Abstract
Purpose
Paclitaxel (PTX) is currently used in combination with cisplatin for Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for the treatment of peritoneal carcinomatosis. Albumin-bound PTX is a promising new drug for HIPEC because of its easy solubility in aqueous perfusion medium and possibly because of the tendency of albumin to cross physiological barriers and accumulate in tumor tissue.
Methods
We tested the feasibility of using nab-paclitaxel in rabbits treated by HIPEC for 60 min compared with the classical formulation at an equivalent PTX dose. Samples of perfusate and blood were collected at different time points and peritoneal tissues were collected at the end of perfusion. PTX concentrations were determined by HPLC. The depth of paclitaxel penetration through the peritoneal barrier was assessed by mass spectrometry imaging.
Results
PTX after nab-paclitaxel treatment penetrated up to 0.63 mm in the peritoneal wall, but after CRE-paclitaxel, it was not detectable in the peritoneum. Moreover, the peritoneal concentration after nab-paclitaxel was five times that after paclitaxel classical formulation. Despite the high levels reached in the peritoneum, systemic exposure of PTX was low.
Conclusions
Our results show that nab-paclitaxel penetrates into the abdominal wall better than CRE-paclitaxel, in terms of effective penetration and peritoneal tissue concentration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraperitoneal chemotherapy is safe and effective in patients with advanced neoplastic disease and for the treatment and prophylaxis of peritoneal carcinomatosis (1–3). Paclitaxel (PTX) is currently used in combination with cisplatin for intraperitoneal chemotherapy showing clinical benefit, and a good safety profile (4).
We and other groups have reported the highly favorable pharmacokinetics of PTX during Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Intraperitoneal drug administration allows the achievement of high drug concentration in the abdominal cavity, with minimal plasmatic drug shift, and with big differences between plasmatic and intra-abdominal concentrations: PTX concentration was found approximately 1000 times higher in the abdominal wall than in plasma (4, 5).
Hyperthermia appears to increase the efficacy of many drugs, probably, by enhancing penetration through the peritoneum. However, the drug penetration in the abdominal wall is very limited, a few millimeters for many molecules (6), but much less than 1 mm for PTX in patients with peritoneal carcinomatosis from ovarian cancer (4), suggesting that this therapy is indicated only for patients with microscopic residual tumor.
On the other hand, drugs administered intraperitoneally are mainly absorbed through the lymphatic vessels, reaching high concentration in lymphnodes. This could increase their effectiveness against lymphnode metastasis which is the second cause of disease recurrence for many abdominal cancers (7, 8).
Research is therefore important for new molecules that can remain longer in the abdominal cavity and whose pharmacodynamic and pharmacokinetic features are suitable for intra-abdominal administration. Several studies have enlarged the number of molecules for intraperitoneal administration either in humans or in animal models (9–14), or have found out new formulations to obtain better penetration in the abdominal wall. A possible candidate for this approach is nab-paclitaxel. Nab-technology (nanoparticle-albumin-bound) has emerged as a promising approach to encapsulate PTX into albumin-based nanoparticles, thus avoiding the use of surfactant solvent such as CremophorEL (CRE), reducing allergic reactions and side effects (15). Albumin is an ideal carrier for drug delivery because of its tendency to cross natural physiological barriers and accumulate in tumor tissue due to both receptor-mediated transcytosis and enhanced permeability and retention effect (EPR) (16, 17). Moreover, the absence of solvent micelles, which are instead present in formulations containing CRE, could increase tissue distribution (18).
The aim of the present study was to assess whether nab-paclitaxel is a good candidate for HIPEC and has a favorable pharmacokinetic profile compared to the classical formulation of paclitaxel for more effective penetration of the drug in peritoneal tissues thus possibly increasing the therapeutic efficacy against peritoneal carcinomatosis.
Material and Methods
Animals
Procedures involving animals and their care were conducted in conformity with institutional guidelines that comply with national (DL26, March 2014) and international (EEC Council Directive 2010/63, August, 2013) laws and policies and in line with guidelines for the welfare and use of animals in cancer research (19).
Nine female New Zealand White Rabbits were used (mean weight 3.5 ± 0.5 kg = m2 0.26). A conversion table was used to calculate the total Body Surface Area. All interventions were performed in a dedicated veterinary operating room. One rabbit was used to test the infusion system (HIPEC circuit in vivo evaluation using saline solution as perfusate) and another one to collect control biological specimens (CTRL).
HIPEC
The circuit comprised two roller pumps (inflow and outflow), one heat exchanger, a reservoir for chemotherapy solution and two solution temperature probes (to measure the central circuit and the rabbit’s intra-abdominal temperature). During the whole procedure inflow and outflow parameters were set as needed. The procedure started when the solution temperature reached 40–42°C. During perfusion, body and the circulating solution temperature were monitored constantly and reservoir solution temperature adjusted as needed.
HIPEC was performed with nab-paclitaxel injectable suspension (kindly provided by Celgene Europe Limited, Uxbridge, UK) and with the classical paclitaxel formulation in CremophorEL (kindly provided by Mylan Generics, Milan, Italy). All the animals were put under general anesthesia through an intramuscular administration of xilazine and ketamine-HCl at the dose of 1 mg/kg and 15 mg/kg respectively. Maintenance of the anesthesia was achieved by orotracheal intubation and consequent administration of a mixture of isofluorane and oxygen. The animals were kept under general anesthesia and vital parameters (heart rate, oximetry) were continuously recorded. A central venous catheter was placed surgically in the external jugular vein for fluids administration and blood collection during the 1 h procedure.
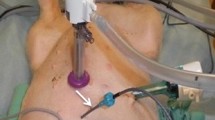
A xifo-pubic laparotomy was performed under sterile conditions and a custom made Coliseum technique was used. Two Jackson Pratt drainages for inflow and outflow chemotherapy solution and an abdominal temperature probe were inserted to ensure homogeneous diffusion of the perfusate in the abdominal cavity (Fig. 1).
Schematic representation of the experimental HIPEC circuit. The equipment consisted of a heat exchanger, two roller pumps (inflow and outflow), a reservoir for chemotherapy solution and two temperature probes. The perfusate, warmed by the heat exchanger, was pumped in the circuit to perfuse the animal and after back in the reservoir.
Consistently with clinical practice, rabbits underwent HIPEC for 60 min at 40–42°C, with nab-paclitaxel (n = 4) or CRE-paclitaxel (n = 3). A dose of 10.83 mg/kg of paclitaxel (extrapolated from the dose of 175 mg/m2 of paclitaxel, commonly used in clinic), corresponding to a mean total dose of 45.5 mg of paclitaxel, was dissolved in 1 L of 0.9% NaCl solution. One rabbit was perfused with 1 L of 0.9% NaCl solution without drug and used to collect control biological samples.
All animals were euthanized with an intravenous overdose of potassium chloride under general anesthesia at the end of the procedure.
Samples Collection
Samples of perfusate solution and blood were collected at different time points in single at 0, 5, 30, 45 and 60 min during HIPEC. Heparinized blood samples were centrifuged for 15 min at 3000 rpm, the plasma was separated and stored frozen at −20°C together with the perfusate samples until HPLC analysis.
At the end of the HIPEC procedure, tissue samples were collected before euthanasia as follows: two 1 cm3 specimens including peritoneum and abdominal muscle layers, one 1 cm3 specimen of liver, kidney, omentum, stomach and mesojejunum tissue. One of the two samples of peritoneum was marked in order to distinguish the portion of tissue adjacent to peritoneal cavity so the sample can be correctly oriented for Matrix Assisted Laser Desorption Ionization (MALDI) imaging analysis.
Tissue samples were gently washed in saline solution and immediately frozen in liquid nitrogen and stored in dry ice or at −80°C until imaging analysis and/or HPLC analysis.
MALDI Mass Spectrometry Imaging and Drug Assay
We assessed the depth of PTX distribution after nab-paclitaxel or CRE-paclitaxel perfusion through the peritoneal barrier by mass spectrometry imaging (MSI). This emerging technique allows molecular visualization of the distribution of drugs and metabolites in biological tissues, providing information on drug localization with high specificity and good spatial resolution (20). For imaging PTX distribution we used a method recently established in our laboratory reported in detail by Morosi et al. (21). Briefly, frozen peritoneal tissues were cut in 10 μm thick sections using a cryo-microtome (Leica Microsystems, Wetzler, Germany) at −20°C and mounted on pre-cooled MALDI plate (Opti-TOF 384 Well insert) by standard thaw-mounting techniques. Three sections were cut for each peritoneal sample for MSI analysis. The plate was dried 1 h under vacuum at room temperature and then sprayed with TiO2 matrix suspension containing deuterated paclitaxel (D5-PTX, Toronto Research, Toronto, Canada), as internal standard (IS), using a BD 180 precision double-action trigger airbrush using nitrogen at 0.2 atm.
MSI analysis was performed using a MALDI 4800 TOF-TOF (AB SCIEX Old Connecticut Path, Framingham, USA), equipped with a 355 nm Nd:YAG laser with a 200 Hz repetition rate, controlled by the 4000 Series ExplorerTM software (AB SCIEX Old Connecticut Path, Framingham, USA). Images of tissue sections were acquired using the 4800 Imaging Tool software (www.maldi-msi.org, M. Stoeckli, Novartis Pharma, Basel, Switzerland), with an imaging raster of 100x100 μm, plotting fragment ion at m/z 284.2 corresponding to the side chain with the amide-acyl group of PTX. The depth of penetration was deduced from the side of a square region of interest (ROI) whose area was drawn and calculated using Tissue View software 1.1 for the portion of tissue reached by the drug.
PTX was measured in plasma, perfusion fluid, peritoneal tissue and other tissues by HPLC (22, 23). Briefly, 0.3 mL of plasma, 0.1 mL of perfusate solution or 0.5 mL of tissue homogenates were spiked with 2 μg of IS (IDN5390, Indena, Milan, Italy) and extracted with 1.5 mL of CH3CN (4 mL for tissue samples). The organic phase was separated, dried under nitrogen, the residue was reconstituted in 150 μL of mobile phase (MP) and injected into the HPLC system (Alliance 2695, Waters, Milford, MA, USA). The chromatographic column was a Symmetry C18, 5 μm, 4.6 × 150 mm (Waters, Milford, MA, USA) and the MP was composed of CH3COONH4 0.01 M pH 5 (50%), CH3CN (40%) and CH3OH (10%). The limits of quantification (LOQ) were 0.04 μg/sample for plasma and 0.08 μg/sample for tissues.
Statistical Analysis
Statistical analysis was performed with GraphPad Prism version 6.01 software (GraphPad software, Inc., La Jolla, CA, USA.). Student’s t test was performed to evaluate differences in PTX concentration in different tissues. P-value <0.05 was considered significant.
Results
Penetration of Drug into Peritoneal Wall
Figure 2 shows the penetration of PTX in peritoneal tissue of rabbits assessed by MSI, after perfusion of nab-paclitaxel or CRE-paclitaxel. The PTX signal, marked in blue, was clearly visible in the side of the peritoneum adjacent to the peritoneal cavity of the rabbits perfused with nab-paclitaxel (A1 - A4). Instead the drug signal is not visible in the rabbits perfused with CRE-paclitaxel. As expected, in the peritoneum of the rabbit perfused with control solution the drug signal is absent. The clarity of signal after nab-paclitaxel treatment allowed us to estimate that PTX penetrated efficiently for up to 0.63 mm in the peritoneal wall (Table I).
Pharmacokinetics Results
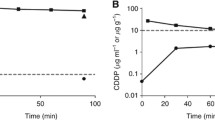
PTX concentrations of the two formulations determined by HPLC in perfusate, plasma, and the total content in peritoneal tissue are compared in Fig. 3. Both formulations warranted high PTX concentrations in perfusate fluid and peritoneum, much higher than the concentrations known to be cytotoxic (5). Looking at the PTX concentration-time curves in the peritoneal fluid it is notable a marked decrease of PTX concentration in the rabbits treated with nab-paclitaxel, marked decrease that is not observed in the rabbits treated with CRE-paclitaxel. As consequence, the mean PTX concentration in the peritoneum of the rabbits treated with nab-paclitaxel (13.22 ± 0.97 μg/g; range: 12.37–14.41 μg/g) was significantly higher (student’s t test p-value = 0.0002) than the concentration measured in the peritoneum of the rabbits perfused with CRE-paclitaxel (3.55 ± 1.75 μg/g; range 2.01–5.45 μg/g) (Fig. 3). PTX systemic exposure was very low for both formu-lations, though slightly higher with nab-paclitaxel than CRE-paclitaxel. PTX concentrations in plasma are consistent with values reported in literature for HIPEC with CRE-paclitaxel in animal model (24). Finally, PTX concentrations achieved in the organs in the peritoneal cavity or extra-peritoneal (the kidney is reported as an example) were higher with nab-paclitaxel than with CRE-paclitaxel (Fig. 4). However even with nab-paclitaxel, the drug concentrations in normal organs are very low −20 and 50 times lower than those achieved after i.v. injection of PTX at equivalent dose in mice and rabbits (25–27)- and thus unlikely toxic.
Discussion
The taxane alkaloid PTX is widely used in the treatment of various cancers either for intravenous or intraperitoneal use. The anticancer activity is related to its binding to microtubules that are stabilized with consequent antimitotic effects.
Paclitaxel is barely soluble in various aqueous solvents and is therefore dissolved as a micellar formulation containing the surfactant Cremophor EL and ethanol, to be subsequently diluted in physiological solution for administration to patients. In humans, Hyperthermic intraperitoneal taxanes administration has mainly been studied in ovarian malignancies (5, 28–34). In particular De Bree et al. and our group reported the intraperitoneal hyperthermic use of docetaxel and PTX in gynecological malignancies (4, 34, 35).
One of the new frontiers of peritoneal carcinosis treatment is search for and application of new molecules to increase the penetration in peritoneal tissue. One possibility is to find out new combination of chemotherapeutics or either drug-carriers or new perfusional strategies. Nab-paclitaxel is a clinically approved nano-formulation of PTX linked to albumin. The conjugation with albumin has several advantages: easy administration due to water-solubility, no need for toxic solvents, improved pharmacokinetics and favored pooling at the cancer site because of the EPR effect and receptor mediate transcytosis. In addition a better antitumor activity of i.p. nab-paclitaxel than CRE-paclitaxel at equitoxic doses was reported in literature (36).
The use of albumin-bound paclitaxel for HIPEC perfusion has never been tested before and the present study in rabbits is thus completely novel. Our results clearly demonstrated the better penetration profile into the abdominal wall of nab-paclitaxel compared to CRE-paclitaxel, both in terms of effective diffusion and concentration achieved in the peritoneum. Nab-paclitaxel has a favorable pharmacokinetic profile for HIPEC which could enhance the drug effect without increasing toxicity. In fact, the high PTX concentration achieved in peritoneal tissue after nab-paclitaxel perfusion, where micrometastasis may be present after cytoreductive surgery, is surely high enough to exert antitumor activity and it is associated with negligible plasma exposure and probably no systemic toxicity. In fact in our cases, we not achieved plasma concentrations of PTX > 0.05 μmol/L (0.04 μg/ml) lasting for more than ten hours, condition reported to be cause of neutropenia, the dose-limiting toxicity for PTX in patients (37). Moreover even if the concentrations in peritoneal organs and in kidneys are higher with nab-paclitaxel than with CRE-paclitaxel, they are much lower than the concentrations achieved after i.v. administration of CRE-paclitaxel in animal models of mice and rabbits (25–27). Consequently an increase in toxicity cannot be considered probable.
Consistently with PK data, MALDI imaging indicated that the albumin-based nanoparticles penetrated better than CRE-paclitaxel in the rabbit peritoneum. In fact we highlighted that paclitaxel penetrates up to 0.63 mm in the peritoneal wall after nab-paclitaxel while it is not detectable after CRE-paclitaxel. The improved penetration of nab-paclitaxel in the abdominal wall is probably due mainly to the solubility of this formulation in the perfusion liquid that ensures major stability and bioavailability of the drug inside the abdomen. Moreover, nab-paclitaxel does not contain CremophorEL which inhibits uptake and transport across endothelial cell monolayer creating micelles that trap the drug (38, 39). In fact a substantial decrease has been observed in the bioavailability of PTX after i.p. administration in the presence of CRE (40). Finally even the receptor mediated transcytosis could be involved in the better nab penetration in normal peritoneal wall. In fact has been reported that albumin transcitosis is mediated manly by Caveolin-1 (17, 41) and that this protein is highly expressed in mesothelial cells in different anatomical sites including peritoneum (42).
This finding together with a better antitumor activity of i.p. nab-paclitaxel described in literature (36), suggests that nab-paclitaxel, when administered by HIPEC, could be more effective than CRE-paclitaxel against peritoneal metastatic cancer cells.
Conclusions
This study shows that in rabbits nab-paclitaxel penetrates through the peritoneum better than the classical PTX formulation when administered by HIPEC. Further experiments in tumor bearing animals should be performed to confirm the improved penetration even in peritoneal metastasis. These data provide a strong rational for a clinical study to assess the efficacy and the safety of nab-PTX by HIPEC.
Abbreviations
- CRE:
-
CremophorEL
- EPR:
-
Enhanced permeation and retention
- HIPEC:
-
Hyperthermic intraperitoneal chemotherapy
- IS:
-
Internal standard
- LOQ:
-
Limit of quantitation
- MALDI:
-
Matrix assisted laser desorption ionization
- MP:
-
Mobile phase
- MSI:
-
Mass spectrometry imaging
- Nab :
-
Nanoparticles-albumin-bound
- PTX:
-
Paclitaxel
- ROI:
-
Region of interest
References
Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2014;40(1):12–26.
Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19(41):6979–94.
Sugarbaker PH, Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol. 2016;7(1):29–44.
Ansaloni L, Coccolini F, Morosi L, Ballerini A, Ceresoli M, Grosso G, et al. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br J Cancer. 2015;112(2):306–12.
de Bree E, Rosing H, Filis D, Romanos J, Melisssourgaki M, Daskalakis M, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol. 2008;15(4):1183–92.
Emoto S, Sunami E, Yamaguchi H, Ishihara S, Kitayama J, Watanabe T. Drug development for intraperitoneal chemotherapy against peritoneal carcinomatosis from gastrointestinal cancer. Surg Today. 2014;44(12):2209–20.
Soma D, Kitayama J, Ishigami H, Kaisaki S, Nagawa H. Different tissue distribution of paclitaxel with intravenous and intraperitoneal administration. J Surg Res. 2009;155(1):142–6.
Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277–83.
Simón-Gracia L, Hunt H, Scodeller PD, Gaitzsch J, Braun GB, Willmore A-MA, et al. Paclitaxel-loaded Polymersomes for enhanced intraperitoneal chemotherapy. Mol Cancer Ther. 2016;15(4):670–9.
Coccolini F, Campanati L, Catena F, Ceni V, Ceresoli M, Jimenez Cruz J, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol. 2015;26(1):54–61.
de Bree E. Optimal drugs for HIPEC in different tumors. J BUON Off J Balk Union Oncol. 2015;20(Suppl 1):S40–6.
Braam HJ, Schellens JH, Boot H, van Sandick JW, Knibbe CA, Boerma D, et al. Selection of chemotherapy for hyperthermic intraperitoneal use in gastric cancer. Crit Rev Oncol Hematol. 2015;95(3):282–96.
Liu L-L, Yi T, Zhao X. Antitumor effect of D-erythrose in an abdominal metastatic model of colon carcinoma. Oncol Lett. 2015;9(2):769–73.
Mehta AM, Van den Hoven JM, Rosing H, Hillebrand MJX, Nuijen B, Huitema ADR, et al. Stability of oxaliplatin in chloride-containing carrier solutions used in hyperthermic intraperitoneal chemotherapy. Int J Pharm. 2015;479(1):23–7.
Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Design, Development and Therapy. 2015;9:3767–77.
Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release Off J Control Release Soc. 2013;170(3):365–72.
Yu X, Jin C. Application of albumin-based nanoparticles in the management of cancer. J Mater Sci Mater Med. 2016;27(1):4.
Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102(11):1555–77.
Morosi L, Zucchetti M, D’Incalci M, Davoli E. Imaging mass spectrometry: challenges in visualization of drug distribution in solid tumors. Curr Opin Pharmacol. 2013;13(5):807–12.
Morosi L, Spinelli P, Zucchetti M, Pretto F, Carrà A, D’Incalci M, et al. Determination of paclitaxel distribution in solid tumors by nano-particle assisted laser desorption ionization mass spectrometry imaging. PloS One. 2013;8(8):e72532.
Fruscio R, Lissoni AA, Frapolli R, Corso S, Mangioni C, D’Incalci M, et al. Clindamycin-paclitaxel pharmacokinetic interaction in ovarian cancer patients. Cancer Chemother Pharmacol. 2006;58(3):319–25.
Cesca M, Frapolli R, Berndt A, Scarlato V, Richter P, Kosmehl H, et al. The effects of vandetanib on paclitaxel tumor distribution and antitumor activity in a xenograft model of human ovarian carcinoma. Neoplasia N Y N. 2009;11(11):1155–64.
Bouquet W, Deleye S, Staelens S, De Smet L, Van Damme N, Debergh I, et al. Antitumour efficacy of two paclitaxel formulations for hyperthermic intraperitoneal chemotherapy (HIPEC) in an in vivo rat model. Pharm Res. 2011;28(7):1653–60.
Eiseman JL, Eddington ND, Leslie J, MacAuley C, Sentz DL, Zuhowski M, et al. Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemotherapy and Pharmacology. 1994;34(6):465–71.
Colombo C, Morosi L, Bello E, Ferrari R, Licandro SA, Lupi M, et al. PEGylated Nanoparticles Obtained through Emulsion Polymerization as Paclitaxel Carriers. Mol Pharm. 2016;13(1):40–6.
Wei Y, Xue Z, Ye Y, Wang P, Huang Y, Zhao L. Pharmacokinetic and tissue distribution of paclitaxel in rabbits assayed by LC-UV after intravenous administration of its novel liposomal formulation. Biomed Chromatogr BMC. 2014;28(2):204–12.
Ansaloni L, Agnoletti V, Amadori A, Catena F, Cavaliere D, Coccolini F, et al. Evaluation of extensive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2012;22(5):778–85.
Muñoz-Casares FC, Rufián S, Arjona-Sánchez Á, Rubio MJ, Díaz R, Casado Á, et al. Neoadjuvant intraperitoneal chemotherapy with paclitaxel for the radical surgical treatment of peritoneal carcinomatosis in ovarian cancer: a prospective pilot study. Cancer Chemother Pharmacol. 2011;68(1):267–74.
Muñoz-Casares FC, Rufián S, Rubio MJ, Díaz CJ, Díaz R, Casado A, et al. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2009;11(11):753–9.
Kim JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Consolidation hyperthermic intraperitoneal chemotherapy using paclitaxel in patients with epithelial ovarian cancer. J Surg Oncol. 2010;101(2):149–55.
Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007;106(1):193–200.
Rufián S, Muñoz-Casares FC, Briceño J, Díaz CJ, Rubio MJ, Ortega R, et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol. 2006;94(4):316–24.
de Bree E, Romanos J, Michalakis J, Relakis K, Georgoulias V, Melissas J, et al. Intraoperative hyperthermic intraperitoneal chemotherapy with docetaxel as second-line treatment for peritoneal carcinomatosis of gynaecological origin. Anticancer Res. 2003;23(3C):3019–27.
de Bree E, Rosing H, Beijnen JH, Romanos J, Michalakis J, Georgoulias V, et al. Pharmacokinetic study of docetaxel in intraoperative hyperthermic i.P. Chemotherapy for ovarian cancer. Anticancer Drugs. 2003;14(2):103–10.
Kinoshita J, Fushida S, Tsukada T, Oyama K, Watanabe T, Shoji M, et al. Comparative study of the antitumor activity of Nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol Rep. 2014;32(1):89–96.
Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47(9):2486–93.
Chen N, Brachmann C, Liu X, Pierce DW, Dey J, Kerwin WS, et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother Pharmacol. 2015;76(4):699–712.
Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer Oxf Engl 1990. 2006;42(1):24–30.
Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, Nooter K, et al. Influence of Cremophor el on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res Off J Am Assoc Cancer Res. 2002;8(4):1237–41.
Gotloib L, Shostak A. Endocytosis and transcytosis of albumin gold through mice peritoneal mesothelium. Kidney Int. 1995;47(5):1274–84.
Shang D, Peng T, Gou S, Li Y, Wu H, Wang C, et al. High Mobility Group Box Protein 1 Boosts Endothelial Albumin Transcytosis through the RAGE/Src/Caveolin-1 Pathway. Sci Rep. 2016;6:32180.
von Ruhland CJ, Campbell L, Gumbleton M, Jasani B, Newman GR. Immunolocalization of caveolin-1 in rat and human mesothelium. J Histochem Cytochem Off J Histochem Soc. 2004;52(11):1415–25.
ACKNOWLEDGMENTS AND DISCLOSURES
We acknowledge support to ED from Cariplo Foundation for the project (Project 2013–0692). The study was supported in part by funding from Celgene Corporation Grant-ITA-039 (M1266). The authors thank J.D. Baggott for editing the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Procedures involving animals and their care were conducted in conformity with institutional guidelines that comply with national (DL26, March 2014) and international (EEC Council Directive 2010/63, August, 2013) laws and policies and in line with guidelines for the welfare and use of animals in cancer research.
Disclosure of Commercial Interest
None of the authors has any financial and personal relationships with other people or organizations to disclose, that could inappropriately influence their work.
Additional information
Federico Coccolini, Fabio Acocella and Lavinia Morosi equally contributed to this work
Rights and permissions
About this article
Cite this article
Coccolini, F., Acocella, F., Morosi, L. et al. High Penetration of Paclitaxel in Abdominal Wall of Rabbits after Hyperthermic Intraperitoneal Administration of Nab-Paclitaxel Compared to Standard Paclitaxel Formulation. Pharm Res 34, 1180–1186 (2017). https://doi.org/10.1007/s11095-017-2132-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2132-4