management issues for example use of antibiotics outside the hospital’s empiric antibiotic guidelines and inappropriate prescription of low molecular weight heparins.
Resolving apparently unintentional discrepancies
The remaining discrepancies (n = 467) were judged to be unintentional and merited clinical pharmacist intervention, as follows.
Endorsement
A minority of the interventions (n = 20; 1 % of meds involving 9 % of patients) took the form of endorsement of the admission prescription. Over three-quarters of endorsements related to the clarification of active ingredients of combination products or formulation type including extended release and enteric coated preparations.
Communication of apparently unintentional discrepancies to the team to facilitate resolution
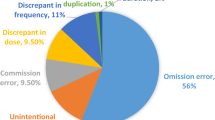
For the majority (91 %) of patients surveyed, there was no explanation in the patient’s healthcare record for the discrepancies identified between the GSPAML and the medications prescribed on admission. These discrepancies were apparently neither intentional nor rational, thus necessitating clinical pharmacist intervention. In total, there were 447 (29 % of meds surveyed) interventions, a mean of 3.3 per patient surveyed. The majority related to medication omission (65.3 %), incorrect dose or frequency (22.5 %) or medication commission (8.9 %). In all cases, the intervention was conveyed to the prescriber by documentation in the patient’s healthcare record detailing the nature of the identified discrepancy with a request for appropriate remedial action to resolve the discrepancy. The discrepancy was also verbally communicated to the team, particularly where it was considered to have the potential to cause serious patient harm. In less urgent cases, verbal communication was opportunistically made to the prescriber if present at the time the discrepancy was identified. Verbal communication occurred for over a quarter (n = 105) of the apparently unintentional discrepancies.
Discrepancies resolved at 48 h
Over half (54 % n = 244) of the clinical pharmacists’ interventions were accepted at 48 h, thereby resolving the discrepancy. In the majority of cases resolution was by means of the admission medication prescription reverting to the original pre-admission regimen. Of the 52 patients who experienced a clinical pharmacist intervention, two had discrepancies resolved at 48 h by the prescriber documenting that the change was intentional. Apparently unintentional discrepancies were more likely to be resolved when also verbally communicated to the team at the time of identification compared to those discrepancies which were not also verbally communicated to the team (Chi-square (1) = 30.029 p < 0.05).
Nature and number of non-reconciliations at 48 h
Less than half of patients (46 %) surveyed experienced an unresolved unintentional discrepancy at 48 h into admission. For most of these (89 %), the medication involved was not indicated for nor used in the management of the patient’s presenting complaint. Medication omission accounted for almost two-thirds (62.5 %) of these, followed by dose/frequency errors (24.3 %) and commissions (9.9 %). The remaining unresolved unintentional discrepancies comprised of route issues and substitution or formulary issues. A statistically significant correlation between the number of pre-admission medications the patient used and the number of unintentional discrepancies was identified (rho (134) = 0.224; r2 = 5 % p < 0.05).
Clinical significance
The majority of the unresolved unintentional discrepancies that were assessed for clinical significance (n = 22) were judged to have the potential to cause minor harm (54.5 %). The remaining cases were judged to have the potential to result in moderate harm (45.5 %). The clinical significance of the resolved unintentional discrepancies (n = 20) were judged to risk the patient experiencing moderate harm (70 %) and minor harm (30 %) had these unintentional discrepancies not been resolved.
Discussion
This study demonstrates the potential contribution that clinical pharmacists can make to medication management on admission to acute hospital care in Ireland, consistent with international literature [25, 26]. Furthermore it provides a measure of the demand for such a service: almost all of the patients surveyed experienced a change to their GSPAML. In some instances, discrepancies were immediately resolved once identified by the clinical pharmacist. However, the majority of pharmacist interventions involved communication about and discussion with the physician regarding apparently unintentional discrepancies. The most common type of discrepancy was omission of a pre-admission medication, followed by dose & frequency issues and commissions, consistent with research in other settings [8, 27]. The clinical significance of most unresolved unintentional discrepancies was assessed as low. This measure has not been routinely reported.
This is the first published study in the Irish setting that details how a clinical pharmacist can contribute to medication management on admission to hospital, including provision of a medication reconciliation service. Data were collected in two Irish hospitals to enhance the external validity of the findings therefore the results are likely generalisable to hospitals providing acute care for adult surgical and medical admissions in Ireland. The findings provide evidence to support clinical pharmacist involvement in medication management on admission. Future research can now build on this foundation. A limitation of the study is the exclusion of patients without the capacity to give consent from the NGH cohort (n = 4). This removed a potentially vulnerable group of patients from the population, as such patients have been identified as susceptible to medication reconciliation errors [11]. It is important that Ethics Committees balance the benefits of obtaining consent for the use of non-identifiable patient data in research against the cost of excluding a group of vulnerable patients from a study which might uncover ways to improve their safety. However, inclusion of such patients within the AMNCH sample allowed representation of this group in the overall sample.
The rigorous methodology employed strengthened the study: Firstly, there was moderate agreement between the investigators at each site compiling the GSPAML. This ensured that the process undertaken at each site was consistent and the two investigators were following a consistent method of developing the GSPAML. This formed the first step of medication reconciliation and ensures a reliable process to be employed by other clinical pharmacists undertaking this role in the future. Secondly, the exact purpose of this study was not disclosed to medical, surgical, pharmacy and nursing staff, thereby minimizing reactive bias. Thirdly, the intentional status of unresolved discrepancies was confirmed by the physician at 48-hours, as recommended in previous medication reconciliation studies [6].
The finding that almost half of the patients surveyed continued to experience an unintentional discrepancy 24 h after the clinical pharmacist’s intervention indicates the need to review the process of medication management on admission. The majority of the unresolved unintentional discrepancies were judged to be of low clinical significance and were unrelated to the presenting complaint. The resolved unintentional discrepancies were assessed as having greater potential to result in harm, which is suggestive that the involvement of the clinical pharmacist in admission medication management may be an effective barrier to medication related morbidity and this should be investigated using a comparative study. For the less clinically significant discrepancies, future studies should investigate the benefit of the pharmacist resolving the discrepancy and communicating the change to the prescriber, rather than relying solely on the prescriber to implement the suggested change. Furthermore as the mode of communication (verbal or written) appeared to influence whether a discrepancy was resolved or not, consideration should be given to testing this as a means to improving outcomes in the overall medicines management process. Pharmacist participation in post-admission ward rounds may provide a forum for communication with physicians which facilitates timely resolution of medication-related issues. Ultimately, medication reconciliation is a resource intensive process and research to establish how best to deliver this with limited resources is warranted.
Conclusion
Clinical pharmacists contribute positively to medication reconciliation on admission to hospital in Ireland and they should be engaged to deliver this service within 24 h of admission.
References
Pippins JR, Gandhi TK, Hamann C, Ndumele CD, Labonville SA, Diedrichsen EK, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med. 2008;23(9):1414–22.
Cornish PL, Knowles SR, Marchesano R, Tam V, Shadowitz S, Juurlink DN, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165:424–9.
Vira T, Colquhoun M, Etchells E. Reconcilable differences: correcting medication errors at hospital admission and discharge. Qual Saf Health Car. 2006;15:122–6.
Rees S, Thomas P, Shetty A, Makinde K. Drug history errors in the acute medical assessment unit quantified by use of the NPSA classification. Pharm J. 2007;279:469–71.
Wong JD, Bajcar JM, Wong GG, Alibhai SMH, Huh J-H, Cesta A, et al. Medication reconciliation at hospital discharge: evaluating discrepancies. Ann Pharmacother. 2008;42:1373–9.
Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ. 2005;173(5):510–5.
Grimes TC, Duggan CA, Delaney TP, Graham IM, Conlon KC, et al. Medication details documented on hospital discharge: cross sectional observational study of factors associated with medication non-reconciliation. Br J Clin Pharmacol. 2011;71(3):449–57.
Fitzsimons M, Grimes T, Galvin M. Sources of pre-admission medication information: observational study of accuracy and availability. Int J Pharm Pract. 2011;19(6):408–16.
Institute for Healthcare Improvement. Accuracy at every step: the challenge of medication reconciliation. Cambridge: Institute of Healthcare Improvement; 2006.
Madden D. Building a culture of patient safety. Report of the commission on patient safety and quality assurance (IE). Department of Health, Ireland; 2008.
National Institute for Health and Clinical Excellence/National Patient Safety Agency. Technical patient safety solutions for medicines reconciliation on admission of adults to hospitals. PSG001; 2007.
National Prescribing Centre (UK). Medicines reconciliation: a guide to implementation. Good practice guide, 5 min guides; 2008.
Campbell F, Karnon J, Czoski C, Jones R. A systematic review of the effectiveness and cost-effectiveness of interventions aimed at preventing medication errors (medicines reconciliation) at hospital admission. The University of Sheffield, School of Health and Related Research. (ScHARR); 2007.
de Winter S, Spriet R, Indevuyst C, Vanbrabant P, Desruelles D, Sabbe M, et al. Pharmacist-versus-physician-acquired medication history: a prospective study at the emergency department. Qual Saf Health Care. 2010;19(5):371–5.
Nester TM, Hale LS. Effectiveness of a pharmacist-acquired medication history in promoting patient safety. Am J Health Syst Pharm. 2002;59:2221–5.
Karnon J, Campbell F, Czoski C. Model-based cost-effectiveness analysis of interventions aimed at preventing medication error at hospital admission (medicines reconciliation). J Eval Clin Pract. 2009;15:299–306.
Gillespie U, Alassaad A, Henrohn D, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomised controlled trial. Arch Intern Med. 2009;169:894–900.
Burnett KM, Scott M, Fleming GF, Clark CM, McElnay JC. Effects of an integrated medicines management program on medication appropriateness in hospitalised patients. Am J Health Syst Pharm. 2009;66:854–9.
Scullin C, Scott MG, Hogg A, et al. An innovative approach to integrated medicines management. J Eval Clin Pract. 2007;13:781–8.
Karapinar-Carkit F, Borgsteede S, Zoer J, et al. Effect of medication reconciliation with and without patient counselling on the number of pharmaceutical interventions among patients discharged from the hospital. Ann Pharmacother. 2009;43:1001–10.
Royal College of Physicians of Ireland/Irish Association of Directors of Nursing and Midwifery/Therapy Professions Committee/Quality and Clinical Care Directorate, Health Service Executive. Report of the National Acute Medicine Programme 2010 (internet) accessed 5th October 2011 http://www.hse.ie/eng/services/Publications/services/Hospitals/AMP.pdf.
Bolas H, Brookes K, Scott M, et al. Evaluation of a hospital-based community liaison pharmacy service in Northern Ireland. Pharm World Sci. 2004;26(2):114–20.
Bowling A. Research methods in health: investigating health and health services. 2nd ed. Buckingham: Open University Press; 2002.
Dean BS, Barber ND. A validated reliable method of scoring the severity of medication errors. Am J Health-Syst Pharm. 1999;56:57–62.
Vasileff HM, Whitten LE, Pink JA, et al. The effect on medication errors of pharmacists charting medication in an emergency department. Pharm World Sci. 2009;31:373–9.
Cohen V, Jellinek SP, Hatch A, Motov S. Effect of clinical pharmacists on care in the emergency department: a systematic review. Am J Health Syst Pharm. 2009;66(15):1353–61.
Bracey G, Miller G, Dean B, et al. The contribution of a pharmacy admissions service to patient care. Clin Med. 2008;8(1):53–7.
Acknowledgments
The work of all the healthcare professionals who undertook the clinical significance testing is greatly appreciated.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galvin, M., Jago-Byrne, MC., Fitzsimons, M. et al. Clinical pharmacist’s contribution to medication reconciliation on admission to hospital in Ireland. Int J Clin Pharm 35, 14–21 (2013). https://doi.org/10.1007/s11096-012-9696-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-012-9696-1