ABSTRACT
Purpose
Drug transfer into milk is of concern due to the unnecessary exposure of infants to drugs. Proposed prediction methods for such transfer assume only passive drug diffusion across the mammary epithelium. This study reorganized data from the literature to assess the contribution of carrier-mediated transport to drug transfer into milk, and to improve the predictability thereof.
Methods
Milk-to-plasma drug concentration ratios (M/Ps) in humans were exhaustively collected from the literature and converted into observed unbound concentration ratios (M/Punbound,obs). The ratios were also predicted based on passive diffusion across the mammary epithelium (M/Punbound,pred). An in vitro transport assay was performed for selected drugs in breast cancer resistance protein (BCRP)-expressing cell monolayers.
Results
M/Punbound,obs and M/Punbound,pred values were compared for 166 drugs. M/Punbound,obs values were 1.5 times or more higher than M/Punbound,pred values for as many as 13 out of 16 known BCRP substrates, reconfirming BCRP as the predominant transporter contributing to secretory transfer of drugs into milk. Predictability of M/P values for selected BCRP substrates and non-substrates was improved by considering in vitro-evaluated BCRP-mediated transport relative to passive diffusion alone.
Conclusions
The current analysis improved the predictability of drug transfer into milk, particularly for BCRP substrates, based on an exhaustive data overhaul followed by focused in vitro transport experimentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
The benefits of breastfeeding are widely accepted based on the results of extensive epidemiological research (1–3). Approximately 90% of women take some form of medication during their first week postpartum (4,5), and, therefore, a general concern is the avoidance of drug transfer into milk and toxicity in breast-fed infants. However, only a few clinical studies have actually investigated drug transfer into milk, necessitating the use of prediction methods to address this issue (6–8).
Rasmussen (9) first reported that the pH difference between milk and plasma governs drug transfer into milk, and assumed that only the unbound, unionized form of a molecule traverses the membranes of the mammary epithelium in a passive diffusion-mediated manner. Based on the pH partition theory, equilibrium dialysis methods were next proposed by Notarianni et al. (10), who determined steady-state drug concentrations in human plasma and reconstituted artificial baby milk across a dialysis membrane. However, these methods were limited by the difficulty in maintaining a pH gradient across the dialysis membrane, considering a milk pH of 7.0 and a plasma pH of 7.4.
Alternative diffusional methods of drug transfer were next proposed to deal with this shortcoming (6,11), where the unbound drug concentration ratio did not require experimental determination. Instead, this ratio could be calculated based on the Henderson-Hasselbalch equation by using the drug pKa, a milk pH of 6.8–7.2, and a plasma pH of 7.4. Unbound drug fractions in milk and plasma were then separately determined in vitro or calculated based on an empirical formula (6,11). Lastly, these values were integrated to predict the milk-to-plasma concentration ratio (M/P). This prediction is generally sound, but there are some exceptions. For example, acyclovir, cimetidine, and nitrofurantoin are all secreted at higher than predicted rates in human subjects (12–14), suggesting that carrier-mediated transport might be involved in the case of these exceptional drugs.
In silico prediction approaches have also been pursued to anticipate drug transfer into milk. Meskin and Lien (15) were the first to take such an approach by focusing on physicochemical parameters. The investigators collected M/P data for 35 drugs in total (20 acidic drugs and 15 basic drugs) from the literature and then constructed best-fit equations centered around the physicochemical parameters of molecular weight, lipophilicity, the pKa of the drug of interest, and so on. Agatonovic-Kustrin et al. (16) extended this method with the aid of an artificial neural network, collecting M/P data for 123 drugs in total and evaluating as many as 71 drug/molecular structure descriptors. Similar quantitative structure/property, relationship/activity methodologies were likewise explored by others (17,18). However, these approaches still did not consider the involvement of carrier-mediated transport in drug transfer into milk.
Experimental animals, including the rat and mouse, have long been used to analyze the mechanism of drug transfer into milk. Importantly, the animal models revealed a similar tendency as the approaches outlined above, i.e., the existence of outlier drugs that are secreted into milk at a rate greater than that expected from passive diffusion alone (19–21). Jonker and colleagues (22) clarified the molecular identity of the putative transporter for these outlier drugs as breast cancer resistance protein (BCRP)/ATP-binding cassette transporter G2 (ABCG2). Jonker et al. found that Bcrp was induced on the apical membrane of normal mammary epithelial cells during lactation, while the transfer of selected Bcrp substrates (i.e., acyclovir, cimetidine, and nitrofurantoin) into milk was attenuated in Bcrp knockout mice (22–24).
In addition to BCRP, our research group has recently demonstrated that the organic cation transporter (OCT)/solute carrier (SLC) family 22A is involved in the transfer of OCT/SLC substrate drugs into milk (25). Moreover, mRNA expression of other transporters (e.g., peptide transporter (PEPT)/SLC15A, concentrative nucleoside transporter (CNT)/SLC28A, and phospholipid transporter ABCA7 are induced in the mammary gland during lactation (20,26–30). If the contribution of these transporters was considered, a more general and dependable prediction method could certainly be established.
Accordingly, the purpose of the current study was to address this issue through a comprehensive and objective assessment of transporter participation in drug transfer into milk, and to apply the obtained information to the improvement of conventional prediction methods. To this end, M/P data were exhaustively collected from the literature, converted into unbound milk-to-plasma concentration ratio (M/Punbound) data, and compared with data calculated by the pH partition theory. Next, an analysis of observed and predicted M/Punbound values (M/Punbound,obs and M/Punbound/pred) confirmed that BCRP primarily contributes to the secretory transfer of drugs into milk. Finally, we set out to improve the predictability of the M/P data with the aid of an in vitro BCRP transport assay.
MATERIALS AND METHODS
Collection of M/P Data and Calculation of Related Parameters
M/P data obtained from clinical observations were adopted from our previous report (31) or from the Hale textbook (32). If the M/P area-under-the-curve ratios (M/P(AUC)) were calculated in our previous report (31), they were preferentially employed in the current investigation. The arithmetical mean value was used if more than two values were reported. The ratio of the unbound drug concentration in the milk to that in the plasma (M/Punbound,obs) was calculated by Eq. 1.
where the f p (unbound fraction of drug in plasma) values were obtained from the DrugBank database (http://www.drugbank.ca/) or the package insert for each drug. The f m,total (unbound fraction of drug in milk) values were obtained from our in vitro spike recovery tests, as described previously (31,33). Briefly, milk was spiked with drug to create a “blank” human milk sample containing an already known drug concentration. After incubation, the drugs were distributed and bound to milk proteins or lipids, or stayed behind as unbound drug fractions in the milk. Each drug concentration (i.e., protein-bound, lipid-bound, or unbound) was quantified by using an ultrafiltration device. In the case of a drug with an unexamined experimental f m,total value, Eq. 2 was selected for the calculation of the f m,total (6), as follows.
The values of f m,protein (fraction of drug free from binding to milk protein) and P app (partition coefficient between milk lipids and water) were also approximated by using Eqs. 3 and 4 (34), respectively. The logD 7.2 (octanol/water partition coefficient at pH 7.2) value was calculated by using the MarvinSketch program (ChemAxon, Budapest, Hungary).
The ratio of predicted unbound drug concentration in milk to that in plasma (M/Punbound,pred) was calculated by Eqs. 5 and 6 for acidic and basic drugs, respectively, based on the pH partition theory (8). For neutral drugs, a ratio of 1.0 was assigned for the M/Punbound,pred. Parameters such as pKa, logD 7.2, and charge at pH 7.0 were calculated by using the MarvinSketch program. Milk pH was assumed to be 6.8–7.2.
The M/Punbound,pred at pH 6.8 and the M/Punbound,pred at pH 7.2 were calculated, and the arithmetic mean of these values was obtained as the M/Punbound,pred. Finally, the ratio of M/Punbound,obs to M/Punbound,pred was defined as Robs/pred (Eq. 7).
Drugs
Acyclovir, bupropion hydrochloride, carbamazepine, metronidazole, nifedipine, ofloxacin and ranitidine hydrochloride were obtained from Wako Pure Chemicals (Osaka, Japan). Clindamycin hydrochloride, doxycycline hydrochloride, levofloxacin, meprobamate, nitrofurantoin, nortriptyline hydrochloride, pefloxacin mesylate dehydrate, and (±)-verapamil hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO, USA). Cefoperazone sodium and ciprofloxacin hydrochloride were purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). Cimetidine and tetracycline hydrochloride were from Nacalai Tesque (Kyoto, Japan). Fluconazole was from LKT Laboratories, Inc. (St. Paul, MO, USA). Moclobemide was from Toronto Research Chemicals (Brisbane, Canada). Levetiracetam was from Tokyo Chemical Industry Co. (Tokyo, Japan). All other reagents were of analytical grade, unless otherwise noted.
Transcellular In Vitro Transport Study
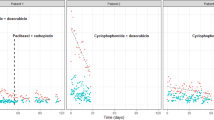
Transcellular transport experiments with human BCRP-expressing cells were performed as described previously, with minor modifications (35). Briefly, Madin-Darby canine kidney (MDCK) II cells were seeded into a 24-well transwell plate (3.0-μm pore size; Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at a density of 2 × 105 cells/well, and then grown for 3 days in Minimum Essential Medium (MEM: Nacalai Tesque, Inc, Kyoto, Japan) containing 10% fetal bovine serum (Biowest, Nuaillé, France), 1% MEM non-essential amino acids (Life Technologies, Carlsbad, CA, USA), and penicillin-streptomycin solution (100 μg/ml) in an atmosphere of 5% CO2/95% air at 37°C. The cells were infected with the recombinant adenovirus expression vector harboring green fluorescent protein (GFP) or human BCRP at a multiplicity of infection of 50. The details for the construction of these recombinant adenoviruses were described in a previous report (36).
After 48 h of culture, GFP-expressing (MDCK II-GFP) cells and BCRP-expressing (MDCK II-BCRP) cells were used for the transport study. The cells were preincubated with Hank’s balanced salt solution (HBSS) at 37°C for 30 min, and transport experiments were then initiated by replacing the MEM-based medium on one side of the cell monolayer with HBSS buffer containing the individual test drugs (each at a concentration of 10 μM). At 1, 2, and 3 h after the initiation of the transport assay, 50-μl aliquots were taken from the opposite side of the cell monolayer and replaced with the same volume of HBSS. For the nitrofurantoin transport experiment, the sampling time was modified to 2, 3, and 4 h instead of 1, 2, and 3 h, because of the limit of detection for the transported drug.
Transcellular transport clearances were calculated in each well from the slopes of the time profiles of apical-to-basal or basal-to-apical drug transport between 1 and 3 h (or between 2 and 4 h for nitrofurantoin). Flux ratios were obtained by dividing the clearance values in the basal-to-apical direction by those in the apical-to-basal direction. Finally, BCRP/GFP flux ratios were obtained by dividing the flux ratios obtained in MDCK II-BCRP cells by those obtained in MDCK II-GFP cells.
Quantification of Drug Concentrations
Drug concentrations were measured as follows. Each drug sample (50 μl) was mixed with acetonitrile (500 μl) containing 500 nM carbamazepine as an internal standard, vortexed, and deproteinized by centrifugation for 10 min at 20,000×g. An aliquot (450 μl) of each supernatant was condensed and dried by using a centrifugal concentrator (SpeedVac, Thermo Fisher Scientific, Bridgewater, NJ, USA). The desiccated sample was then dissolved in 40% acetonitrile (200 μl), vortexed, and centrifuged for 5 min at 20,000×g, and an aliquot of the sample (160 μl) was applied to the sampling plates for liquid chromatography-tandem mass spectroscopy (LC-MS/MS) analysis.
Next, LC-MS/MS analysis was performed by using an ultra-performance liquid chromatography (UPLC) system and a Quattro Premier XE mass spectrometer (Waters, Milford, MA, USA) with a 1.7-μm particle Acquity UPLC™ bridged ethyl hybrid C18 analytical column (2.1 × 100 mm, Waters). Samples were kept at 4°C in the sample injector, and aliquots of 7.5 μl were injected. The oven temperature was maintained at 40°C, and the flow rate was maintained at 0.3 ml/min. Acetonitrile or methanol was selected as the organic solvent in the mobile phase. The mobile phase consisted of formic acid/acetonitrile (0.1:99.9, v/v) or formic acid/methanol/20 mM ammonium acetate (0.1:97.9:2.0, v/v/v) (see Supplementary Table I for a summary of detailed LC-MS/MS/UPLC analytical conditions).
Statistical Analysis
Quantifiable data are presented as the mean ± the standard error of the mean (SEM). GraphPad Prism 5 software (GraphPad Software, Inc., La Jolla, CA, USA) was selected to perform all statistical analyses.
Results
Observed and Predicted M/P Values Obtained from the Literature
M/P data for 51 drugs were adopted from our previous study, in which the M/P(AUC) values of the corresponding drugs were calculated (31). These data were preferentially employed for the current analysis, anticipating a more accurate evaluation relative to that obtained from a single sampling point alone. We extended our survey to include data reported in the most prevalent textbook in the field of breastfeeding and medications, Medications and Mother’s Milk, 13th edition (32). This textbook documents the M/P values of 227 drugs. Collectively, we obtained the M/P values for a total of 230 drugs.
Calculation of the M/Punbound,obs values required knowledge of the unbound fractions of the drugs in the plasma (f p) and the milk (f m,total). Unfortunately, f p values were not reported for 14 out of 230 drugs, and so these drugs were excluded from further analysis. Among the remaining 216 drugs, 50 more were also excluded from further analysis, because the f m,total/f p values were lower than 0.37 or higher than 11. These exclusion criteria were based on our previous analysis (33), in which we determined f m,total values for 44 drugs in vitro and calculated the f m,total/f p values from these data as being in the range of 0.37 to 11. After excluding these 64 drugs, we finally obtained reliable M/Punbound,obs values for 166 drugs. These 166 drugs were subjected to a comparative analysis of M/Punbound,obs and M/Punbound,pred values (Supplementary Table II). The ratios of these two values (Robs/pred) are shown in the far right-hand column of Supplementary Table II.
Most of the BCRP Substrates are Secreted into Milk More Extensively Than Predicted by the pH Partition Theory
Total 166 drugs were classified to 5 groups based on Robs/pred values (Table I). Theoretically, if the value is around 1, asymmetrical transport across the mammary epithelial cells is negligible. On the other hand, if the values are lower or higher than 1, asymmetrical transport is not negligible. To avoid false positive estimates, the lower and higher Robs/pred threshold were set as 0.5 and 1.5, respectively for the middle group. As much as 63 out of 166 drugs were classified to the middle group with 0.5≦Robs/pred < 1.5, while 51 and 52 drugs were classified to the lower groups with Robs/pred < 0.5 and the higher groups with 1.5≦Robs/pred, respectively (Table I). Next, we particularly focused on BCRP because it is the only transporter at present known to involve in drug transfer into milk. Among the 166 drugs examined, 16 were known BCRP substrates as highlighted by gray in Supplementary Table II; nitrofurantoin (37), zidovudine (38,39), bupropion (40), hydroxychloroquine (41), cimetidine (42), ciplofloxacin (43,44), pefloxacin (35), ofloxacin (43,44), cefoperazone (45), acyclovir (46), hydrochlorothiazide (47), lamivudine (48), zonisamide (49), levetiracetam (49), cefazolin (45), and chlorothiazide (50). It is notable that BCRP substrates are much more concentrated in the groups with Robs/pred values higher than 1.5 (Table I).
In Vitro Transport Assay of Test Drugs
We arbitrarily selected 12 drugs from Supplementary Table II (8 drugs known as BCRP substrates and 4 drugs not known as BCRP substrates) and performed an in vitro transcellular transport assay by using BCRP- and GFP-expressing MDCK II cells (Fig. 1). BCRP-mediated transport activity was assessed by calculating the BCRP/GFP flux ratio (Table II). Values were in the range of 6.26 to 0.95, with the highest value observed for nitrofurantoin (6.26 ± 3.85), a typical BCRP substrate, and the lowest value observed for metronidazole (0.95 ± 0.16), a negative control drug. Moreover, we tried to find crypt-BCRP substrates from the drugs with Robs/pred values higher than 1.5 but not known as BCRP substrates. We selected nifedipine, doxycycline, nortriptyline, tetracycline, meprobamate, levofloxacin, ranitidine, and clindamycin, then performed an in vitro transcellular transport assay. Finally, we found levofloxacin as a novel BCRP substrate (Fig. 1) with BCRP/GFP flux ratio of 1.63 ± 0.28 (Table II). BCRP-mediated transports were not observed for the other 7 drugs tested (Supplementary Fig. 1). After adding levofloxacin, total 13 drugs (9 BCRP substrates and 4 BCRP non-substrates) were subjected to the following analyses.
Transcellular transport across MDCK II cell monolayers expressing BCRP. Transcellular transport of drugs (10 μM) across MDCK II cell monolayers expressing BCRP (MDCK II-BCRP, squares) or GFP (MDCK II-GFP, circles) was determined at the designated time in the basal-to-apical direction (closed symbols) and the apical-to-basal direction (open symbols). Each data point represents the mean ± the SEM.
Correlation of Robs/pred Values and the BCRP/GFP Flux Ratio
Given the quantitative contribution of BCRP to the transfer of substrate drugs into milk, BCRP/GFP flux ratios would positively correlate with the Robs/pred values. Figure 2a shows that this was indeed the case for the 13 drugs tested (y = 1.918x - 0.8973, r 2 = 0.93005).
Correlation of BCRP-mediated transport in vitro and contribution of carrier-mediated transport to drug transfer into milk in vivo. a BCRP/GFP flux ratios and Robs/pred values of the 13 selected drugs shown in Table II were plotted to give the linear regression equation (y = 1.918x - 0.8973, r 2 = 0.93005). b Five drugs (ciprofloxacin, bupropion, ofloxacin, fluconazole, metronidazole) were arbitrarily selected from the 13 drugs shown in a) and plotted to give the linear regression equation (y = 1.6243x - 0.4951, r 2 = 0.65399).
Improvement of the M/P Prediction by Considering BCRP-Mediated Transport
It is useful if we could correctly predict M/P values for secretory type drugs. Among the 13 drugs shown in Table II, 5 (ciprofloxacin, bupropion, ofloxacin, fluconazole, metronidazole) were arbitrary selected as construction set, while the other 8 were used as validation set. At first, a linear regression equation was obtained using the construction set (Fig. 2b) (y = 1.6243x - 0.4951 and r 2 = 0.65399, where y = Robs/pred and x = BCRP/GFP flux ratio). By using this equation, R’obs/pred values were calculated for the validation set drugs (nitrofurantoin, acyclovir, pefloxacin, cimetidine, levofloxacin, cefoperazone, verapamil, moclobemide) (Table II). To assess whether the predictability of drug M/P values was improved, we calculated the predicted M/P (M/Ppred) values based on the following two methods (Table II).
M/Punbound,pred and f m,total/f p values were adapted from Supplementary Table II. Figure 3 demonstrated that the correlation was improved when the new method was used (r 2 = 0.88982 for the new method vs. r 2 = 0.091 for the conventional method). To verify validity of our new method, we set another 6 extreme conditions from set 2 to set 7 shown in Supplementary Table III. Although the degree of improvements were varied depending on the combination, predictabilities were more or less improved by correcting the BCRP-mediated transport in all the conditions tested (Supplementary Table III and Supplementary Fig. 2).
Comparison of the predictabilities of M/P values based on conventional method and new method. a Relationship between the M/P values observed in vivo of 8 drugs (a, nitrofurantoin; b, cimetidine; c, acyclovir; d, levofloxacin; e, pefloxacin; f, verapamil; g, moclobemide; h, cefoperazone) (Supplementary Table II) and the M/P values predicted based on the conventional method; pH partition theory alone (M/Ppred (pH-partition)). b Relationship between the M/P values observed in vivo of the same 8 drugs shown in a) and the M/P values predicted based on the new method; pH partition theory, followed by correction for BCRP-mediated transport (M/Ppred (corrected by BCRP)). Linear regression lines and correlation coefficients are shown.
Discussion
Carrier-mediated transport has long been accepted as potentially responsible for the transfer of drugs into milk with higher or lower than predicted in vivo M/P ratios. However, no method has been established for the indisputable Identification of such transporter-facilitated drugs and the prediction of their M/P values. The ability to predict M/P values is particularly important for those drugs with higher than expected M/P values (i.e., drugs that are secreted into milk via active transport), because drug transfer into milk increases the risk of unnecessary drug exposure to the infant.
The current investigation paid particular attention to M/Punbound values, given that the M/P ratio provides an index of asymmetrical transport across the mammary epithelium. Our major achievements are as follows: 1) We have reconfirmed that BCRP is the predominant drug transporter involved in the secretory transfer of substrate drugs into human milk; and 2) the predictability of drug M/P values was improved herein by considering the BCRP-mediated transport activity determined by our in vitro transport assay.
Known BCRP substrates were frequently found in the highly secreted drug group (i.e., Robs/pred ≧ 1.5); however, three drugs were located in a group with lower secretion values (R obs/pred < 1.5). Those include levetiracetam (R obs/pred = 1.12), cefazoline (R obs/pred = 0.25), and chlorothiazide (R obs/pred = 0.17) (Table I and Supplementary Table II). There are two possibilities explaining these exceptional drugs whose Robs/pred are lower than other BCRP-substrates. Firstly, it is possible that the transport activity of human BCRP for these drugs might be too small to affect the M/Punbound ratios in vivo (45,50). Indeed, BCRP-mediated transport was not clearly observed in our in vitro assay system for levetiracetam (data not shown). Secondary, inappropriate sampling time point of milk and plasma in vivo might underestimate M/Punbound. M/P value of chlorothiazide was determined at only 1, 2, and 3 h after oral drug administration (51). However, hydrochlorothiazide, another BCRP substrate with a very similar structure to chlorothiazide, does not appear in the milk until 5 h after oral administration (52), whereas the concentration in the milk is almost double that in the serum at 8 to 24 h after administration. As a result, the calculated M/P(AUC) value of hydrochlorothiazide was sometimes as high as 0.99 (Supplementary Table II). Hence, underestimation of the in vivo M/P value might pertain to chlorothiazide.
Thirty-nine out of 52 drugs that exhibited Robs/pred values of ≧1.5 are at first not known as BCRP substrates (Table I). We thought there were at least three possibilities explaining why these 39 drugs were secreted into milk than estimated from passive diffusion; i) unbound fraction in milk (f m,total) was over-estimated, ii) they were crypt-BCRP substrates, iii) transporter(s) other than BCRP was involved. As to the possibility i), we think it most plausible for drugs whose f m,total values were not determined by in vitro experiment. Alternatively, f m,total values were calculated empirically using logD 7.2 and f p. This calculation does not always give correct value. For example, if there was a specific binding protein in the milk, f m,total value would be over-estimated. As many as 6 in the top 12 drugs (Robs/pred≧4.5), f m,total/f p ratios were higher than 6.0; dyphylline (6.03), nifedipine (9.33), doxycycline (9.48), nortriptyline (8.16), valproic acid (6.41), hydralazine (7.06) (Supplementary Table II). Notably, these are not known as BCRP-substrate. For these 6 drugs, we should be more careful when discussing about the contribution of transporter-mediated secretion into milk. As to the possibility ii), we have done in vitro BCRP-transport assay for 8 drugs (nifedipine, doxycycline, nortriptyline, tetracycline, meprobamate, levofloxacin, ranitidine, clindamycin) whose Robs/pred values were higher than 1.5 but not known as BCRP substrates. We successfully found that levofloxacin was a novel BCRP substrate. Such crypt-BCRP substrates could partially explain the case. Based on above considerations, we think that possibility iii) may be at least applicable for mesalamine, foscarnet, phencyclidine and acebutolol (R obs/pred ≧ 4.5), though we don’t have any idea about the concrete candidate transporters.
It is possible that reabsorption process is involved for the 51 drugs whose Robs/pred values were less than 0.5 (Table I). For example, PEPT2 is induced during lactation in rat and human and localized in the rat epithelial cells of ducts and glands (29). Given that PEPT2 is a proton-coupled high affinity di- and tri-peptide uptake transporter and that milk pH is slightly acidic, PEPT2 may involve in reabsorption of substrates from the milk. In line with the hypothesis, Robs/pred values of PEPT2 substrates are extremely low; captopril (0.11), cephalexin (0.09), and cefadroxil (0.04) (supplementary Table II). While it is interesting hypothesis, we don’t have any further evidence at present.
Here, we modified the conventional prediction method to facilitate the prediction of outlier drugs that were more extensively secreted into milk than expected by taking advantage of a human BCRP-expressing cell system to determine R’obs/pred values in vitro. The R’obs/pred values were then integrated with experimentally determined f p and f m,total values, and the M/Punbound,pred values were readily calculated by applying the Henderson-Hasselbalch equation in silico. From an ethical standpoint, our modified methodology is beneficial because it does not require clinical studies employing lactating women or investigations utilizing whole-animal models. Especially regarding the use of experimental animals, the trend is now replacement, refinement, or reduction in the risk assessment of chemicals (53).
BCRP accepts various types of compounds as substrates, including sulfated hormone metabolites, flavonoids, vitamin B2, and drug compounds (54). Indeed, as much as 27.3% of the drug dose can be recovered in the milk of lactating rats up until 32 h after the oral administration of sorafenib (package insert of NEXAVAR® (sorafenib)), a recently developed tyrosine kinase inhibitor. This degree of sorafenib secretion into milk would inevitably exert pharmacological actions on the offspring. A similar phenomenon would be expected if human mothers received sorafenib during lactation, because the drug is a good substrate of human BCRP as well as rat Bcrp (55). Although breastfeeding is contraindicated in nursing mothers when taking tyrosine kinase inhibitors, it is important to consider the contribution of BCRP when any drug of interest is to be administered to a breastfeeding mother. The new method described here provides a way to estimate the extent of drug transfer into milk for such peculiar cases.
In conclusion, this study successfully improved the prediction of drug transfer into milk, particularly for BCRP substrates, on the basis of an exhaustive analysis of literature data followed by focused in vitro transport experimentation. Although other unidentified transporters might be involved in secretory drug transfer into milk together with BCRP, we anticipate that our BCRP-grounded method will prove extremely useful in advancing newly developed medications that are efficacious for nursing mothers and safe for infants during lactation.
Abbreviations
- ABCG2:
-
ATP-binding cassette transporter G2
- BCRP:
-
Breast cancer resistance protein
- CNT:
-
Concentrative nucleoside transporter
- f m,protein :
-
Fraction of drug free from binding to milk protein
- f m,total :
-
Unbound drug fraction in milk
- f p :
-
Unbound drug fraction in plasma
- GFP:
-
Green fluorescent protein
- HBSS:
-
Hank’s balanced salt solution
- LC-MS/MS:
-
Liquid chromatography-tandem mass spectroscopy
- logD 7.2 :
-
Octanol/water partition coefficient at pH 7.2
- M/P:
-
Milk-to-plasma concentration ratio
- M/P(AUC):
-
M/P area-under-the-curve ratio
- M/Punbound :
-
Unbound M/P
- M/Punbound,obs :
-
Observed unbound M/P
- M/Punbound,pred :
-
Predicted unbound M/P
- MDCK:
-
Madin-Darby canine kidney
- MEM:
-
Minimum Essential Medium
- OCT:
-
Organic cation transporter
- P app :
-
Partition coefficient between milk lipids and water
- PEPT:
-
Peptide transporter
- Robs/pred :
-
Ratio of M/Punbound,obs to M/Punbound,pred
- SEM:
-
Standard error of the mean
- SLC:
-
Solute carrier
- UPLC:
-
Ultra-performance liquid chromatography
REFERENCES
Flacking R, Ewald U, Starrin B. “I wanted to do a good job”: experiences of ‘becoming a mother’ and breastfeeding in mothers of very preterm infants after discharge from a neonatal unit. Soc Sci Med. 2007;64:2405–16.
Jones G, Steketee RW, Black RE, Bhutta ZA, Morris SS. How many child deaths can we prevent this year? Lancet. 2003;362:65–71.
Kramer MS. “Breast is best”: the evidence. Early Hum Dev. 2010;86:729–32.
Anderson PO. Drug use during breast-feeding. Clin Pharm. 1991;10:594–624.
Matheson I. Drugs taken by mothers in the puerperium. Br Med J (Clin Res Ed). 1985;290:1588–9.
Atkinson HC, Begg EJ. Prediction of drug distribution into human milk from physicochemical characteristics. Clin Pharmacokinet. 1990;18:151–67.
Begg EJ, Atkinson HC, Duffull SB. Prospective evaluation of a model for the prediction of milk: plasma drug concentrations from physicochemical characteristics. Br J Clin Pharmacol. 1992;33:501–5.
Fleishaker JC. Models and methods for predicting drug transfer into human milk. Adv Drug Deliv Rev. 2003;55:643–52.
Rasmussen F. Mammary excretion of benzylpenicillin, erythromycin, and penethamate hydroiodide. Acta Pharmacol Toxicol (Copenh). 1959;16:194–200.
Notarianni LJ, Belk D, Aird SA, Bennett PN. An in vitro technique for the rapid determination of drug entry into breast milk. Br J Clin Pharmacol. 1995;40:333–7.
Fleishaker JC, Desai N, McNamara PJ. Factors affecting the milk-to-plasma drug concentration ratio in lactating women: physical interactions with protein and fat. J Pharm Sci. 1987;76:189–93.
Gerk PM, Kuhn RJ, Desai NS, McNamara PJ. Active transport of nitrofurantoin into human milk. Pharmacotherapy. 2001;21:669–75.
Lau RJ, Emery MG, Galinsky RE. Unexpected accumulation of acyclovir in breast milk with estimation of infant exposure. Obstet Gynecol. 1987;69:468–71.
Oo CY, Kuhn RJ, Desai N, McNamara PJ. Active transport of cimetidine into human milk. Clin Pharmacol Ther. 1995;58:548–55.
Meskin MS, Lien EJ. QSAR analysis of drug excretion into human breast milk. J Clin Hosp Pharm. 1985;10:269–78.
Agatonovic-Kustrin S, Ling LH, Tham SY, Alany RG. Molecular descriptors that influence the amount of drugs transfer into human breast milk. J Pharm Biomed Anal. 2002;29:103–19.
Katritzky AR, Dobchev DA, Hur E, Fara DC, Karelson M. QSAR treatment of drugs transfer into human breast milk. Bioorg Med Chem. 2005;13:1623–32.
Zhao C, Zhang H, Zhang X, et al. Prediction of milk/plasma drug concentration (M/P) ratio using support vector machine (SVM) method. Pharm Res. 2006;23:41–8.
Dostal LA, Weaver RP, Schwetz BA. Excretion of high concentrations of cimetidine and ranitidine into rat milk and their effects on milk composition and mammary gland nucleic acid content. Toxicol Appl Pharmacol. 1990;102:430–42.
Gerk PM, Oo CY, Paxton EW, Moscow JA, McNamara PJ. Interactions between cimetidine, nitrofurantoin, and probenecid active transport into rat milk. J Pharmacol Exp Ther. 2001;296:175–80.
McNamara PJ, Burgio D, Yoo SD. Pharmacokinetics of cimetidine during lactation: species differences in cimetidine transport into rat and rabbit milk. J Pharmacol Exp Ther. 1992;261:918–23.
Jonker JW, Merino G, Musters S, et al. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat Med. 2005;11:127–9.
Merino G, Jonker JW, Wagenaar E, van Herwaarden AE, Schinkel AH. The breast cancer resistance protein (BCRP/ABCG2) affects pharmacokinetics, hepatobiliary excretion, and milk secretion of the antibiotic nitrofurantoin. Mol Pharmacol. 2005;67:1758–64.
van Herwaarden AE, Schinkel AH. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol Sci. 2006;27:10–6.
Ito N, Ito K, Ikebuchi Y, et al. Organic cation transporter/solute carrier family 22a is involved in drug transfer into milk in mice. J Pharm Sci. 2014;(in press).
Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303:487–96.
Farke C, Meyer HH, Bruckmaier RM, Albrecht C. Differential expression of ABC transporters and their regulatory genes during lactation and dry period in bovine mammary tissue. J Dairy Res. 2008;75:406–14.
Gilchrist SE, Alcorn J. Lactation stage-dependent expression of transporters in rat whole mammary gland and primary mammary epithelial organoids. Fundam Clin Pharmacol. 2010;24:205–14.
Groneberg DA, Doring F, Theis S, Nickolaus M, Fischer A, Daniel H. Peptide transport in the mammary gland: expression and distribution of PEPT2 mRNA and protein. Am J Physiol Endocrinol Metab. 2002;282:E1172–9.
Ito S, Alcorn J. Xenobiotic transporter expression and function in the human mammary gland. Adv Drug Deliv Rev. 2003;55:653–65.
Koshimichi H, Ito K, Hisaka A, Honma M, Suzuki H. Analysis and prediction of drug transfer into human milk taking into consideration secretion and reuptake clearances across the mammary epithelia. Drug Metab Dispos. 2011;39:2370–80.
Hale TW. Medications and mother’s milk. 13th ed. TX: Hale Publishing; 2008.
Ito N, Ito K, Koshimichi H, et al. Contribution of protein binding, lipid partitioning, and asymmetrical transport to drug transfer into milk in mouse versus human. Pharm Res. 2013;30:2410–22.
Atkinson HC, Begg EJ. Relationship between human milk lipid-ultrafiltrate and octanol-water partition coefficients. J Pharm Sci. 1988;77:796–8.
Kodaira H, Kusuhara H, Fujita T, Ushiki J, Fuse E, Sugiyama Y. Quantitative evaluation of the impact of active efflux by p-glycoprotein and breast cancer resistance protein at the blood–brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a surrogate. J Pharmacol Exp Ther. 2011;339:935–44.
Kondo C, Suzuki H, Itoda M, et al. Functional analysis of SNPs variants of BCRP/ABCG2. Pharm Res. 2004;21:1895–903.
Barrera B, Otero JA, Egido E, et al. The anthelmintic triclabendazole and its metabolites inhibit the membrane transporter ABCG2/BCRP. Antimicrob Agents Chemother. 2012;56:3535–43.
Pan G, Giri N, Elmquist WF. Abcg2/Bcrp1 mediates the polarized transport of antiretroviral nucleosides abacavir and zidovudine. Drug Metab Dispos. 2007;35:1165–73.
Wang X, Furukawa T, Nitanda T, et al. Breast cancer resistance protein (BCRP/ABCG2) induces cellular resistance to HIV-1 nucleoside reverse transcriptase inhibitors. Mol Pharmacol. 2003;63:65–72.
Hemauer SJ, Patrikeeva SL, Wang X, et al. Role of transporter-mediated efflux in the placental biodisposition of bupropion and its metabolite, OH-bupropion. Biochem Pharmacol. 2010;80:1080–6.
Marki-Zay J, Tauberne Jakab K, Szeremy P, Krajcsi P. MDR-ABC transporters: biomarkers in rheumatoid arthritis. Clin Exp Rheumatol. 2013;31:779–87.
Pavek P, Merino G, Wagenaar E, et al. Human breast cancer resistance protein: interactions with steroid drugs, hormones, the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo(4,5-b)pyridine, and transport of cimetidine. J Pharmacol Exp Ther. 2005;312:144–52.
Merino G, Alvarez AI, Pulido MM, Molina AJ, Schinkel AH, Prieto JG. Breast cancer resistance protein (BCRP/ABCG2) transports fluoroquinolone antibiotics and affects their oral availability, pharmacokinetics, and milk secretion. Drug Metab Dispos. 2006;34:690–5.
Ando T, Kusuhara H, Merino G, Alvarez AI, Schinkel AH, Sugiyama Y. Involvement of breast cancer resistance protein (ABCG2) in the biliary excretion mechanism of fluoroquinolones. Drug Metab Dispos. 2007;35:1873–9.
Kato Y, Takahara S, Kato S, et al. Involvement of multidrug resistance-associated protein 2 (Abcc2) in molecular weight-dependent biliary excretion of beta-lactam antibiotics. Drug Metab Dispos. 2008;36:1088–96.
Gunness P, Aleksa K, Koren G. Acyclovir is a substrate for the human breast cancer resistance protein (BCRP/ABCG2): implications for renal tubular transport and acyclovir-induced nephrotoxicity. Can J Physiol Pharmacol. 2011;89:675–80.
Hasegawa M, Kusuhara H, Adachi M, Schuetz JD, Takeuchi K, Sugiyama Y. Multidrug resistance-associated protein 4 is involved in the urinary excretion of hydrochlorothiazide and furosemide. J Am Soc Nephrol. 2007;18:37–45.
Kim HS, Sunwoo YE, Ryu JY, et al. The effect of ABCG2 V12M, Q141K and Q126X, known functional variants in vitro, on the disposition of lamivudine. Br J Clin Pharmacol. 2007;64:645–54.
Nakanishi H, Yonezawa A, Matsubara K, Yano I. Impact of P-glycoprotein and breast cancer resistance protein on the brain distribution of antiepileptic drugs in knockout mouse models. Eur J Pharmacol. 2013;710:20–8.
Beery E, Rajnai Z, Abonyi T, et al. ABCG2 modulates chlorothiazide permeability–in vitro-characterization of its interactions. Drug Metab Pharmacokinet. 2012;27:349–53.
Werthmann Jr MW, Krees SV. Excretion of chlorothiazide in human breast milk. J Pediatr. 1972;81:781–3.
Miller ME, Cohn RD, Burghart PH. Hydrochlorothiazide disposition in a mother and her breast-fed infant. J Pediatr. 1982;101:789–91.
Lilienblum W, Dekant W, Foth H, et al. Alternative methods to safety studies in experimental animals: role in the risk assessment of chemicals under the new European Chemicals Legislation (REACH). Arch Toxicol. 2008;82:211–36.
Noguchi K, Katayama K, Sugimoto Y. Human ABC transporter ABCG2/BCRP expression in chemoresistance: basic and clinical perspectives for molecular cancer therapeutics. Pharmgenomics Pers Med. 2014;7:53–64.
Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, Schinkel AH. Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther. 2010;9:319–26.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by a research scholarship from the Japan Research Foundation for Clinical Pharmacology, and a Grant-in-Aid for Scientific Research on Innovative Areas HD-Physiology from the Ministry of Education, Science and Culture of Japan (Grant No. 22136015).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(PDF 67.0 kb)
Supplementary Fig. 2
(PDF 146 kb)
Supplementary Table I
(PDF 57.6 kb)
Supplementary Table II
(PDF 102 kb)
Supplementary Table III
(PDF 59.8 kb)
Rights and permissions
About this article
Cite this article
Ito, N., Ito, K., Ikebuchi, Y. et al. Prediction of Drug Transfer into Milk Considering Breast Cancer Resistance Protein (BCRP)-Mediated Transport. Pharm Res 32, 2527–2537 (2015). https://doi.org/10.1007/s11095-015-1641-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-015-1641-2