Abstract
Purpose
Characterize the contents of distal ileum and cecum in healthy adults under conditions simulating the bioavailability/bioequivelance studies of drug products in fasted and fed state.
Methods
Twelve males participated in a two-phase crossover study. Phase I: subjects remained fasted overnight plus 4.5 h in the morning prior to colonoscopy. Phase II: subjects remained fasted overnight, consumed breakfast in the morning, and abstain from food until colonoscopy, 4.5 h after breakfast. Upon sampling, volume, pH and buffer capacity were measured; after ultracentrifugation, supernatant was physicochemically characterized and non-liquid particles diameter was measured.
Results
In distal ileum, pH is ~8.1 and size of non-liquid particles is ~200 μm, regardless of dosing conditions; in fed state, liquid fraction was lower whereas osmolality and carbohydrate content were higher. In cecum, the environment was similar with previously characterized environment in the ascending colon; in fasted state, size of non-liquid particles is smaller than in distal ileum (~70 μm). Fluid composition in distal ileum is different from cecum, especially in fasted state.
Conclusion
Differences in luminal environment between distal ileum and cecum may impact the performance of orally administered products which deliver drug during residence in lower intestine. Dosing conditions affect cecal environment more than in distal ileum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Luminal environment in the lower intestine is important for the performance of orally administered active pharmaceutical ingredients (APIs) with extended and/or delayed absorption kinetics. Examples are immediate release products of highly dosed APIs and modified release drug products. Due to the limited free water volume in the transverse colon (1) regions of primary interest in regard to drug/dosage form performance in the lower gut are the distal small intestine, the cecum, and the ascending colon.
To date, performance of dosage forms is assessed with in vivo bioavailability (BA) / bioequivalence (BE) studies in healthy adults which are time consuming and costly. One way to reduce the likelihood of failing an in vivo BA/BE study, it would be to screen the performance in vitro in a simulated environment. In recent years our knowledge on the environment in healthy adult gastrointestinal (GI) tract under conditions simulating BA/BE studies has been improved substantially, for the upper GI lumen and to a limited extent for the lower gut (e.g. 2). It is interesting to note that physicochemical characteristics of contents of ascending colon differ from those in upper intestinal lumen under both fasted and fed state conditions to an extent that may affect API and/or dosage form performance (3–5). Although in clinical practice a distinction between fasted and fed state may not be relevant, in BA/BE studies food effects are of primary concern. Studies have shown that solubility of lipophilic APIs in the lumen of the ascending colon can be different than in the contents of stomach and/or upper intestine during fasting state (6) and such differences together with subsequent difference in luminal dissolution rates may be crucial for the prediction of intraluminal behaviour of highly dosed APIs (7).
In the present investigation the environment in distal ileum was evaluated versus the environment in cecum, two regions in the lower intestine which have been poorly characterized to date. Although bacteria may also affect the fate of drugs and dosage forms, in the present study we characterized the contents on a physicochemical basis without specifically considering the microbes.
Materials and Methods
Materials
Dulcolax® (disacodyl tablets, 5 mg/tablet) was from Boehringer Ingelheim (Athens, Greece). All chemicals were of analytical grade and purchased from Sigma Aldrich Chemie GmbH (Germany), except for egg phosphatidylcholine (for constructing standard curves), which was donated by Lipoid GmbH (Germany). All solvents were of HPLC grade. Total protein content was determined using a commercially available kit (BCA®, Thermo Scientific, Rockford, USA using bovine serum albumin as reference) whereas samples were pretreated with Compact-Able™ Protein Assay Preparation Reagent set (Pierce, Rockford, USA).
Human Study
The study was conducted in the Red Cross Hospital of Athens after receiving approvals by the Scientific and the Executive Committee of the Hospital (AP 30799, 06 December 2012). The study was performed according to the Declarations of Helsinki for biomedical research involving human subjects.
Inclusion Criteria
Willingness of the subject to participate as indicated by his signed informed consent, age 18–60 years, weight within 20% of ideal body weight as determined by Metropolitan Life Tables, verification of suitability by a general physical examination and ability to abstain from cigarette smoking, alcohol, and over-the-counter and prescription medication(s) for 3 days prior to and throughout the Colonoscopy Day. In addition, a blood sample was taken to assess electrolyte balance, kidney and liver function, blood morphologic characteristics, and lipid levels, and the subject had to be found healthy in all these examinations to qualify.
Exclusion Criteria
Existence of a major health problem (cardiovascular, pancreatic, hepatic, thyroid etc.) and/or existence of any condition requiring prescription drug therapy, recent history of gastrointestinal symptom regardless of the severity (e.g. heartburn, constipation, haemorrhoids, etc.), receipt of an investigational agent (new or generic) within 30 days prior to the initiation of study, presence of antibodies indicating active acute or chronic HIV, HBV, or HCV infection, use of medication which may affect GI function (including antibiotics) within 30 days of the study, and irregular bowel habits.
Subjects
Twelve healthy males with a mean age of 28 (range: 22–42 years) gave informed consent and participated in this two-phase crossover study.
Study Protocol
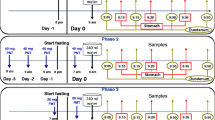
Subject work-up prior to either Phase involved the administration of 10 mg of bisacodyl 50 h prior to colonoscopy plus 10 mg of bisacodyl 44 h prior to each colonoscopy. With this regimen, bisacodyl effects on the intracolonic environment at the time of colonoscopy have been shown to be non-significant (4). One subject received an additional 5 mg bisacodyl tablet 30 h prior to each colonoscopy. From the time of first bisacodyl administration until the night prior to each colonoscopy day, subjects ate only liquified food (e.g. fish/chicken/rice soups, fruit juices) and white bread ad libitum. From the night prior to colonoscopy day until arrival to the clinic, i.e. for at least 12 h, subjects remained fasted (Fig. 1). Subject workup prior to colonoscopy did not have major effect on stools consistency. Based on Bristol Stool Chart (8) stools consistency at distal colon during colonoscopy varied from Type 3 (like a sausage or snake, but with cracks on its surface) to Type 6 (fluffy pieces with ragged edges, a mushy stool).
In Phase I, subjects reported to the clinic at 8:00 am and then continued fasting for an extra 4.5 h. Colonoscopy was started at ≈ 12:30 pm (fasted state). In Phase II, subjects were fasted overnight, reported to the clinic at 8:00 am, and were given a standard breakfast (8:00–8:30 am). Four and a half hours after breakfast, colonoscopy was started (fed state). Breakfast consisted of two eggs fried in butter, two strips of bacon, two slices of toasted bread with butter, four ounces of fried potatos, and eight ounces of milk and 150 ml of water (960 kcal: approximately 150, 250 and 560 cal of protein, carbohydrate and fat, respectively) (4,9). In order to control water consumption on Colonoscopy Day non-carbonated water was administerd to subjects according to the schedule described in Fig. 1. Total amount of water from arrival to the clinic until colonoscopy was 900 ml (Fig. 1).
A few minutes prior to colonoscopy, 0.3 mg alphentanyl hydrochloride (0.5 mg/ml, Rapifen®) and 2.0 mg of midazolam (5 mg/ml, Dormicum®) were administered intravenously to induce conscious sedation. Since conscious sedation was induced 10–40 min prior to sample collection, its potential impact on intestinal transit times requires consideration. In preclinical species the effect of opiates on intestinal transit times is unclear (10) whereas the effect of alfentanyl, specifically, is dose-dependent (11). In cats, midazolam administered at 0.1 mg/kg dose level, i.e. much higher than the dose used in the present study on mg/kg basis, increased significantly oro-caecal transit times (12), i.e. the impact on intestinal transit specifically cannot be evaluated with certainty. These data suggest that the impact on intestinal transit of alfentanyl and midazolam, administered in the present study at low doses to induce conscious sedation, is not clear.
In both Phases, after placement of the colonoscope, collection of contents from the distal ileum and cecum was performed under anaerobic conditions and lasted approximately 20 min in total; approximately 5 min and 15 min were sufficient to collect practically all of the contents from terminal ileum and cecum, respectively. Ileal samples were collected from the distal ileum approximately 20–30 cm from the ileocecal valve and after completion of collection of ileal samples, and discarding all the ileal contents from the colonoscope, cecal samples, i.e. contents from the region between the ileocecal valve up to the end of the cecum (length of about 8–9 cm (13) were collected. Sampling from each region aimed at collecting the entire volume of contents. After removal of the colonoscope, 2 mg of flumazenil (0.1 mg/ml, Anexate®) was administered as antidote to midazolam. In all cases, colonoscopy lasted for about 1 h in total.
Handling of Samples
Sample volume, pH and buffer capacity were measured immediately upon collection. After adding a methanolic cocktail of 50 mM diisopropylfluorophosphate, 50 mM diethyl(p-nitrophenyl)phosphate, 50 mM acetophenone, and 250 mM phenylboronic acid [2% (v:v)] to terminate the supernatant’s lipolytic and proteolytic activity (14), the sample was ultracentrifuged (30,000 g, 15 min, 25°C) under anaerobic conditions and the % aqueous content was measured. Ultracentrifugation conditions were appropriate for eliminating both solids and bacteria (15,16) without affecting the structure of bacteria, i.e. without liberating intracellular components which could contribute to degradation in the supernatant (17). To avoid second freeze—thawing cycle of samples, the supernatant was divided in 6 portions that were kept at −70°C until analysis of each of the following six parameters was performed: osmolality, total protein content, total carbohydrate content, short chain fatty acids, ten individual bile acids and lipids. Precipitate was stored under anaerobic conditions at −70°C and in a part of this material non-liquid particles diameter was measured.
Analysis of Samples
pH values were measured by a pH electrode (Schott, model CG842, Mainz, Germany). Buffer capacity was measured by dropwise addition of HCl solution and was calculated as described previously (3).
Osmolality, total protein content and total carbohydrate content, were measured as described previously (3,4).
Individual bile acids, i.e., taurocholic acid (TC), glycocholic acid (GC), taurochenodeoxycholic acid (TCDC), ursodeoxycholic acid (UDC), glycochenodeoxycholic acid (GCDC), cholic acid (C), glycodeoxycholic acid (GDC), chenodeoxycholic (CDC), deoxycholic (DC) and and lithocholic acid (LC) were quantified by a gradient HPLC method using a Charged Aerosol Detector (4).
Lipids, i.e. triglycerides (TGs), diglycerides (DGs), monoglycerides (MG), fatty acids (FAs), phosphatidylcholine (PC), lyso-phosphatidylcholine (Lyso-PC) and cholesterol (CHO) were assayed by using a previously developed gradient HPLC method using a Charged Aerosol Detector (4). Glycerol trioleate was used for quantifying TGs, 1,2 dioleoyl-rac-glycerol and dipalmitin and dioleylglycerol were used for quantifying DGs, and 1-monooleyl-rac-glycerol was used for quantifying MGs. Oleic acid, linoleic acid and stearic acid were used for quantifying FAs.
Seven short chain fatty acids (SCFAs), i.e. acetic acid (AA), propionic acid (PA), iso-butyric acid (IBA), n-butyric acid (BA), iso-valeric acid (IVA), valeric acid (VA), and caproic acid (CA) were quantified by using a previously developed gas chromatography method (4,18).
Size of non-liquid luminal components was measured using the Malvern Mastersizer apparatus (Malvern Instruments Ltd. Uk). After ultracentrifugation of the luminal samples, a sample from the precipitate was transferred in an Eppendorf tube, 1 mL of water was added, and after vortexing, the sample was transferred in the Malvern Mastersizer volume sample dispersion unit (water volume in the unit: 120 ml, stirrer speed: 2000 rpm), and the volume mean diameter (D[4,3]) was measured. Due to volume availability restrictions, precipitates from five volunteers in the fasted state and six volunteers in the fed state were used; one to four samples from each precipitate were subjected to measurements. A preliminary experiment had showed that same results are obtained if a sample from the precipitate (volunteer #8, cecum sample, fed state, D[4,3] = 271 ± 65 μm) or 1 ml of total luminal sample is used (volunteer #8, cecum sample, fed state, D[4,3] = 269 ± 38 μm). Because of the small volume of collected luminal samples, the use of precipitates was preferred.
Data Analysis
Data are presented as Box-Whisker plots showing the median value, the 10th, 25th, 75th and 90th percentiles, and the individual outlying data points, with dotted lines indicating the mean value.
For each parameter, differences between fasting and fed state and between distal ileum and cecum were evaluated with the paired t-test or the Wilcoxon test, depending on the results of normality and equal variance tests. Type I error was set to 0.05. All statistical comparisons were performed using SigmaStat 3.5 (SPSS Science Inc., New York, USA).
Results and Discussion
Characteristics of Contents of Distal Ileum and Cecum
Total Volume
The volume of contents recovered from distal ileum and from cecum varied significantly with the prandial state (p = 0.007 and p = 0.010 for ileal and cecal contents, respectively, Fig. 2). Mean(SD) volumes of samples collected from distal ileum were 3.8(2.3) ml and 7.3(3.3) ml in the fasted and in the fed state, respectively, whereas the corresponding volumes collected from the cecum were 5.0(2.1) ml and 8.0(2.7) ml, respectively (Fig. 2). There was no statistical difference between ileal and cecal volumes at the same prandial state (p = 0.058 and 0.485 in the fasted and in the fed state, respectively). As the collection of ileal contents lasted approximately 5 min, the values of collected volumes from distal ileum are in line with Ladas et al. (19) where the ileal flow was measured in 5 healthy persons in fasted state and after a long chain triglyceride rich meal and it was found to be 62.4 ± 24.7 ml/h and 96.1 ± 20.2 ml/h. It should be noted that the estimated daily endogenous ileal flow in healthy adults under normal dietary intakes is approximately 2 l per day (20). Compared to volumes recovered from the ascending colon of healthy volunteers [22.3(7.7) ml and 30.8(10.6) ml, in fasted and fed state, respectively (4)], cecal volumes were statistically lower in both dosing conditions (p < 0.001). It is interesting to note that, based on mean values, total volume of contents in the ascending colon is 5.9 and 4.2 times bigger than the total volume of contents in distal ileum.
Volume, non-liquid particles diameter, pH, and buffer capacity of the contents of distal ileum (light yellow boxes) and of cecum (dark green boxes) of healthy volunteers measured in the fasted and in the fed state on a crossover basis. n is the number of subjects contributed to the construction of box plots on a crossover basis.
pH and Buffer Capacity
Median pH value of ileal contents was 8.1 regardless the dosing conditions (Fig. 2). Information regarding ileal luminal pH is limited and has been collected either from patients with ileostomy or from healthy adults using the radiotelemetric capsules. The median values estimated from data in the present study are slightly higher than literature data, according to which ileal pH varies between 7 and 8, but in line with literature data indicating no effect of dosing conditions (21). Furthermore, these data confirm the trend of increasing pH values along the small intestine (2,22). pH of the duodenal contents has been measured by direct sampling in several studies and ranges between 6 and 7 (2,3,21,23) whereas in the jejunum it ranges from 6.5 to 7.8 (2,21) in the fasted state. In the fed state, pH values have been reported to be 5.0–6.5 both in the duodenum (2,3,24,25) and in the jejunum (2,21). Using a radiotelemetry device, pH gradually increases along the small intestine with reported median values being 6.6 in the proximal part, 7.0 in the mid part and 7.3 in the distal part of the small intestine (22).
In cecum, pH of contents varied significantly with prandial state (p = 0.003) (Fig. 2). Median(range) pH values of cecal contents were 7.4 (range: 6.2–8.5) and 6.4 (range: 5.5–7.9) in the fasted and in the fed state, respectively, and these values differ from pH values of ileal contents (p < 0.001 at corresponding prandial states) (Fig. 2). Using a similar protocol for collection of contents from the ascending colon, Diakidou et al. (4) have reported values [pH 7.6, fasted state and pH 6.2, fed state] which are not statistically different from values measured in cecum in the present study (p > 0.245). The trend of decreasing pH values from small intestine to large intestine is in line with pH data obtained with radiotelemetry device (22).
As with pH, buffer capacity also did not differ significantly between prandial states in distal ileum [8.9(3.6) mmol/l/ΔpH in the fasted state and 15.2(8.4) mmol/l/ΔpH in the fed state, p = 0.230] but it differed in cecum [19.2(10.2) mmol/l/ΔpH in the fasted state and 33.6(13.1) mmol/l/ΔpH in the fed state, p = 0.025] (Fig. 2). It is interesting to note that buffer capacity in distal ileum is similar with values previously reported for duodenal and jejunal contents in the fasted state [2.8 mmol/l/ΔpH (19) to 13 mmol/l/ΔpH (3,26)] and similar with the value in ileal contents of a patient with polyposis [6.4 mmol/l/ΔpH (27)]. Compared to cecal contents, ileal contents had significantly lower buffer capacity only in the fed state (p = 0.031). On the other hand, cecal contents had similar buffer capacity with contents in ascending colon in the fasted state [21.5(7.9) mmol/l/ΔpH, p = 0.246] and in the fed state [37.7(15.5) mmol/l/ΔpH, p = 0.563] (4).
Liquid Fraction
The liquid fraction of contents in distal ileum was significantly lower (p = 0.005) in the fed state [68.6(13.2)%] compared to the fasted state [89.9(10.0)%] but in cecum the trend did not reach significance [(63.7(13.1) % in the fed state vs. 69.7(19.2) % in the fasted state, p = 0.436] (Fig. 3). However, the liquid fraction in the fed ascending colon was significantly lower than in the fasted ascending colon (4). Liquid fractions in conjuction with the very small total volumes in the distal ileum and cecum show that liquid volumes in the region are extremely small. In the fasted state, ileal contents had significantly higher proportion of fluids than cecal contents (p < 0.001), but similar in the fed state (p = 0.352). It should be noted that the liquid fraction of cecal contents was not statistically different from previously measured values in contents of ascending colon [(70.3(17.0) % and 56.0(9.0) % in the fasted state and in the fed state, respectively (4)] but statistical power of relevant comparisons was low (0.050 for the fasted state and 0.239 for the fed state comparisons). It is interesting to note that, based on mean values, liquid volume in the ascending colon is 15.6 ml in the fasted state and 18.5 ml in the fed state whereas corresponding liquid volumes in distal ileum are 3.4 and 5.1 ml, respectively. Therefore, ileal liquid volumes are increased 4.6 and 3.6 times upon arrival to the ascending colon in the fasted and in the fed state, respectively. It should also be clarified that liquid volumes reported in this section refer to specific regions at the intestinal lumen, i.e. 20–30 cm of distal ileum, cecum or ascending colon, and, therefore, they are lower than liquid volumes expected to be present in the entire small intestine or colon.
Percent liquid fraction of the contents, and osmolality, total protein content and total carbohydrate content of supernatant after ultracentrifugation of contents of distal ileum (light yellow boxes) and of cecum (dark green boxes) of 12 healthy volunteers in the fasted state and in the fed state on a crossover basis.
Physicochemical Characteristics of Supernatant After Ultracentrifugation of Contents of Distal Ileum and Cecum
Size of Non-liquid Particles (Solids and Bacteria)
Volume mean diameter (D[4,3]) of non-liquid particles in distal ileum was not statitistically different between the fasted state [205(16) μm] and fed state [248(35) μm, p = 0.052]. On the other hand, D[4,3] of non-liquid particles in the cecum was statistically lower in the fasted state [74(89) μm] compared with the fed state [263(52) μm, p = 0.002]. In the fasted state, the diameter of non-liquid particles in distal ileum was significantly higher than in the cecum (p = 0.012) but it did not differ between the two regions in the fed state (p = 0.581).
Osmolality
After ultracentrifugation of ileal contents, supernatants were generally hypoosmotic and values in the fasted state were significantly lower than in the fed state [60(50) mOsmol/Kg] vs. [252(245) mOsmol/Kg, p = 0.034] (Fig. 3). Ileal osmolality was lower than that measured in upper small intestine at corresponding dosing conditions (3,26). Data from this study are in agreement with earlier data by Fodtran and Locklear (28) who have reported osmolality values in the mid and lower intestine, after a steak meal to healthy volunteers, between 270 and 310 mOsmol/kg.
Supernatants of ultracentrifuged cecal contents collected in the fasted and in the fed state did not have statistically different osmolalities [144(65) mOsmol/Kg vs 267(197) mOsmol/Kg, p = 0.112] (Fig. 3). Osmolality differed significantly between cecum and distal ileum in the fasted state (p = 0.023) but not in the fed state (p = 0.775). Osmolality in the supernatants of ultracentrifuged cecal contents (this study) is similar with values in ascending colon (4) at corresponding dosing conditions.
Protein Content
Protein content in supernatants of ultracentrifuged ileal samples did not differ significantly between fasted state [5.1(3.3) mg/ml] and fed state [3.39(0.74) mg/ml] state (Fig. 3). In supernatants of ultracentrifuged cecal samples, protein content was higher in the fasted state [10.2(2.2) mg/ml] than in the fed state [6.2(3.2) mg/ml] (p = 0.023, Fig. 2). These data are in line with protein content measured in ultracentrifuged samples collected from ascending colon of healthy volunteers in the fasted [9.8(4.6) mg/ml] and in the fed [6.9(2.4) mg/ml] state (4). Trend for decreased protein content in fed state in ascending colon has been related to the fact that in the fed state digestion and absorption of peptides and proteins administered with the meal are almost complete by the end of the small intestine and the liquid that arrives in the colon in the fed state primarly dilutes existing proteins in the ascending colon (4). It is interesting to note that in the fasted state, protein content in samples collected from the duodenum is approximately 1 mg/ml (23,25), rises in distal ileum and, then, it is almost doubled in cecal contents (p = 0.030). The increase in protein concentration in cecum compared to distal ileum is also observed in the fed state (p = 0.020).
Carbohydrate Content
Mean(SD) carbohydrate content of ileal samples was significantly lower in the fasted state [1.55(0.99) mg/ml] than in the fed state [12.7(5.3) mg/ml] (p < 0.001, Fig. 3). Similar trend was observed in cecal contents; mean(SD) carbohydrate levels were 2.3(1.0) mg/ml and 9.8(7.0) mg/ml, in the fasted and in the fed state, respectively (Fig. 3). In the fasted state, there is statistically significant increase in carbohydrate content from distal ileum to cecum (p = 0.042, Fig. 3) and from cecum to the ascending colon where the concentration of soluble carbohydrates was 8.1(8.6) mg/ml [p = 0.042 (4)]. This trend is not observed in the fed state where mean level of soluble carbohydrates in cecal contents was similar with that observed in the supernatant of colonic contents of fed healthy adults [14.0(7.4) mg/ml] (p = 0.174 (4)].
Short Chain Fatty acids
Total SCFA concentration was statistically higher in cecum than in distal ileum both in the fasted state (p < 0.001) and in the fed state (p < 0.001) (Fig. 4). This is in line with the significantly lower pH values in the cecum compared to the distal ileum. The higher buffer capacity of cecal contents compared to ileal contents could also be attributed (at least partly) to the higher levels of SCFAs.
Concentrations of individual short chain fatty acids in the supernatant after ultracentrifugation of contents of distal ileum (light yellow boxes) and from cecum (dark green boxes) of 12 healthy volunteers in the fasted state (a) and in the fed state (b) on a crossover basis. Asterisk (*) indicates that the difference between ileal and cecal data is significant. Key: AA, acetic acid; PA, propionic acid; IBA, iso-butyric acid; BA, n-butyric acid; IVA, iso-valeric acid; VA, valeric acid; CA, caproic acid.
Mean(SD) total SCFAs levels in distal ileum were 8.6(6.6) mM and 5.8(4.7) mM in the fasted and in the fed state, respectively, whereas corresponding concentrations in cecal contents were 32.2(17.6) mM and 29.3(15.4) mM indicating that the presence of SCFAs did not differ significantly with the dosing conditions in the two regions (Fig. 4). This is in contrast with the singnificant food effect observed previously in contents of ascending colon (4), and may be attributed to a smaller efficiency of bacteria in distal ileum and cecum to digest food remnants, compared with bacteria in the ascending colon where mean(SD) concentration of total SCFAs has been measured to be 30.9(15.4) mM and 48.1(23.8) mM in fasted state and fed state, respectively (4).
Acetate dominated (69–83%) and it was followed by propionate and butyrate whereas caproate was found only in trace amounts (Fig. 4). Similar observations have been made also in fluids collected from the ascending colon of healthy volunteers (4,29).
Bile Acids
In the supernatants of ultracentrifuged contents of distal ileum, cholic acid and chenodeoxycholic acid were the most abundant (mean values are 26 and 13 μM, respectively) in the fasted state, and glycocholic and glycochenodeoxycholic in fed state (mean values are 51 and 28 μM, respectively) (Fig. 5). In cecum, the most abundant bile acids were cholic acid, chenodeoxycholic acid and deoxycholic acid both in the fasted state (mean values are 54, 32, and 51 μM, respectively) and in the fed state (mean values are 100, 70, and 41 μM, respectively) (Fig. 5). These data indicate that deconjugation of bile acids is almost complete in the fasted distal ileum and confirm previous data implying that deconjugation of bile acids is almost complete by entering the large intestine, regardless of dosing conditions (4).
Concentrations of individual bile acids in the supernatant after ultracentrifugation of contents of distal ileum (light yellow boxes) and from cecum (dark green boxes) of 12 healthy volunteers in the fasted state (a) and in the fed state (b) on a crossover basis. Key: TC, Taurocholic acid; GC, Glycocholic acid; TCDC, Taurochenodeoxycholic acid; UDC, Ursodeoxycholic acid; GCDC, Glycochenodeoxycholic acid; C, Cholic acid; GDC, Glycodeoxycholic acid; CDC, Chenodeoxycholic acid; DC, Deoxycholic acid;LC, Lithocholic acid.
In distal ileum, mean(SD) total bile acid concentration in the fasted state and in the fed state was 71(151) μM and 182(132) μM, respectively; this difference did not reach significance (p = 0.078), possibly due to the low power of the test (power = 0.320). In the cecal contents, mean(SD) total bile acid concentration was 183(221) μM and 280(305) μM in the fasted state and in the fed state, respectively, again with the difference not being significant (p = 0.419 / power = 0.050) .
Total bile acid concentration in fasted cecum is similar to that in ascending colon [117(123) μΜ (4), p = 0.373] but significantly higher than that measured in distal ileum (p = 0.004). In contrast, in the fed state, total bile acid concentration in cecal contents is similar to ileal contents (p = 0.470) but lower to recently reported values in colonic contents from healthy volunteers in which the same meal with the present study was administered [587(413) μM (4), p = 0.023].
It has been reported that the concentration of bile acids in the distal ileum is influenced by the type of administered meal. Ladas et al. (30) found that after a long chain triglyceride meal the bile acid concentrations in distal ileum were higher (median = 1.0 mM) than after a medium chain triglyceride meal (median = 0.5 mM) (30). As expected, due to bile acid reabsorption in distal ileum (31) these values are significantly lower than in the upper small intestine where concentrations in the fasted state are 2–5 mM (2,3,21,23,25,26) and in the fed state are 8–20 mM (2,3,21,24,25).
Cholesterol, Phosphatidylcholine, Free Fatty Acids and Glycerides
Mean(SD) concentration of cholesterol in distal ileum was 413(309) μM and 317(426) μM in the fasted and in the fed state, respectively, whereas corresponding concentrations in cecum were 1004(1072) μM and 640(771) μM (Fig. 6a). Within each region concentration of cholesterol does not differ significantly with the dosing conditions. Cholesterol concentration was higher in cecum compared with distal ileum both in the fasted state (p = 0.036) and in the fed state (p = 0.044). This is in agreement with the higher concentration of SCFAs, and mainly of acetates, in cecum compared to distal ileum, as acetate is the primary substrate for cholesterol synthesis (32). Although cholesterol levels were similar in cecum and ascending colon (4) in the fasted state (p = 0.260), in the fed state cholesterol concentration in the ascending colon was higher [1882(1502) μM, (4)] than in the cecum (p = 0.006).
Concentrations of cholesterol (a), phosphatidylcholine (b), lyso- phosphatidylcholine (c), long chain free fatty acids (d), monoglycerides (e) and diglycerides (f) in the supernatant after ultracentrifugation of contents of distal ileum (light yellow boxes) and of cecum (dark green boxes) of 12 healthy volunteers measured in the fasted state and in the fed state on a crossover basis.
Phosphatidylchloline was quantified in all supernatants after ultracentrifugation collected from distal ileum and cecum (Fig. 6b). Lyso-phosphatidylchloline was quantified in all supernatants in the fasted state but in only 3 out 12 ileal and in 5 out 12 cecal supernatants in the fed state (Fig. 6c). Concentration of total phospholipids in distal ileum did not differ in the fasted state compared to the fed state [73(41) μM vs 40(51) μΜ, p = 0.170]. In cecum, the total concentration of phospholipids was higher in the fasted state compared to the fed state [166(110) μΜ vs 82(77) μΜ, p = 0.033]. In the fasted state, total concentration of phospholipids increases significantly from distal ileum to cecum (p < 0.001) and, as previous data indicate from cecum to ascending colon [362(210) μM, (4)]. In the fed state, there is no difference in phospholipids concentration between distal ileum and cecum (p = 0.110) but there is an increase in the ascending colon [539(393) μM, (4)]. It should be noted that no lyso-phosphatidylchloline could be identified in supernatants of samples collected from the ascending colon (4) and its absence indicated that phosphatidylchloline was not of pancreatic origin as phosphatidylchloline is converted to lyso-phosphatidylchloline by pancreatic phospholipase A in the upper small intestine (4). Data from the present study confirm this hypothesis for the fed state, but it is unclear if in the fasted state lyso-phospahtidylcholine in ileum and cecum is partly of pancreatic origin.
Free fatty acids were quantified in the supernatants of collected contents as the sum of oleic, linoleic and steatic acid and there was no statistically significant difference between prandial states. Mean(SD) concentrations in distal ileum were 63(47) μM and 64(72) μM, in the fasted and fed state. In cecum, corresponding concentrations were 143(118) μM and 150(141) μM (Fig. 6d). Supernatants from cecal contents contained almost double concentration of free fatty acids than ileal contents both in fasted state (p = 0.030) and in the fed state (p = 0.044) with concentrations being similar with those in supernatants from ascending colon (4).
Regardless of the dosing conditions, triglycerides were below detection limit in the supernatant after ultracentrifugation of ileal and cecal contents and were found in traces only in ileal supernatant in 3 out of 12 fed subjects. Minimal concentrations of monoglycerides and diglycerides were observed in the fasted state both in the distal ileum and in the cecum, especially in the fed state (Fig. 6e and f). Similar observations have been made previously for the contents of the ascending colon (4).
Concluding Remarks
In the present investigation we characterized the contents of distal ileum and cecum in healthy adults under conditions simulating the bioavailability/bioequivelance studies of drug products in fasted and fed state.
Fluid composition in distal ileum is different from cecum, especially in the fasted state.
In distal ileum, pH seems to be approximately 8.1 and size of non-liquid particles approximately 200 μm, regardless of dosing conditions. Liquid fraction is lower whereas osmolality and carbohydrate content is higher in fed state. Other components exist at low concentrations, not significantly affected by dosing conditions; therefore, they are unlikely to affect drug product performance in the region.
In cecum, dosing conditions affect the environment more than in distal ileum, i.e. pH, protein content and phospholipids are lower in the fed state whereas buffer capacity is higher in the fed state. Relevant data are qualitatively and quantitatively in line with previously reported data for the conditions in the ascending colon (4). In the fasted state, size of non-liquid particles is much smaller than in distal ileum (approximately 70 μm).
Data collected in this study provide a basis for developing in vitro methods to evaluate the impact of luminal environment in distal ileum and in cecum on the performance of orally administered highly dosed active pharmaceutical ingredients and modified release drug products.
Abbreviations
- AA:
-
Acetic acid
- API:
-
Active pharmaceutical ingredient
- BA:
-
n-Butyric acid
- BA/BE:
-
Bioavailability/Bioequivalence
- BIS:
-
Bisacodyl
- C:
-
Cholic acid
- CA:
-
Caproic acid
- CDC:
-
Chenodeoxycholic acid
- CHO:
-
Cholesterol
- DC:
-
Deoxycholic acid
- DG:
-
Diglyceride
- FA:
-
Fatty acid
- GC:
-
Glycocholic acid
- GCDC:
-
Glycochenodeoxycholic acid
- GDC:
-
Glycodeoxycholic acid
- GI:
-
Gastrointestinal
- HBV:
-
Hepatitis B virus
- HCV:
-
Hepatitis C virus
- HIV:
-
Human Immunodeficiency Virus
- HPLC:
-
High performance liquid chromatography
- IBA:
-
Iso-butyric acid
- IVA:
-
Iso-valeric acid
- LC:
-
Lithocholic acid
- Lyso-PC:
-
Lyso-phosphatidylcholine
- MG:
-
Monoglyceride
- PA:
-
Propionic acid
- PC:
-
Phosphatidylcholine
- SCFA:
-
Short chain fatty acid
- SD:
-
Standard deviation
- TC:
-
Taurocholic acid
- TCDC:
-
Taurochenodeoxycholic acid
- TG:
-
Triglyceride
- UDC:
-
Ursodeoxycholic acid
- VA:
-
Valeric acid
References
Schiller C, Froehlich C-P, Giessmann T, Siegmund W, Moennikes H, Hosten N, et al. Intestinal fluid volumes and transit of dosage forms as assessed by numeric resonance imaging. Aliment Pharmacol Ther. 2005;22:971–9.
Bergström CA, Holm R, Jørgensen SA, Andersson SB, Artursson P, Beato S, et al. Early pharmaceutical profiling to predict oral drug absorption: current status and unmet needs. Eur J Pharm Sci. 2014;57:173–99.
Kalantzi L, Goumas K, Kalioras V, Abrahamsson B, Dressman JB, Reppas C. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm Res. 2006;23(1):165–76.
Diakidou A, Vertzoni M, Goumas K, Söderlind E, Abrahamsson B, Dressman J, et al. Characterization of the contents of ascending colon to which drugs are exposed after oral administration to healthy adults. Pharm Res. 2009;26:2141–51.
Vertzoni M, Goumas K, Söderlind E, Abrahamsson B, Dressman J, Poulou A, et al. Characterization of the ascending colon fluids in ulcerative colitis. Pharm Res. 2010;27:1620–6.
Vertzoni M, Diakidou A, Chatzilias M, Söderlind E, Abrahamsson B, Dressman JB, et al. Biorelevant media to simulate fluids in the ascending colon of humans and their usefulness in predicting intracolonic drug solubility. Pharm Res. 2010;27:2187–96.
Petrakis O, Vertzoni M, Angelou A, Kesisoglou F, Bentz K, Goumas K, et al. Identification of key factors affecting the oral absorption of salts of lipophilic weak acids: a case example. J Pharm Pharmacol. 2015;67(1):56–67.
Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–4.
European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on the investigation of bioequivalence. January 2010. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed 28 Dec 2014.
Kohn CW, Muir 3rd WW. Selected aspects of the clinical pharmacology of visceral analgesics and gut motility drugs in the horse. J Vet Intern Med. 1988;2:85–91.
Dhasmana KM, Banerjee AK, Erdmann W. Gastrointestinal transit following intrathecal or subcutaneous narcotic analgecics. Arch Int Pharmacodyn. 1987;286:152–61.
Sparkes AH, Papasouliotis K, Viner J, Cripps PJ, Gruffyd – Jones TJ. Assessment of orocaecal transit time in cats by the breath hydrogen method: the effects of sedation and a comparison of definitions. Res Vet Sci. 1996;60:243–6.
Washington N, Washington C, Wilson C. Physiological pharmaceutics: biological barriers to drug absorption. 2nd ed. London:Taylor and Francis; 2001.
Hernell O, Staggers JE, Carey MC. Physical-Chemical behaviour of dietary and biliary lipids during intestinal digestion and absorption. 2. Phase analysis and aggregation states of luminal lipids during duodenal fat digestion in healthy adult human beings. Biochemistry. 1990;29:2041–56.
MacFarlane GT, MacFarlane S, Gibson GR. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb Ecol. 1998;35:180–7.
Apajalahti JH, Saerkilahti LK, Maeki BR, Heikkinen JP, Nurminen PH, Holben WE. Effective recovery of bacterial DNA and percent-guanine-plus-cytosine-based analysis of community structure in the gastrointestinal tract of broiler chickens. Applied. Environ Microbiol. 1998;64:4084–8.
Vertzoni M, Carlsson A, Abrahamsson B, Goumas K, Reppas C. Degradation kinetics of metronidazole and olsalazine by bacteria in ascending colon and in feces of healthy adults. Int J Pharm. 2011;413:81–6.
Zhao G, Nyman M, Jinsson A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed Chromatogr. 2006;20:674–82.
Ladas SD, Isaacs PE, Murphy GM, Sladen GE. Fasting and postprandial ileal function in adapted ileostomates and normal subjects. Gut. 1986;27(8):906–12.
Debongnie JC, Phillips SF. Capacity of the human colon to absorb fluid. Gastroenterology. 1978;74(4):698–703.
Sjögren E, Abrahamsson B, Augustijns P, Becker D, Bolger MB, Brewster M, et al. In vivo methods for drug absorption—Comparative physiologies, model selection, correlations with in vitro methods (IVIVC), and applications for formulation/API/excipient characterization including food effects. Eur J Pharm Sci. 2014;57:99–151.
Fallingborg J, Christensen LA, Ingeman-Nielsen M, Jacobsen BA, Abildgaard K, Rasmussen HH. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment Pharmacol Ther. 1989;3(6):605–13.
Psachoulias D, Vertzoni M, Goumas K, Kalioras V, Beato S, Butler J, et al. Precipitation in and supersaturation of contents of the upper small intestine after administration of two weak bases to fasted adults. Pharm Res. 2011;28:3145–58.
Vertzoni M, Markopoulos C, Symillides M, Goumas C, Imanidis G, Reppas C. Luminal lipid phases after administration of a triglyceride solution of danazol in the fed state and their contribution to the flux of danazol across caco-2 cell monolayers. Mol Pharm. 2012;9:1189–98.
Persson EM, Gustafsson AS, Carlsson AS, Nilsson RG, Knutson L, Forsell P, et al. The effects of food on the dissolution of poorly soluble drugs in human and in model small intestinal fluids. Pharm Res. 2005;22(12):2141–51.
Perez de la Cruz Moreno M, Oth M, Deferme S, Lammert F, Tack J, Dressman J, et al. Characterization of fasted-state human intestinal fluids collected from duodenum and jejunum. J Pharm Pharmacol. 2006;58:1079–89.
Fadda HM, Sousa T, Carlsson AS, Abrahamsson B, Williams JG, Kumar D, et al. Drug solubility in luminal fluids from different regions of the small and large intestine of humans. Mol Pharm. 2010;7(5):1527–32.
Fordtran JS, Locklear TW. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966;11(7):503–21.
Kumar V, Sinha AK, Makkar HP, de Boeck G, Becker K. Dietary roles of non-starch polysaccharides in human nutrition: a review. Crit Rev Food Sci Nutr. 2012;52(10):899–935.
Ladas SD, Isaacs PE, Murphy GM, Sladen GE. Comparison of the effects of medium and long chain triglyceride containing liquid meals on gall bladder and small intestinal function in normal man. Gut. 1984;25(4):405–11.
Davenport HW. Physiology of the digestive tract. An introductory text. 5th ed. Chicago: Year Book Medical Publishers, Inc; 1982.
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40(3):235–43.
ACKNOWLEDGMENTS AND DISCLOSURES
This work would not have been possible without the participation of reliable volunteers and authors would like to express their sincere appreciation.
Authors would like to thank Ms. Maria Koursari for her excellent technical assistance during colonoscopies.
Parts of the present work have been presented as an oral presentation at the “Progress within the IMI OrBiTo project—predictive tools for oral Biopharmaceutics” meeting (Academy of Pharmaceutical Sciences, Stevenage, UK, 13 May 2014) and as a poster at AAPS Annual Meeting, November 2–6, 2014, San Diego, California, USA.
This work was performed within the OrBiTo project (http://www.orbitoproject.eu) which is funded by the Innovative Medicines Initiative Joint Undertaking under Grant Agreement No 115369.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reppas, C., Karatza, E., Goumas, C. et al. Characterization of Contents of Distal Ileum and Cecum to Which Drugs/Drug Products are Exposed During Bioavailability/Bioequivalence Studies in Healthy Adults. Pharm Res 32, 3338–3349 (2015). https://doi.org/10.1007/s11095-015-1710-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1710-6