ABSTRACT
Purpose
Genistein, the major bioactive isoflavone of soybeans, acts as a radiosensitizer for prostate cancer (PCa) both in vitro and in vivo. However, pure genistein promoted increased metastasis to lymph nodes. A mixture of soy isoflavones (genistein, daidzein, glycitein) did not cause increased metastasis, but potentiated radiotherapy. We tested whether daidzein could negate genistein-induced metastasis.
Methods
Mice bearing PC-3 prostate tumors were treated with daidzein, genistein or both, and with tumor irradiation. Primary tumors and metastases were evaluated. The effects of each isoflavone and soy were compared in vitro using PC-3 (AR−) and C4-2B (AR+) androgen-independent PCa cell lines.
Results
Daidzein did not increase metastasis to lymph nodes and acted as a radiosensitizer for prostate tumors. Daidzein inhibited cell growth and enhanced radiation in vitro but at doses higher than genistein or soy. Daidzein caused milder effects on inhibition of expression and/or activities of APE1/Ref-1, HIF-1α and NF-κB in PC-3 and C4-2B cells.
Conclusions
Daidzein could be the component of soy that protects against genistein-induced metastasis. Daidzein inhibited cell growth and synergized with radiation, affecting APE1/Ref-1, NF-κB and HIF-1α, but at lower levels than genistein and soy, in AR+ and AR- PCa cells, suggesting it is an AR-independent mechanism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
We have shown in several studies that soy isoflavones, which are safe dietary agents, act as potent radiosensitizers in prostate cancer (PCa) both in vitro and in vivo (1–6). High consumption of soy in Asian populations was associated with low incidence of PCa in epidemiological studies (7,8). The incidence in PCa is high in the United States; it is estimated that 192,280 new cases of PCa will be diagnosed in 2009, and 27,360 men will die of the disease (9–11). The majority of cases of PCa now diagnosed in the United States present with clinically localized disease (12). Localized PCa is sensitive to conventional radiotherapy, yet this treatment was reported to be insufficient to eradicate PCa in a proportion of patients, resulting in clinical recurrence (12–14). Recurrent tumors reflect the limitations of radiotherapy and are caused by radioresistance of tumor cells, which is also partly due to focal areas of radioresistant hypoxic cells.
To improve the local control of PCa, we have explored the combination of radiation with additional anti-tumor agents targeting cell survival pathways. Soy isoflavones were found to act as natural anti-cancer agents for PCa (15,16). The isoflavones in soybeans mainly include genistein, daidzein and glycitein. Genistein is the major compound of soy and the most bioactive. Daidzein is also a significant isoflavone in the soybean and has shown anti-cancer activity, whereas glycitein is a minor component (17,18). We have shown that genistein inhibited the cell growth of androgen-dependent (LNCaP) and androgen-independent (PC-3) human PCa cell lines in vitro, establishing it as a cytotoxic agent for prostate cancer (19,20).
The potential of genistein to enhance the therapeutic efficacy of radiotherapy for PCa was extensively investigated. We have previously demonstrated that pure genistein potentiates radiation-induced tumor cell killing of human PC-3 PCa cell line in vitro, when given prior to radiation (1,2,4–6). In vivo, using a metastatic orthotopic PC-3 tumor model in nude mice, we showed that treatment with genistein combined with prostate tumor irradiation significantly enhanced inhibition of prostate tumor growth and increased mouse survival (3). Paradoxically, in the course of these studies, while testing the effect of pure genistein given as a single modality treatment, we observed increased spontaneous metastasis to regional lymph nodes (3). Genistein treatment caused a two-fold increase in the size of para-aortic lymph nodes over that observed in untreated mice (3). These large nodules showed heavy tumor infiltration by histology staining and remnants of lymphoid tissues (3). Immunostaining of lymph nodes for Ki-67 proliferation marker suggested that genistein does not increase tumor cell proliferation in lymph nodes compared to control lymph nodes but probably causes increased metastasis (3).
To rule out whether the increased metastasis by genistein is unique to the PC-3/nude mouse xenograft model as a result of impaired immune system, these studies were repeated in a syngeneic murine RM-9 orthotopic PCa model in immuno-competent C57BL/6 mice (21). In this RM-9 model, treatment with genistein alone also showed a trend toward increased lymph node metastasis (21). Furthermore, genistein treatment also demonstrated a tendency to increase the incidence of metastasis to the mesentery lining the bowel in an orthotopic xenograft metastatic KCI-18 renal cell carcinoma (RCC) model (22).
Our novel findings on promotion of metastasis by treatment with pure genistein were a concern for soy-based clinical trials for cancer patients. Nevertheless, the soy isoflavones pills, used in human interventions, contain a mixture of genistein, daidzein and glycitein isoflavones and not only pure genistein (23). Therefore, a similar mixture of soy isoflavones, consisting of 43% genistein, 21% daidzein and 2% glycitein, extracted from soybeans, was tested in vivo in the PC-3 orthotopic PCa model. In contrast to pure genistein, the soy isoflavones mixture did not cause a further increase in the size of metastatic para-aortic lymph nodes compared to lymph nodes obtained from untreated control mice (5).
The goal of the current study was to determine whether daidzein, the second major isoflavone component of the soy formulation, protected against the adverse metastatic effects of genistein. The soy isoflavones mixture was also effective at enhancing prostate tumor radiation effect causing significant inhibition of tumor growth and metastasis, as previously observed with pure genistein combined with radiation (3–5). We have now tested whether pure daidzein contributes to this process of radiosensitization for PCa.
We have previously demonstrated that like pure genistein, the mixture of soy isoflavones potentiates radiation-induced cell killing in vitro and in vivo (4–6,24). We have identified three molecular targets, APE1/Ref-1, NF-κB and HIF-1α, which are activated by tumor cells as a survival response to radiation. These survival signaling pathways were inhibited by soy isoflavones, leading to increased cell killing and tumor growth inhibition (4–6,24). Apurinic/apyrimidinic (AP) endonuclease 1/redox factor-1 (APE1/Ref-1) is a protein involved in DNA repair that also functions as a redox activator of cellular transcription factors, including NF-κB and HIF-1α (24,25). We tested whether these molecules are affected by daidzein by comparing the effect of daidzein to genistein and to the mixture of soy isoflavones.
Advanced PCa is androgen independent and not responsive to hormone ablation; however, PCa tumor cells could still express AR. Our mechanistic studies in vitro were performed using the androgen-independent cell line PC-3, which is androgen-receptor (AR) negative. In the current study, we investigated whether the inhibition of cell growth and potentiation of radiation cell killing mediated by soy isoflavones also occur in the C4-2B PCa cell line, which is AR+ and androgen independent (26,27).
MATERIALS AND METHODS
Prostate Cancer Cells
PC-3 human PCa cell line (AR− and non-responsive to androgen) was cultured in F-12K culture medium (CM) containing 7% heat-inactivated fetal bovine serum (FBS) with supplements. The C4-2B human PCa cell line (AR+ and non-responsive to androgen) was cultured in RPMI 1640 CM containing 10% heat-inactivated FBS with supplements (6).
Orthotopic Implantation of PC-3 Cells In Vivo
PC-3/PI prostate tumor cell lines were generated by passaging PC-3 cells in the prostate of nude mice, as previously described (3,5). PC-3/PI tumor cells were injected, at a concentration of 5 × 105 cells in 20 µl HBSS, into the prostate of 5–6-week-old male Balb/c nu/nu nude mice (Harlan Sprague Dawley, Indianapolis, IN). Prostate implantation was performed as previously detailed (3,5). The prostate of anesthetized mice was exposed through a midline laparotomy incision and by anterior retraction of the bladder and male sex accessory glands. Injection of cells was performed with a 27-gauge needle inserted in the prostatic lobe located at the base of the seminal vesicles (3,5). The abdominal wound was sutured using 5.0 vicryl absorbable sutures for the skin inner layer and 5.0 prolene non-absorbable sutures for the skin outer layer. Mice were housed and handled under sterile conditions in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. Mice received Lab Diet # 5021 (Purina Mills, Inc., Richmond, IN). The animal protocol was approved by the Institutional Animal Care and Use Committee of Wayne State University.
In Vivo Daidzein/Genistein/Soy Isoflavones Treatment
Pure daidzein (Biomol International) or pure genistein (LKT, Toronto, Canada) was purchased, and a formula of soy isoflavones was obtained from NIH. The soy isoflavones mixture G2535 consisted of 43% genistein, 21% daidzein, 2% glycitein, 2.5 % protein, 11.9% fat, 1.7% water, with the remainder being carbohydrate (manufactured by Organic Technologies and obtained from NIH). Pure daidzein, pure genistein and the soy isoflavones mixture were dissolved in 0.1 mol/L Na2CO3 and mixed with sesame seed oil at a 2:1 ratio just prior to treatment to facilitate gavage and avoid irritation of the esophagus by Na2CO3. Mice were treated by gavage either with 1 mg/day (50 mg/kg body weight/day) soy isoflavones, an equivalent genistein dose of 0.43 mg/day (21.5 mg/ kg body weight/day) or an equivalent daidzein dose of 0.21 mg/day (10.5 mg/kg body weight/day). Control and radiation-only treated groups received Na2CO3 and sesame seed oil.
Experimental Protocol in PC-3 Tumor Model
Following prostate implantation with PC-3/PI cells, before initiating treatment, a few mice were killed to monitor and confirm tumor growth and size in the prostate. By day 8 after cell injection, mice had established 0.4-cm prostate tumors compared with 0.2-cm normal prostate. At that time point, mice were pretreated for 3 days with oral daidzein, genistein or soy isoflavones on days 8–10. On day 11, mice received 5 Gy photon radiation to the tumor-bearing prostate, as previously detailed (3,5). Prostate tumor irradiation was also provided on day 11 to mice treated with radiation only. One day after radiation, daidzein, genistein or soy isoflavones were resumed and administered daily for the duration of the experiment (3,5). Six to nine mice were used per experimental group. Mice were killed on day 36 after PC-3/PI cell injection, necropsied and examined for gross tumors in the prostate. The tumor-bearing prostates and para-aortic lymph nodes were resected and weighed.
Tumor Cell Growth Assays and Clonogenic Analysis of Cell Survival In Vitro
For in vitro assays, pure daidzein, pure genistein and soy isoflavones powders were dissolved in 0.1 mol/L Na2CO3 and were further diluted in CM to obtain final concentrations of 5–100 µmol/L. Control cells were incubated with equivalent dilutions of Na2CO3 in CM. Cells plated in 6-well plates were treated when ∼75% confluent. Radiation treatment was done with photons using a 60Co unit (AECL Theratron 780), as previously detailed (1,2). For cell count assays, PC-3 or C4-2B cells were plated at 105 cells per well in duplicate wells, in 6-well plates (1,2). A day later, cells were treated with daidzein, genistein or soy isoflavone mixture for 72 h, and then, cells from each well were harvested. Viable cells were counted using trypan blue exclusion dye (1,2). To assess long-term cell survival by clonogenic assay, PC-3 cells were treated with daidzein, genistein or soy isoflavone mixture for 24 h and then irradiated with 3 Gy photons (2,4). After radiation, cells were plated in triplicate wells in 6-well plates in 2 mL CM as follows: 500 cells per well for control, 1,000 cells per well for isoflavones or radiation alone, 2,000 cells per well for daidzein + radiation and 3,000 cells per well for genistein or soy + radiation treatments (2,4,5). The different number of cells plated for single and combined treatments was determined based on cell titrations assays to predict a measurable survival fraction. Cells were incubated for 10 days in the presence of isoflavones at 37°C in a 5% CO2/5% O2/90% N2 incubator. Colonies were fixed, stained using 2% crystal violet and counted (clones of at least 50 cells were counted as 1 colony) (4,5). The plating efficiency was calculated for each well, and the surviving fraction was normalized to control cells (4,5). In this assay, the plating efficiency was about 36%. For C4-2B cells, cells were treated with isoflavones for 48 h and then irradiated with 3 Gy photon radiation prior to plating in a colony assay. Because the C4-2B cells grow very loosely, a soft agar colony assay was used. In each well of 6-well plates, the bottom layer consisted of 0.5% agar in CM (1.5 ml per well), the middle layer consisted of 0.3% agar containing the C4-2B cells in CM (1.5 ml per well) and the top layer had 1 ml CM. Using these conditions, the plating efficiency for C4-2B cells was about 20%. Therefore, larger numbers of cells were plated in triplicate wells at a concentration of 3,000 cells per well for control, 6,000 cells per well for radiation or isoflavones alone, 8,000 cells per well for radiation + isoflavones and 10,000 cells per well for combined radiation + daidzein + genistein. C4-2B cells were incubated for 17 days in the presence of isoflavones at 37°C in a 5% CO2/5% O2/90% N2 incubator. Colonies were stained using 5 mg/ml para-iodonitrotetrazolium violet overnight at 37°C. Colonies were counted in each well using a Nikon Eclipse TS 100 inverted microscope.
Western Blot Analysis
For Western blot analysis, cells were washed with phosphate-buffered saline (PBS) and scraped. For protein extraction, cell lysis was done using modified RIPA buffer. Protein samples (20–40 μg) were loaded and separated on 7.5%, 10% or 12% SDS-PAGE gel and transferred to Trans-Blot membranes (Bio-Rad, Hercules, CA). Membranes were immunoblotted with primary antibodies (Ab) directed against HIF-1α (R&D Systems, Minneapolis, MN), APE1/Ref-1 (Novus Biologicals, Littleton, CO) and PARP, poly-APD-ribose-polymerases, (BIOMOL, Plymouth Meeting, PA) (4,6). Antibodies were diluted in a range of 1:500 to 1:2500. Membranes were incubated in IgG-HRP secondary Abs. Membranes were re-probed with anti-β-actin Ab (Santa Cruz, CA) as an internal control. Membranes were incubated in SuperSignal® West Pico Chemiluminescent Substrate (Pierce, Rockford, IL), and analysed in Bio-Rad ChemiDoc XRS imaging system (6).
Immunofluorescence Staining of Cells
C4-2B cells were cultured onto cover slips in six-well plates and treated with 30 μM soy isoflavones for 24 h, then irradiated with 3 Gy. Five hours after radiation, cells were fixed with 4% formaldehyde, permeabilized with 0.5% Triton X100 and incubated overnight at 4°C with anti-HIF-1α Abs (Exalpha Biologicals). After washing with PBS, cells were exposed for 2 h to FITC-conjugated secondary goat anti-mouse IgG (Alexa Fluor 488, Invitrogen, Carlsbad, CA) prepared in DAPI and 0.05% Triton X 100 (6). Stained cells were analyzed using NIKON E-800 UV microscope for detection of DAPI and FITC staining.
Electrophoretic Mobility Shift Assay
Nuclear extracts were prepared from treated cells using Sigma CelLytic™ NuCLEAR™ Extraction Kit (Sigma-Aldrich, St. Louis, MO) (2,4,6). Electrophoretic mobility shift assay (EMSA) was accomplished as previously detailed. Briefly, 5 µg of nuclear proteins were incubated with IRDye-700 labeled NF-κB oligonucleotide or IRDye-700 labeled HIF-1α oligonucleotide (LI-COR Biosciences, Lincoln, NE) (4,6). The DNA-protein complex was run on 8.0% native polyacrylamide gel using TBE buffer, pH 8.4. The bands were visualized by Odyssey Infrared Imaging System using Odyssey Software Release 1.1. AlphaEaseFC™ imaging software (AlphaInnotech, San Leandro, CA) was used to quantify resultant bands. Equal protein loading was ensured by immunoblotting 10 µg of nuclear protein with retinoblastoma antibody.
Statistical Analysis
For in vivo data analysis, differences in the weights of prostate tumors and lymph nodes among the various treatments groups were analyzed by two-tailed unpaired student’s t-test (3). Comparisons between means of various treatment groups in cell growth and clonogenic assays, Western blot and EMSA assays were analyzed by two-tailed unpaired student’s t-test (2,4). A p-value less than 0.05 was considered statistically significant.
RESULTS
Daidzein Protects Against Genistein-Induced Lymph Node Metastasis
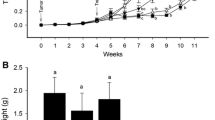
We have previously shown that treatment of PC-3 prostate tumors with pure genistein inhibits primary tumor growth inhibition by about 30%, but this treatment increased by two-fold the size of regional para-aortic lymph nodes compared to metastatic lymph nodes in control mice (3). However, in contrast to pure genistein, treatment of PC-3 prostate tumors with a natural formulation of soy isoflavones (43% genistein, 21% daidzein and 2% glycitein) did not cause increased spontaneous metastasis (5). We have now tested whether daidzein, the second main isoflavone of soybeans, was the component of the soy isoflavone mixture that interfered with genistein-induced lymph node metastasis. Mice bearing established PC-3 prostate tumors (0.4 × 0.25 × 0.2 cm in size and 19 mg weight) were treated with the mixture of isoflavones at 1 mg/day and compared to an equivalent dose of 0.43 mg/day of pure genistein or 0.21 mg/day of pure daidzein or both 0.21 mg/day daidzein and 0.43 mg/day genistein combined. Mice were killed on day 36 after cell injection to assess prostate tumors and lymph node sizes (Fig. 1A). Either daidzein or genistein or both combined caused tumor growth inhibition in the range of 30–50% compared to control mice (p ≤ 0.05) (Fig. 1B). No statistical significance was obtained when comparing the difference in tumor weights between groups treated with daidzein, genistein or both combined, and the extent of tumor growth inhibition was comparable to that obtained with the mixture of soy isoflavones (Fig. 1B). However, the effect of isoflavones on metastasis to para-aortic lymph nodes showed striking differences between treatment groups (Fig. 1C). Pure genistein reproducibly caused a two-fold increase in the weight/size of lymph nodes compared to control mice (p = 0.03), as previously observed (3,5). In contrast to genistein-induced increased metastasis, the mean size of metastatic para-aortic lymph nodes following treatment with pure daidzein was comparable to that of control mice (Fig. 1C). Interestingly, when both daidzein and genistein were combined, the mean weight of lymph nodes was comparable to that observed with daidzein alone with no further increase, which is consistent with the effect of the mixture of soy isoflavones on lymph node metastasis (Fig. 1C) (5).
Treatment of PC-3 prostate tumors with isoflavones and tumor irradiation. A Treatment schedule diagram. On day 8–10 after PC-3 cell injection in prostate, mice bearing established prostate tumors were treated daily with oral daidzein, genistein or a mixture of soy isoflavones. For combination therapy with tumor irradiation, prostate tumors were irradiated with 5 Gy photons on day 11. One day later, isoflavones treatment with daidzein or daidzein + genistein was resumed and given every day. Mice treated with isoflavones only without radiation received treatment on a daily basis up to day 36 post cell injection. On day 36, mice were killed, and the prostates, including the tumors, were resected and weighed. B Response of primary prostate tumors following daidzein, genistein or a mixture of soy isoflavones alone or combined with radiation. The mean tumor weights ± SE from 6–9 mice per treatment group are reported. *p < 0.05; **p < 0.0001 represent significance of each treatment group relative to control group. C Response of para-aortic lymph node metastases. At least 2 lymph nodes were obtained from each mouse and weighed. The mean lymph nodes weights ± SE from 6–9 mice per treatment group are reported. *p < 0.05 relative to control group.
When mice were treated with tumor irradiation in conjunction with daidzein only or daidzein combined with genistein, as shown in the schedule diagram in Fig. 1A, a significant 90% tumor growth inhibition was observed (p < 0.0001), much greater than with daidzein alone or daidzein + genistein (p < 0.001) (Fig. 1B). Furthermore, treatment with daidzein and radiation inhibited metastasis to the lymph nodes, as previously observed with genistein or soy mixture (Fig. 1C) (5).
Daidzein is Less Effective than Genistein or Soy in Mediating Cell Killing
We have shown that daidzein is as biologically active as genistein for causing PC-3 prostate tumor growth inhibition and potentiating the effect radiation (Fig. 1B). Moreover, we found that daidzein did not promote lymph node metastasis, unlike genistein (Fig. 1C). We have previously shown that the mixture of soy isoflavones or pure genistein inhibited PC-3 cell growth in vitro and potentiated radiation-induced cell killing (1,2,4,6). To study further the role of daidzein in the anti-tumor activity mediated by soy isoflavones, we compared the effects of pure daidzein and pure genistein to the soy mixture. These assays were performed in both the PC-3 (AR−) and C4-2B (AR+) cell lines, which differ by expression of AR even though both are androgen-independent cell lines. The short-term effects of doses of 15–100 µM of each single isoflavone or the soy mixture on cell growth were assessed by counting viable cells after 72 h treatment in vitro (Fig. 2A). In PC-3 cells, daidzein inhibited cell growth in a dose-dependent manner. However, compared to 30 µM genistein or 30 µM soy mixture, higher doses up to 100 µM of daidzein were needed to significantly inhibit 70% of cell growth (p = 0.01 relative to control) (Fig. 2A). Based on these data, to assess the long-term effects of daidzein using clonogenic assays, we compared doses of 5–60 µM daidzein to the bioactive doses of 30 µM genistein and 30 µM soy mixture (Fig. 2B). Whereas genistein and soy at 30 µM caused a strong 75–85% inhibition of cell survival, daidzein caused only about 28–35% significant inhibition with doses of 30 and 60 µM (p < 0.05) (Fig. 2B). When daidzein was combined with 3 Gy radiation, only a dose of 60 µM daidzein showed a greater inhibition of cell survival than with radiation alone or daidzein alone (p = 0.001), which was comparable to the cell survival inhibition of 85–90% observed with much lower doses of 15 µM of genistein or the soy mixture combined with radiation (Fig. 2C).
PC-3 cell growth inhibition by isoflavones and radiation. A Cell growth of PC-3 cells treated with isoflavones. PC-3 cells were treated with daidzein, genistein or soy at concentrations of 15, 30, 60 and 100 µM for 72 h, and viable cells were counted. Bars represent the mean number of cells of duplicate wells calculated as the percentage of control cells ± SE. *p < 0.01 relative to control group. B Survival of PC-3 cells treated with isoflavones. PC-3 cells were treated with 5, 10, 15, 30 and 60 µM of daidzein or 30 µM of genistein or soy for 72 h and plated in a clonogenic assay. Bars represent the mean survival fraction of triplicate wells ± SE. *p < 0.01; **p < 0.001 represent significance of each treatment group relative to control group. C Survival of PC-3 cells treated with isoflavones and radiation. PC-3 cells were pre-treated with 10, 15, 30 and 60 µM of daidzein (D) or 15 µM of genistein (G15) or soy (S15) for 24 h, then irradiated with 3 Gy photons and plated in a clonogenic assay in the presence of the same concentrations of isoflavones. Bars represent the mean survival fraction of triplicate wells ± SE. **p < 0.001 represent significance of each treatment group relative to control group.
Compared to PC-3 cells, we found that C4-2B cells were more sensitive to daidzein, genistein or soy (Fig. 3A). The soy mixture showed the most effective inhibition of cell growth of 80–95% at doses of 15 and 30 µM (p < 0.001). Such effect was seen with 60 µM of pure genistein (p < 0.001), whereas daidzein caused 70% inhibition at a higher dose of 100 µM (p < 0.001) (Fig. 3A). C4-2B cells were also radiosensitive, and 3 Gy radiation caused 70% cell growth inhibition (Fig. 3B). This inhibition was significantly augmented to 80–85% with doses of 30 µM daidzein (p < 0.05) and lower doses of 15 µM genistein (p < 0.01) or only 10 µM soy mixture (p < 0.01) compared to radiation alone or to either isoflavones alone (p = 0.01–0.001) (Fig. 3B). Clonogenic assays performed with C4-2B cells showed that, when combined with radiation, a low dose of 10 µM daidzein or genistein enhanced significantly inhibition of cell survival to 75 and 87%, respectively, compared to about 40% with isoflavones (p = 0.001) and 60% with radiation (p < 0.05) (Fig. 3C). Combining both daidzein and genistein with radiation showed the same effect as with genistein + radiation and was comparable to that of the soy mixture combined with radiation (Fig. 3C).
C4-2B cell growth inhibition by isoflavones and radiation. A Cell growth of C4-2B cells treated with isoflavones. C4-2B cells were treated with daidzein, genistein or soy at concentrations of 15, 30, 60 and 100 µM for 72 h, and viable cells were counted. Bars represent the mean number of cells of duplicate wells calculated as the percentage of control cells ± SE. *p < 0.001 relative to control group. B Cell growth of C4-2B cells treated with isoflavones and radiation. C4-2B cells were treated with 30 µM of daidzein (D30), 15 µM of genistein (G15) or 10 µM of soy (S10) for 24 h, then irradiated with 3 Gy photons and further incubated for 72 h. Viable cells were counted. Bars represent the mean number of cells of duplicate wells calculated as the percentage of control cells ± SE. *p < 0.01 relative to control group. C Survival of C4-2B cells treated with isoflavones and radiation. C4-2B cells were pre-treated with 10 µM of daidzein (D) or 10 µM of genistein (G) or 10 µM of soy (S) for 48 h, then irradiated with 3 Gy photons and plated in a clonogenic assay in the presence of the same concentrations of isoflavones. Bars represent the mean survival fraction of triplicate wells ± SE. *p < 0.001; **p < 0.0001 represent significance of each treatment group relative to control group.
Daidzein Causes a Milder Effect on PARP Cleavage, HIF-1α and APE1/Ref-1 Expressions
We have previously shown that both pure genistein and the soy mixture cause apoptotic PC-3 cell death in a dose-dependent manner, which was marked with a dose of 60 µM (4,5). We now assessed whether daidzein cause apoptotic cell death by measuring PARP cleavage from a 116 kDa molecule to a 65 kDa molecule, which is indicative of late stages of apoptosis. PC-3 and C4-2B cell lines were treated with different concentrations of 15, 30 and 60 µM of pure daidzein in vitro and 60 µM genistein or 60 µM soy mixture for 72 h (Fig. 4A). Daidzein caused mild PARP cleavage detectable in both cell lines at 60 µM in contrast to greater PARP cleavage induced by genistein and the soy mixture in both PC-3 and C4-2B cell lines (Fig. 4A).
Effect of daidzein and radiation compared to genistein and soy on PARP, HIF-1α and APE1/Ref-1 in PC-3 cells and C4-2B cells. A, B Effect of isoflavones in PC-3 cells and C4-2B cells. Cells were either untreated (C for control) or treated with 15, 30 and 60 µM of daidzein (D15, D30 and D60) or 60 µM of genistein (G60) or 60 µM of soy (S60) for 72 h. and samples were processed for Western blot to detect PARP and cleaved PARP (A); HIF-1α and APE1/Ref-1 (B). C Effect of isoflavones and radiation in PC-3 cells and C4-2B cells. Cells were untreated (Con) or pre-treated with 30 µM of daidzein (Daid) or genistein (Gen) or soy (Soy) alone, or with radiation alone (Rad), or combined with radiation (D+R, G+R, S+R). Pre-treatment with isoflavones was done for 72 h, and then cells were irradiated with 3Gy radiation. At 3 h after radiation, cells were processed for Western blot analysis to detect HIF-1α and APE1/Ref-1. β-actin protein levels served as loading control.
In previous studies, we found that the expression of the cell survival molecules HIF-1α and APE1/Ref-1 were drastically inhibited in PC-3 cells by pure genistein and the mixture of soy isoflavones (4,6). To assess the role of daidzein on the effect mediated by the soy mixture, cells were treated with 15, 30 and 60 µM of pure daidzein and 60 µM genistein or 60 µM soy mixture for 72 h. Whereas expression of HIF-1α was completely inhibited by genistein or the soy mixture, a lower level of inhibition was observed with 60 µM daidzein. Daidzein at 60 µM decreased expression of APE1/Ref-1, and this effect was comparable to that observed with genistein and the soy mixture (Fig. 4B). The effects of isoflavones on HIF-1α and APE1/Ref-1 were similar in both PC-3 and C4-2B cell lines.
Both HIF-1α and APE1/Ref-1 molecules were found to be upregulated by radiation, but this effect was inhibited by pre-treatment with pure genistein or the soy mixture in PC-3 cells (4,6). We now showed that pre-treatment of the cells with daidzein also inhibits radiation-induced activation of HIF-1α and APE1/Ref-1 akin to our findings with genistein and soy mixture (Fig. 4C). Furthermore, our new data showed that this effect was reproduced in both PC-3 (AR−) cells and C4-2B (AR+) cells (Fig. 4C).
Inhibition of Radiation-Induced HIF-α Expression in C4-2B Cells Treated with Soy Isoflavones
We recently reported that treatment of PC-3 cells with soy isoflavones inhibits increased cellular expression and nuclear localization of HIF-1α induced by radiation using immunofluorescence staining studies (6). These studies were repeated using C4-2B cells. Cells were pre-treated with 30 µM of the soy isoflavones mixture, then with 3 Gy radiation and incubated for 5 h prior to immunofluorescence staining. C4-2B cells showed expression of HIF-1α in the cytoplasm, and some cells also showed nuclear expression (Fig. 5A). This expression was greatly enhanced both in the cytoplasm and nucleus of the cells by radiation (Fig. 5B) as previously observed with PC-3 cells (6). When C4-2B cells were treated with soy isoflavones, HIF-1α staining was mostly observed in the cytoplasm with minimal nuclear staining (Fig. 5C). A similar pattern of staining was observed when cells were pre-treated with soy then irradiated, with much lower levels of HIF-1α nuclear staining compared to the high intensity staining seen in cells treated with radiation only (Fig. 5D).
Soy isoflavones decrease nuclear protein expression and inhibit HIF-1α radiation-induced increase in C4-2B cells. C4-2B cells were pre-treated with 30 μM soy isoflavones for 24 h followed by 3 Gy radiation. At 5 h after radiation, cells were processed for HIF-1α immunostaining. A Control cells show HIF-1α cytoplasmic staining and minimal nuclear staining. B Radiation-treated cells exhibit intense nuclear and cytoplasmic HIF-1α staining. C Cells treated with soy show cytoplasmic staining but minimal nuclear HIF-1α staining. D Cells treated with soy combined with radiation have a significant decrease in nuclear HIF-1α staining compared to radiation-treated cells. All magnifications ×40.
Radiation-Induced DNA Binding Activity of HIF-1α and NF-κB is Inhibited by Pre-treatment of Daidzein or Genistein or Soy in Both PC3 and C4-2B Cell lines
We have previously shown that treatment with pure genistein or the soy mixture in PC-3 (AR−) cells significantly inhibited the DNA binding activities of both HIF-1α and NF-κB transcription factors and their activation by radiation (2,4,6). In the current study, we tested whether isoflavones also alter these transcription pathways in the C4-2B (AR+) cells and whether pure daidzein isoflavone plays a role in the inhibition mediated by the mixture of soy isoflavones. Both PC-3 and C4-2B cells were treated with 30 µM of either pure daidzein, or genistein or the soy mixture as single treatment modality for 48 h or followed by radiation and tested for DNA binding activity by EMSA. Daidzein effect on HIF-1α DNA binding activity was milder than genistein or soy in PC-3 cells, whereas genistein or soy significantly inhibited both HIF-1α DNA binding activity (p < 0.0001) (Fig. 6A). In C4-2B cells, the soy mixture was more effective at decreasing HIF-1α DNA binding activity (Fig. 6A). In both cell lines, radiation caused an increase in HIF-1α DNA binding activity that was effectively inhibited by soy isoflavones or genistein (p ≤ 0.0001), but the effect of daidzein was milder (Fig. 6A). Comparable findings were observed with the effect of isoflavones on NF-κB DNA binding activity, showing greater inhibition mediated by the mixture of soy isoflavones and pure genistein than with daidzein (Fig. 6B). Inhibition of radiation-induced activation of NF-κB DNA binding activity was also more prominent with the soy mixture or pure genistein, and a milder effect of daidzein was observed (Fig. 6B). These data were consistently observed both in C4-2B (AR+) and PC-3 (AR−) cell lines.
Pre-treatment with soy isoflavones inhibits HIF-1α and NF-κB DNA-binding activity induced by radiation in both PC-3 and C4-2B cells. A Effect of treatment on HIF-1α DNA binding activity in PC-3and C4-2B cells. Both cell lines were treated with each modality alone or pre-treated with 30 μM daidzein (Daid or D), 30 μM genistein (Gen or G) or 30 μM soy (Soy or S) for 48 h followed by 3 Gy radiation (Rad or R). At 5 h after radiation, cell nuclear extracts were subjected to EMSA for analysis of HIF-1α DNA binding activity. Data from untreated control (con) are also shown. B Effect of treatment on NF-κB DNA binding activity in PC-3 and C4-2B cells. Nuclear extracts from the experiment presented in panel A were subjected to EMSA for analysis of NF-κB DNA binding activity. In panels A and B, data are also presented as the mean I.D.V. of the band per μg protein loaded (±S.E.). *p < 0.05; ** p < 0.01 represent significance of each treatment group relative to control group. Western blot for retinoblastoma (Rb) protein in the nuclear extract was performed as an internal loading control.
DISCUSSION
Most animal studies have emphasized the role of genistein in cancer prevention for prostate cancer and mammary tumors (28–31). Controversial findings describing beneficial or adversary effects of pure genistein have been reported. Feeding mice with soy phytochemical concentrate (SPC, containing genistein, daidzein and glycitein) reduced tumor growth of s.c. implanted human LNCaP cells in SCID mice (32). In a prevention study, using an orthotopic model of LNCaP cells in SCID mice similar to our model, SPC was more effective than genistein or soy protein (33). In contrast, other studies showed increase in tumor growth by genistein in experimental colon cancer in rats, orthotopic mouse mammary tumors and rat prostate tumors (34–36). However, our studies in metastatic PCa and RCC animal models are not based on using genistein for chemoprevention but for therapy of established tumors. Our findings in orthotopic PCa models suggested that pure genistein promoted lymph node metastasis, when given as a treatment modality (3,21). However, a mixture of soy isoflavones, consisting of genistein, daidzein and glycitein, which is more representative of the soy pills used in human interventions, did not increase metastasis to regional lymph nodes in the PC-3 orthotopic model (5). This soy mixture was also effective at enhancing prostate tumor radiation effect, causing significant inhibition of tumor growth and metastasis, as previously observed with pure genistein (3,5). In the current study, we investigated the role of daidzein, the second major isoflavone in the soybeans, in negating the increased metastasis by genistein, and in contributing to radiosensitization for PCa. We found that daidzein protected against the adverse metastatic effects of genistein in the PC-3 orthotopic PCa model. Treatment with pure daidzein did not cause a further increase in the size of para-aortic lymph nodes, beyond that measured in metastatic lymph nodes from mice treated with vehicle (control) or with soy mixture. These findings with pure daidzein are in contrast to the two-fold increase in the mean size of lymph nodes that was reproducibly observed following treatment with pure genistein (3,5). Moreover, when both daidzein and genistein were combined, the mean size of lymph nodes was comparable to that observed with daidzein alone with no further increase, which is consistent with the effect of the mixture of soy isoflavones on lymph node metastasis. These data indicate that pure daidzein could be the component of the soy isoflavones mixture that protects against genistein-induced lymph node metastasis. We also showed that daidzein is as effective as pure genistein at enhancing prostate tumor radiation effect, causing significant inhibition of tumor growth and metastasis. We conclude that, like genistein, daidzein also acts as a potent radiosensitizer for orthotopic prostate tumor in mice, and it also probably contributes to the effect mediated by the mixture of soy isoflavones.
To elucidate the role of daidzein, as a component of soy isoflavones, in the mechanism of PCa cells growth inhibition, we compared the effect of each isoflavone and the soy mixture in PCa cell lines in vitro on cell killing and survival signaling pathways. We also compared two androgen-independent human PCa cell lines, which differ by expression of the androgen receptor, the PC-3 (AR−) and the C4-2B (AR+), which has a phenotype more representative of advanced refractory PCa (26,27). Our goal was to determine whether AR+ hormone refractory PCa is sensitive to the cytotoxic effect of isoflavones alone and combined with radiation, as previously demonstrated with PC-3 (AR−) cells. We found that daidzein inhibited cell growth and synergized with radiation in cell growth assays and long-term clonogenic assays, but this effect was observed at doses 3–4-fold higher than those of genistein or soy mixture. C4-2B cells were more sensitive to growth inhibition by treatment with daidzein, genistein or the soy mixture given alone and in combination with radiation. These data confirmed that AR+ PCa tumor lines are also responsive to cell killing by isoflavones and radiation akin to AR− PCa lines, and this process is independent of AR expression. These findings are in agreement with a recent study examining the growth inhibition of androgen-dependent and -independent PCa cell lines (37). Compared to the effects of genistein or the soy mixture, daidzein treatment caused milder effects on PARP cleavage in both PC-3 and C4-2B cell lines, in agreement with the lower cytotoxicity of daidzein observed in cell growth assays.
In our previous mechanistic studies, we have demonstrated that genistein or the soy mixture causes tumor cell apoptosis and enhances radiation killing by inhibiting cell survival pathways activated by radiation including the repair enzyme/redox activator APE1/Ref-1 and the transcription factors HIF-1α and NF-κB (2,4,6,24). In addition to its effects as a DNA repair enzyme, APE1/Ref-1 functions as a redox activator of cellular transcription factors, including NF-κB and HIF-1α, and facilitates their DNA binding via the reduction of a cysteine residue to a sulfhydryl state (24). Both NF-κB and HIF-1α transcription factors promote the synthesis of molecules critical for cell survival in response to cellular stress (24). We and others have shown that APE1/Ref-1, NF-κB and HIF-1α molecules are upregulated and/or activated by radiation and have been implicated in radioresistance of cancer cells (4,24,38–40). We found that expression and/or activities of these molecules are inhibited by pure genistein and by the soy isoflavones mixture (4,6). These findings were confirmed in the current study, but in addition, we showed that daidzein also caused inhibition of expression of APE1/Ref-1 and HIF-1α; however, its effects were milder than those observed with genistein or soy. Pre-treatment of cells with genistein or soy isoflavones inhibited upregulation of expression of APE1/Ref-1 and HIF-1α induced by radiation, as previously reported (4,6). We now showed that pre-treatment of cells with daidzein also inhibits radiation-induced activation of HIF-1α and APE1/Ref-1. These data suggest that in addition to genistein, daidzein is also a bioactive component of soy isoflavones and contributes to its effect in blocking survival molecules activated by radiation. Soy isoflavones also inhibited radiation-induced HIF-1 α and APE1/Ref-1 in C4-2B (AR+) cells, as observed in PC-3 (AR−) cells (6). It is interesting to note that, as previously shown with PC-3 cells, soy also inhibited the activation and nuclear translocation of HIF-1α protein induced by radiation in C4-2B cells. These data indicate that modulation of HIF-1α and APE1/Ref-1 molecules by soy isoflavones and/or radiation occurs independently of the status of AR.
We have demonstrated that downregulation of APE1/Ref-1 expression by soy isoflavones altered the DNA binding activity of NF-κB and HIF-1α and blocked their activation induced by radiation (4,6). This mechanism could potentially contribute to radiosensitization of PCa by blocking NF-κB and HIF-1α transcriptions of important molecules essential for tumor growth, progression and angiogenesis. The effect of daidzein on NF-κB and HIF-1α DNA binding activities was milder than that observed with genistein or the soy mixture.
In the current study, we have demonstrated that both daidzein and genistein could contribute to the radiosensitization of PCa by soy isoflavones via mechanisms leading to inhibition of cell survival pathways, which are activated in response to oxidative stress and DNA damage induced by radiation. Although the effects of daidzein were milder than genistein or soy in vitro, its effects in vivo were as potent as those of genistein, causing tumor growth inhibition and enhancing the effect of tumor irradiation. Our novel observation that the presence of daidzein, in the soy mixture, protects against genistein-induced increased lymph node metastasis is intriguing, and its mechanism remains to be clarified. This mechanism could include other pathways, in vivo, in the tumor microenvironment, which are differently affected by daidzein and genistein.
Abbreviations
- Ab:
-
Antibody
- APE/Ref-1:
-
Apurinic/Apyrimidinic Endonuclease 1/Redox Factor-1
- AR:
-
Androgen Receptor
- AR−:
-
Androgen Receptor negative
- AR+:
-
Androgen Receptor positive
- CM:
-
Culture medium
- EMSA:
-
Electrophoretic mobility shift assay
- FBS:
-
Fetal bovine serum
- HIF-1α:
-
Hypoxia Inducible factor 1 alpha
- NF-κB:
-
Nuclear Factor kappa B
- PARP:
-
poly-APD-ribose-polymerases
- PBS:
-
Phosphate buffered saline
- PCa:
-
Prostate Cancer
- RCC:
-
Renal cell carcinoma
REFERENCES
Hillman GG, Forman JD, Kucuk O, Yudelev M, Maughan RL, Rubio J et al. Genistein potentiates the radiation effect on prostate carcinoma cells. Clin Cancer Res. 2001;7:382–90.
Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6:107.
Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M et al. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3:1271–9.
Raffoul JJ, Banerjee S, Singh-Gupta V, Knoll ZE, Fite A, Zhang H et al. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67:2141–9.
Raffoul JJ, Banerjee S, Che M, Knoll ZE, Doerge DR, Abrams J et al. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007;120:2491–8.
Singh-Gupta V, Zhang H, Banerjee S, Kong D, Raffoul JJ, Sarkar FH et al. Radiation-induced HIF-1alpha cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int J Cancer. 2009;124:1675–84.
Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metastasis Rev. 2002;21:265–80.
Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: a cross-national study. J Natl Cancer Inst. 1998;90:1637–47.
Nelson WG, De Marzo AM, Isaacs WB. Prostate cancer. N Engl J Med. 2003;349:366–81.
Walsh PC, DeWeese TL, Eisenberger MA. Clinical practice. Localized prostate cancer. N Engl J Med. 2007;357:2696–705.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49.
Zietman AL, DeSilvio ML, Slater JD, Rossi Jr CJ, Miller DW, Adams JA et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294:1233–9.
Swanson GP, Hussey MA, Tangen CM, Chin J, Messing E, Canby-Hagino E et al. Predominant treatment failure in postprostatectomy patients is local: analysis of patterns of treatment failure in SWOG 8794. J Clin Oncol. 2007;25:2225–9.
Morgan PB, Hanlon AL, Horwitz EM, Buyyounouski MK, Uzzo RG, Pollack A. Radiation dose and late failures in prostate cancer. Int J Radiat Oncol Biol Phys. 2007;67:1074–81.
Sarkar FH, Li Y, Wang Z, Kong D. Cellular signaling perturbation by natural products. Cell Signal. 2009;21:1541–7.
Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–50.
Lo FH, Mak NK, Leung KN. Studies on the anti-tumor activities of the soy isoflavone daidzein on murine neuroblastoma cells. Biomed Pharmacother. 2007;61:591–5.
Choi EJ, Kim GH. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683–90.
Davis JN, Singh B, Bhuiyan M, Sarkar FH. Genistein-induced upregulation of p21WAF1, downregulation of cyclin B, and induction of apoptosis in prostate cancer cells. Nutr Cancer. 1998;32:123–31.
Davis JN, Kucuk O, Sarkar FH. Genistein inhibits NF-kappa B activation in prostate cancer cells. Nutr Cancer. 1999;35:167–74.
Wang Y, Raffoul JJ, Che M, Doerge DR, Joiner MC, Kucuk O et al. Prostate cancer treatment is enhanced by genistein in vitro and in vivo in a syngeneic orthotopic tumor model. Radiat Res. 2006;166:73–80.
Hillman GG, Wang Y, Che M, Raffoul JJ, Yudelev M, Kucuk O et al. Progression of renal cell carcinoma is inhibited by genistein and radiation in an orthotopic model. BMC Cancer. 2007;7:4.
Hussain M, Banerjee M, Sarkar FH, Djuric Z, Pollak MN, Doerge D et al. Soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2003;47:111–7.
Raffoul JJ, Sarkar FH, Hillman GG. Radiosensitization of prostate cancer by soy isoflavones. Curr Cancer Drug Targets. 2007;7:759–65.
Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461:83–108.
Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–81.
Bhuiyan MM, Li Y, Banerjee S, Ahmed F, Wang Z, Ali S et al. Down-regulation of androgen receptor by 3, 3′-diindolylmethane contributes to inhibition of cell proliferation and induction of apoptosis in both hormone-sensitive LNCaP and insensitive C4–2B prostate cancer cells. Cancer Res. 2006;66:10064–72.
Mentor-Marcel R, Lamartiniere CA, Eltoum IE, Greenberg NM, Elgavish A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res. 2001;61:6777–82.
Aronson WJ, Tymchuk CN, Elashoff RM, McBride WH, McLean C, Wang H et al. Decreased growth of human prostate LNCaP tumors in SCID mice fed a low-fat, soy protein diet with isoflavones. Nutr Cancer. 1999;35:130–6.
Lamartiniere CA, Murrill WB, Manzolillo PA, Zhang JX, Barnes S, Zhang X et al. Genistein alters the ontogeny of mammary gland development and protects against chemically-induced mammary cancer in rats. Proc Soc Exp Biol Med. 1998;217:358–64.
Lamartiniere CA, Cotroneo MS, Fritz WA, Wang J, Mentor-Marcel R, Elgavish A. Genistein chemoprevention: timing and mechanisms of action in murine mammary and prostate. J Nutr. 2002;132:552S–8.
Zhou JR, Gugger ET, Tanaka T, Guo Y, Blackburn GL, Clinton SK. Soybean phytochemicals inhibit the growth of transplantable human prostate carcinoma and tumor angiogenesis in mice. J Nutr. 1999;129:1628–35.
Zhou JR, Yu L, Zhong Y, Nassr RL, Franke AA, Gaston SM et al. Inhibition of orthotopic growth and metastasis of androgen-sensitive human prostate tumors in mice by bioactive soybean components. Prostate. 2002;53:143–53.
Rao CV, Rivenson A, Simi B, Zang E, Hamid R, Kelloff GJ et al. Enhancement of experimental colon carcinogenesis by dietary 6-phenylhexyl isothiocyanate. Cancer Res. 1995;55:4311–8.
Cohen LA, Zhao Z, Pittman B, Scimeca J. Effect of soy protein isolate and conjugated linoleic acid on the growth of Dunning R-3327-AT-1 rat prostate tumors. Prostate. 2003;54:169–80.
Wietrzyk J, Mazurkiewicz M, Madej J, Dzimira S, Grynkiewicz G, Radzikowski C et al. Genistein alone or combined with cyclophosphamide may stimulate 16/C transplantable mouse mammary cancer growth. Med Sci Monit. 2004;10:BR414–9.
Tepper CG, Vinall RL, Wee CB, Xue L, Shi XB, Burich R et al. GCP-mediated growth inhibition and apoptosis of prostate cancer cells via androgen receptor-dependent and -independent mechanisms. Prostate. 2007;67:521–35.
Wouters A, Pauwels B, Lardon F, Vermorken JB. Review: implications of in vitro research on the effect of radiotherapy and chemotherapy under hypoxic conditions. Oncologist. 2007;12:690–712.
Fishel ML, Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol Aspects Med. 2007;28:375–95.
Harada H, Kizaka-Kondoh S, Li G, Itasaka S, Shibuya K, Inoue M et al. Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene. 2007;26:7508–16.
ACKNOWLEDGEMENTS
This study was supported by the Fund for Cancer Research (grant to G.G.H.), American Cancer Society (grant ROG-06-097-01 to G.G.H.) and the American Institute for Cancer Research (grant 03B108 to G.G.H.). We thank Amit Patel for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh-Gupta, V., Zhang, H., Yunker, C.K. et al. Daidzein Effect on Hormone Refractory Prostate Cancer In Vitro and In Vivo Compared to Genistein and Soy Extract: Potentiation of Radiotherapy. Pharm Res 27, 1115–1127 (2010). https://doi.org/10.1007/s11095-010-0107-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0107-9