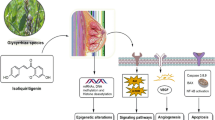
Abstract
Genistein, a commonly occurring isoflavone, has recently gained popularity owing to its ever-expanding spectrum of pharmacological benefits. In addition to health benefits such as improved bone health and reduced postmenopausal complications owing to its phytoestrogen properties, it has been widely evaluated for its anti-cancer potential. Several studies have established the potential for its usage in the management of breast, lung, and prostate cancers, and its usage has significantly evolved from early applications in traditional systems of medicine. This review offers an insight into its current status of usage, the chemistry, and pharmacokinetics of the molecule, an exploration of its apoptotic mechanisms in cancer management, and opportunities for synergism to improve therapeutic outcomes. In addition to this, the authors have presented an overview of recent clinical trials, to offer an understanding of contemporary studies and explore prospects for a greater number of focused trials, moving forward. Advancements in the application of nanotechnology as a strategy to improve safety and efficacy have also been highlighted, with a brief discussion of results from safety and toxicology studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genistein is a widely abundant isoflavone that commonly exists in a variety of soy-based products. Popular in Chinese and Ayurvedic traditional systems of medicine, it is widely consumed in Asian diets and is slowly gaining popularity worldwide (Smeriglio et al. 2019). Its benefits have been studied in different diseases including diabetes, cardiovascular, and obesity-related conditions as well as age-related disorders, chiefly menopausal complications for women and prostate cancer for men (Islam et al. 2020). As the incidence of cancer continues to increase globally, there is a need to explore alternative treatment strategies (Hazafa and Rehman K-U-, Jahan N, Jabeen Z. 2020). Owing to the rising rates of cancer, there is an increased pressure on the healthcare system and a substantial decrease in the overall quality of life of patients. This has directed focus to phytoconstituents such as isoflavones, possessing a broad spectrum of therapeutic benefits. Soy isoflavones have been widely studied for their potential in regulating bone health and improved respiratory and cardiac functioning, as well as neurological actions (Kim et al. 2021). Regular supplementation has been linked with the exertion of a protective effect, and as research continues to grow, a variety of cancers and signaling pathways have been targeted using genistein.
Genistein has found extensive applications in the management of breast and prostate cancer and has been dubbed as one of the “big five” chemicals targeting stem cells (Naujokat and McKee 2021). This has been attributed to its ability to target various signaling cascades and control the expression of numerous biomarkers and genes, as discussed further in the review. Genistein has been observed to chiefly inhibit the expression of inflammation-promoting markers and reactive oxygen species (ROS) (Obinu et al. 2021), which are linked with oxidative damage and tumor proliferation (Křížová et al. 2019). By inducing apoptosis and controlling the migration and spread of neoplasms, it has found applications as a popular chemopreventive agent (Kim 2021). In addition to its clinical potential in isolation, genistein has been evaluated for its synergistic effects with a variety of natural and synthetic anti-cancer agents. The results from these studies have been promising, indicating opportunities for further research and expansion of the conditions that may be managed by its usage (Abdulridha et al. 2020). Another growing avenue is the usage of nanotechnological interventions to get rid of the issues involved with the delivery of phytoconstituents, such as poor aqueous solubility and propensity for metabolism, thereby reducing beneficial outcomes. Various nanoformulations of genistein, including nanosuspensions, liposomes, nanoparticles, and structured nanovesicles have been evaluated (Dutta et al. 2018). Besides the clinical efficacy of this agent, it is also essential to discuss its safety and toxicology profile, to determine optimum dosing and develop an understanding of adverse effects associated with its usage, if any. This review offers a holistic overview of the chemistry of this molecule, the key pathways targeted for cancer management, and recent updates on clinical trials and nanotechnological interventions, as well as future perspectives.
Chemistry and pharmacokinetics of genistein
Chemistry of genistein
Genistein (5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one), a well-studied isoflavone, has its place in the class of aglycones. Soy products contain this isoflavone, which is a metabolite of soybeans. Also known by its chemical name, 4′,5,7-trihydroxyisoflavone, genistein was first isolated in 1899 from plants belonging to the family Fabaceae and represents 60% of the total soy isoflavone content (Tuli et al. 2019). It is a secondary metabolite consisting of 2 aromatic benzene rings and one non-aromatic heterocyclic pyran ring (Garbiec et al. 2022). Genistein’s basic carbon skeleton also consists of a C2–C3 double bond and an oxo group at the C4 position of the C ring. Figure 1 showcases the skeleton structure of genistein, with the three cyclic rings (A, B, C) highlighted. In addition to this, it shows the presence of three OH groups at the C5, C7, and C4′ positions of rings A and B, respectively. Isoflavones in their natural sources exist in glycosylated forms, but they become physiologically active only in aglycone form. In mammals, isoflavones may show effects similar to estrogen. Genistein too potentiates an estrogen-like effect owing to C4 and C7 on the phenol ring that is comparable functionally and structurally to the phenol groups in E2, allowing both estrogen receptor isoforms to bind with equal potential (Sharifi-Rad et al. 2021). Several derivatives of genistein are being made to improve its anti-cancerous potential such as 2-alkyl substituted fluorinated genistein derivatives are developed to selectively inhibit breast cancer cells, whereas genistein-1,3,5-triazine analogs have shown anti-proliferative activities against various cancer cell lines including breast, cervical, prostate, and liver (Zhu et al. 2022; Zou et al. 2023).
Pharmacokinetic profile
Genistein shows poor aqueous solubility; hence, a study has suggested that increasing the dose has no significant effect on its bioavailability. Genistein is cleaved by phlorizin hydrolase in the brush border cells or by enteric microflora into its biologically active aglycone form. Its oral bioavailability is roughly 10%, has a low absorption potential, and is therefore transported passively through the intestinal membrane, undergoing post-absorption metabolism. (Yu et al. 2021) Retinal distribution was observed to be higher in diabetic rats, due to increased blood–retinal barrier permeability (Hakami et al. 2021). Owing to its poor bioavailability, an array of advanced nano-based drug carriers are being explored to improve its water solubility and stability and make its bioavailability more efficient (Rasheed et al. 2022). An example of this is the formulation of solid lipid nanosuspensions, aiding the bypassing of the first-pass metabolism and preferentially reaching the lymphatic system of the intestine, thereby improving bioavailability (Obinu et al. 2021). Genistein is observed to undergo phase II biotransformation reactions, encompassing methylation, glycosylation, glucuronidation, acetylation, and sulphonation reactions in rats. The main metabolic products observed in human plasma following ingestion were genistein-7-glucuronide, 4′-glucuronide, 7-sulfate, 4′-sulfate, 4′,7-diglucuronide, and 7-glucuronide-4′-sulfate. The malonyl glucoside conjugation pathway is a conserved pathway for isoflavones. Malonyl genistein is also a metabolic product observed wherein the malonyl group replaces the hydroxy group; however, it is not a major metabolic pathway. Genistein is also reported to undergo enterohepatic circulation (Yang and Tsai 2019). Genistein is known to be excreted into breast milk in very minute quantities and not in significant amounts. It is excreted by urine within 1 day of intake (Yu et al. 2021). Developing an understanding of the pharmacokinetic profile of phytoconstituents is essential, as it enables the detection of any potential interactions, as well as aids the designing of suitable carriers for optimal delivery.
Apoptotic mechanisms of genistein in cancer
The Fas-FasL pathway
The apoptotic effect in the cells is generated via the cross-talk between the intrinsic and extrinsic apoptotic pathways as depicted in Fig. 2 (Petak and Houghton 2001; Slee et al. 1999). The intrinsic apoptotic pathways are associated with intracellular cysteine protease or caspases (CASP) (Yeh et al. 2007) such as mitochondria-dependent pathways regulated by CASP-9. CASP-9 is activated by the conjugation of apoptotic protease-activating factor 1 (Apaf-1), an adaptor molecule with cytochrome c and procaspase-9 in the presence of ATP (Fig. 2) (Chen and Wang 2002; Budihardjo et al. 1999). Alternatively, the extrinsic apoptotic pathways are commenced by CASP-8 and regulated via death receptors (DR) like tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and Fas ligand (FasL) (Fig. 2) (Petak and Houghton 2001; Chen and Wang 2002; Budihardjo et al. 1999). In an experiment performed by Yeh et al. (Yeh et al. 2007), it was shown that genistein moderately increased the levels of procaspase-9 in human hepatocellular carcinoma (Hep3B cells). However, no apoptosis via extrinsic or intrinsic pathways was observed in Hep3B cells when the cells were exposed to 100 µM genistein for 24 h as there was no change in the expression of death receptors like DR4, DR5, and Fas and their associated ligands: Apo-2L/TRAIL, and FasL. Further, genistein-treated cells for 48 h altered the levels of various proteins including Bad and Mcl-1, leading to cell death. The apoptotic mechanisms of genistein in different cancers are described in detail in Table 1.
The TRAIL-DR pathway
TRAIL is a cytokine that specifically prompts cell death of tumor cells over healthy counterparts through interacting with DR4 and DR5 (Oishi et al. 2013; Dai et al. 2015). TRAIL belongs to the TNF family, and it can exist in both soluble form and on cytotoxic T-lymphocytes/natural killer (NK) cells, thereby provoking immune surveillance against tumor cells (Yang et al. 2019; Girisa et al. 2019). The interaction between TRAIL on the NK cells and DR on the target cells initiates the activation of cascade of proteins ultimately leading to the cleavage of CASP-8. Further, CASP-8 activates CASP-3 and Bid to commit the cell to apoptosis. Genistein has been known to potentiate the cytotoxic effects of TRAIL in various types of human malignancies. Szliszka and colleagues demonstrated that cervical tumor cells are resistant to TRAIL-associated cytotoxicity, whereas the combination of genistein and TRAIL showed additive cytotoxicity indicating that genistein may potentiate the cytotoxic effects of apoptosis-inducing agents (Szliszka et al. 2008). Similarly, the combinational treatment of indole-3-carbinol, genistein, and TRAIL upregulated the level of DR4 and DR5 and thereby significantly promoted cell death of endometrial tumor cells (Parajuli et al. 2013). The combination of genistein and TRAIL substantially regressed cancerous growth in orthotopic pancreatic mice models with caspase-3 activation (Nozawa et al. 2004). Interestingly, dexamethasone was found to potentiate cell death of pancreatic β-cells by upregulating the levels of TRAIL and DR5, whereas the combination of dexamethasone and genistein decreased the levels of TRAIL and DR5 and rescued the β-cells from undergoing apoptosis indicating that genistein can serve as a cytoprotective agent in normal cells (Suksri et al. 2022).
Activation of anti-apoptotic mechanisms is the primary means by which cancer cells escape from cell death, and therefore, reversal of anti-apoptosis is crucial to promote cell death of tumor cells. Interestingly, pre-treatment of cholangiocarcinoma cells with genistein led to a substantial elevation in the ability of NK cells to induce apoptosis which was evidenced by upregulation in the level of FasR, DR4, and DR5 in cholangiocarcinoma cells upon treatment with genistein (Chiawpanit et al. 2022). Genistein also sensitized hepatocellular carcinoma (HCC) cells to TRAIL, promoted the cleavage of Bid, and reverted resistance to TRAIL (Jin et al. 2009a). Genistein enhanced TRAIL-driven cell death of advanced glioma cells by promoting the proteasomal degradation of the short isoform of c-FLIP (FLICE (FADD-like IL-1β-converting enzyme)-inhibitory protein) without affecting the viability of normal astrocytes (Siegelin et al. 2009). C-FLIP is a prominent apoptosis-inhibiting protein offering resistance against drug/cytokine-driven apoptosis in cancer cells (Safa 2013). The p38-MAPK pathway drives cell proliferation and anti-apoptosis, and inhibition of p38-MAPK could be a good strategy to counteract cell proliferation and induce apoptosis. Genistein inhibited the p38-MAPK pathway in HCC cells and upregulated the TRAIL-driven apoptosis (Jin et al. 2009b). Similarly, TRAIL-mediated apoptosis potentiating effects of genistein were found in diverse cancerous cell lines, including HCC, lung cancer, and gastric cancer cells (Jin et al. 2011, 2007; Nazim and Park 2015).
The TNF-α-TNFR1 pathway
There is no doubt that cancer causes inflammation of the cells (Tuli et al. 2019). Genistein is known to induce inflammation inhibitory effect by reducing the release of IL-8, IL-6, and IL-1β from MH7A cells elicited by TNF-α. It also inhibited the cell viability and proliferation by suppressing the TNF-α-induced AMPK inhibition, phosphorylation of IκB kinase-α/β and IκBα, and translocation of TNF-α-induced NF-κB into the nucleus (Fig. 3) (Li et al. 2014). Further, genistein inhibited the levels of TNF-α and IL-1β in lipopolysaccharide-stimulated BV2 microglia by inactivating toll-like receptor-4 and NF-κB (Jeong et al. 2014). In another study, 1.04 or 1.3 mg/day of genistein abrogated inflammation by lowering the level of IL-6 and TNF-α in a murine model of peritoneal endometriosis (Sutrisno et al. 2018). The anti-cancer and inflammation inhibitory effects have been also reported in diethylnitrosamine-mediated HCC in mice when they were treated with genistein for longer periods (Lee et al. 2019a).
Modulating Bcl2-Bax pathway
The apoptotic activity of genistein in oral squamous cell carcinoma (OSCC) was demonstrated in a study using genistein-loaded lactalbumin nanoparticles (GLNPs). The GLNPs destroyed the mitochondrial membrane in OSCC by the accumulation of ROS making it permeable to proapoptotic proteins, such as Bcl2-Bax and CASP-3. The increased expression of these proapoptotic proteins causes cytochrome c translocation to the cytosol from the mitochondria leading to apoptosis (Fig. 3) (Dev et al. 2021). Similar results were seen in different studies where genistein was administered orally for the in vitro treatment of colorectal cancer on SW620 and SW480 cell lines (Rendón et al. 2022) and HT29 and LoVo colon cancer cell lines (Luo et al. 2014). Another study found that 50 µM of genistein causes ER-α-dependent cell death in MCF-7 BC cells by increased of Bcl2–Bax ratio and cyclin D1 downregulation (Jiang et al. 2018). Genistein (0.01–100 µM) changes the antioxidant enzyme expression to impede oxidative stress and increase the Bcl2–Bax ratio, promoting autophagy-dependent apoptosis in MCF-7 breast cancerous cells (Lavigne et al. 2008). In other studies, genistein potentiated cell death in tumor cells by reducing the Bcl2–Bax ratio and increasing the ATM phosphorylation and expression of tumor suppressor gene p73 (Xu and Loo 2001) and upregulating the p53 and poly-(ADP-ribose)-polymerase (Shim et al. 2007; Sohel et al. 2022).
Targeting PI3K-Akt-mTOR pathway
The PI3K-Akt-mTOR signaling mechanism is a crucial signaling pathway of tumor proliferation, dissemination, and angiogenesis and is considered a significant therapeutic target for treating human cancers, and new medications are in development to inhibit specific components of this signaling pathway (Ahmad et al. 2013; Joshi et al. 2023; Tuli et al. 2023). As the name suggests, this signaling pathway contains three main components PI3K (phosphoinositide-3-kinase), Akt (protein kinase B), and mTOR (mammalian target of rapamycin); inactivation of these targets induces apoptosis and reduces cell survival as illustrated in Fig. 3. Suppression of Akt phosphorylation by genistein causing impaired PI3K-Akt-mTOR signaling cascade promotes G2/M cell cycle seize and increased expression of p21 which led to suppression of cancerous growth and potentiates cell death in various tumor cell lines, including breast cancer, NSCLC, human esophageal squamous carcinoma, and prostate cancer (Akimoto et al. 2001; Lian et al. 1998, 1999; Li et al. 1999a, 1999b). Genistein exerted its effects through the inhibition of Akt stimulation induced by epidermal growth factor (EGF) and inhibition of Akt-induced NF-κB activation via disrupting the cross-talk between Akt and NF-κB in prostate cancer, breast cancer, and myeloma (Li and Sarkar 2002; Gong et al. 2003; He et al. 2009). In a mechanistic study of genistein, it was revealed that Akt inhibition causes decreased telomerase enzyme activity as well as an elevated level of cell cycle progression inhibitor (i.e., p27) leading to apoptosis activation in breast cancer (Chinni et al. 2003). Similarly, genistein inactivates Akt protein in colon cancer cells via stimulation of the Foxo3 transcription factor that finally increased the p27 expression levels (Qi et al. 2011). Recently, genistein plus centchroman inhibited the phosphorylation of PI3K, NF-κB, and Akt which subsequently promoted apoptosis in breast adenocarcinoma by following events such as PARP cleavage, elevated Bax/Bcl2 ratio, and stimulation of caspases 3 and 9 (Kaushik et al. 2019). Another synergistic study indicates that genistein combined with isoprenoid perillyl alcohol has a more potent inhibitory activity for PI3K-Akt-mTOR signaling cascade compared to individual PI3K and mTOR inhibitors in prostate and colon carcinoma (Peffley et al. 2007). Taken together, it was concluded that genistein alone or in combination with other inhibitors abrogates the PI3K-Akt-mTOR signaling mechanism which successively potentiates the apoptosis in multiple tumor cell lines.
Targeting the JAK-STAT3 signal pathway
Signal transducer and activator of transcription (STAT3) is a transcription factor that is involved in relaying signals for cell proliferation, prosurvival, anti-apoptosis, angiogenesis, invasion, migration, and metastasis (Mohan et al. 2022, 2021a; Lee et al. 2020a). STAT3 undergoes activation upon receiving extracellular stimulus from upstream cytokines (IL-6 family cytokines) and growth factors (EGF), and the signal is mediated through Janus kinases (JAKs), epidermal growth factor receptor (EGFR), oncostatin M receptor, and other related cytokine receptors as represented in Fig. 3 (Mohan et al. 2021b; Sajith et al. 2021; Arora et al. 2021). Persistent activation of STAT3 is seen in different human cancers which contributes to cancerous growth and progression (Lee et al. 2020b, 2019b; Malojirao et al. 2020). Abrogation of the STAT3 signaling cascade has been identified as a good strategy to induce cytotoxicity in STAT3-positive tumor cells (Lee et al. 2019c; Baburajeev et al. 2016; Mohan et al. 2014). Genistein was found to display differential action against STAT3 activity, and the majority of studies have presented genistein to have inhibitory action towards the STAT3 signaling pathway. Gao and colleagues demonstrated that genistein suppresses JAK/STAT3 axis by downregulating the expression of EGFR in esophageal carcinoma cells and abrogating tumor growth in the xenograft mice model (Gao et al. 2020). Genistein was reported to inhibit the constitutive stimulation of the STAT3 signaling cascade in pancreatic tumor cells (Lian et al. 2004). In another study, it was found to impart anti-cancer function by activating STAT3 and increasing the levels of ROS in pancreatic tumor cells, whereas the treatment with ascorbic acid (a good antioxidant) reverted the genistein-induced generation of ROS (Bi et al. 2018). Sharma and colleagues performed molecular dynamic simulations and indicated that genistein displays excellent interaction with the IL-6/IL-6Rα to suppress the STAT3 pathway (Sharma et al. 2022). Pinski and coworkers demonstrated that genistein induces neuroendocrine differentiation of prostate cancer cells which was associated with the elevation of MAPK and STAT3 signaling cascades (Pinski et al. 2006). The normal prostate tissue comprises only < 1% of neuroendocrine cells, and the number of these cells significantly increases in prostate cancer. Neuroendocrine differentiation is correlated with disease progression and prognosis in individuals with prostate cancer (Hu et al. 2015). On the other hand, some studies have indicated that genistein can activate the STAT3 pathway. Zhen and coworkers demonstrated that genistein triggers the phosphorylation of STAT3 and increases the interaction of STAT3 with the hepcidin promoter in human hepatocytes (Zhen et al. 2013). Hepcidin is a peptide hormone and a critical regulator of iron metabolism whose expression is elevated in some types of human cancers (Fan et al. 2021; Julián-Serrano et al. 2021). Additionally, numerous experiments have targeted on the modulation of STAT3 signaling in different disease conditions including liver fibrosis, leiomyoma, epilepsy-induced brain injury, and rheumatoid arthritis (Xu et al. 2021; Shushan et al. 2007; Hu et al. 2021; Cheng et al. 2020).
Synergism of genistein
The synergism of genistein in combination with various therapeutic agents has been studied. This section offers insight into a few significant studies, as well as the potential for further research. A key advantage offered by therapeutic synergism is the improved cells’ susceptibility to radiotherapy. Tang et al. explored the synergism of genistein and AG1024, a tyrosine kinase inhibitor, intending to improve treatment outcomes. These agents were observed to trigger cellular apoptosis and improved the radiosensitivity of cells, offering a significant advantage over monotherapy (Tang et al. 2018). The effects of the co-administration of genistein and sulforaphane have been evaluated as well, and these compounds have been observed to decrease cellular proliferation and trigger cell death. In vitro evaluation indicated the downregulation of biomarkers such as histone deacetylase (HDAC), chiefly HDAC2 and HDAC3, along with human telomerase reverse transcriptase (hTERT) levels. These results were further strengthened by in vivo testing in transgenic mice, and a marked reduction in tumor size and volume was observed (Paul et al. 2018). Concerning ovarian cancer, the cytotoxic effects of genistein in synergy with centchroman, a selective estrogen receptor modulator, have been evaluated. These agents have been observed to downregulate Bax and Bcl2 levels, as well as inflammatory markers such as caspases. Following a comparative in vivo analysis in a mouse breast cancer model, it was concluded that combined usage of these agents was more effective than singular delivery (Kaushik et al. 2019). In a study undertaken by Lee et al., genistein was seen to exert an antiadipogenic effect, in combination with atorvastatin. The combination was observed to lower the levels of key adipogenic markers, such as mitogen-activated protein kinases (MAPKs), and peroxisome proliferator-activated receptor γ (PPARγ). This positive outcome offers potential for the usage of genistein in the management of metabolic disorders, chiefly in menopausal women (Lee et al. 2021).
The therapeutic potentials of the analogs of genistein have been explored as well. A study by Mesmar et al. explored the benefits of AXP107-11, a genistein analog, in improving the sensitivity of cells to chemotherapy. An in vivo study indicated an enhancement in cellular sensitivity to gemcitabine, following treatment with genistein. This indicates an interesting avenue for synergistic therapy of genistein, in combination with conventional chemotherapeutic agents (Mesmar et al. 2019). Administration of genistein alongside doxorubicin has been evaluated as well, and key benefits include improved chemosensitivity in various cancers, such as lymphomas (Mohammad et al. 2003), as well as a marked reduction in the toxicity of synthetic chemotherapeutic agents (Chen et al. 2019a). Moving forward, there is a need to investigate the combinatorial effects of various natural and synthetic agents in combination with genistein, to assess adverse effects, synergistic mechanisms, and potentials for repurposing and improving the overall survival time and quality of life of patients.
Overview of recent clinical trials
As shown in Table 2, it gives an insight into recent clinical trials undertaken to evaluate the efficacy and safety profile of genistein. A major drawback in these studies was observed to be the relatively small size of the patient pool and scattered studies. There is a need to conduct a large number of multi-center clinical trials with diverse subject groups, to evaluate the safety and efficacy of genistein, and establish an optimum dosage for cancer management.
Nanodelivery of genistein
Despite the wide range of pharmacological benefits offered by genistein, it suffers from a variety of drawbacks commonly faced by phytoconstituents, such as weak water solubility, and a high first-pass effect in the native form. These greatly reduce its bioavailability, posing a challenge to formulators. Nanotechnology has been harnessed as a promising strategy to overcome these pitfalls and improve treatment outcomes (Joshi et al. 2019; Elmowafy et al. 2022). In addition to curative effects, genistein has also been evaluated for its prophylactic benefits. Landauer et al. evaluated the efficacy of a nanosuspension of genistein in radioprotection, in a mouse model. At a dosage of 150 mg/kg, in multiple intramuscular doses, genistein was observed to exert a protective action against exposure to full-body radiation (Landauer et al. 2019). Another study by Salem et al. evaluated the benefits of the administration of a genistein nanosuspension, through different routes. While nanotechnological interventions improve the bioavailability of the compound and offer a greater degree of radioprotection, there is a need to undertake further studies to establish efficacy pre- and post-exposure and to determine an optimal dosing and most suitable route of administration (Salem et al. 2022).
Other interesting advancements include the formulation of genistein-encapsulated nanoparticles using solvent-exchange methods, to improve surface characteristics to obtain an optimum release profile and safety (Soleimanpour et al. 2020). Kamel et al. explored the pulmonary delivery of genistein–lipid nanoparticles for lung cancer management, to provide a better release profile and improved uptake (Kamel et al. 2019). Additionally, there is potential to explore the delivery of genistein in combination with conventional chemotherapeutic agents, to reduce toxicity, overcome resistance, improve selectivity, and provide a synergistic action (Xue et al. 2014). These outcomes may be achieved by the application of nanotechnological interventions.
To enhance bioavailability and aqueous solubility, genistein-loaded mixed micelles have been designed. Post-encapsulation, improved pharmacokinetic properties were reported, including enhanced aqueous solubility and membrane permeability. In addition to this, a two-fold increase in oral bioavailability was reported, indicating that nanomicelles could be leveraged to deliver genistein (Shen et al. 2018). Nano-structured lipid carriers, developed with the aid of solvent emulsification and evaporation, were also studied, and it was revealed that they showed sufficient plasma concentration for a longer period and better distribution in rat ovarian tissues (Mittal et al. 2019). In a recent study, it was found that signal sensing, carrier-free, and triple combination nanomedicine developed provide improved drug loading and high permeability against NSCLC (Wang et al. 2022). So, this approach could be used to deposit genistein at specific cancer sites with a specific dose to alleviate the toxicity problems. However, while these novel technologies continue to gain popularity, there is a need to address challenges associated with their scalability and toxicity. This may be overcome by conducting a greater number of clinical studies, as well as designing technologies to facilitate easier translation from laboratories to a commercial scale. Table 3 offers a recent update of genistein nanoformulations, for cancer management. In addition to the composition of the nanoformulation, the cell line on which its action was evaluated and key benefits have also been documented.
Safety and toxicology studies
Isoflavones have been generally recognized to be non-toxic, according to the outcomes obtained from clinical experiments. However, mild side effects, primarily involving the gastrointestinal system, have been observed. These include nausea, constipation, and bloating. While there have been negative results concerning the safety of isoflavones such as S-equol in animal reproductive tissues, it is safe in human reproductive systems (Chen et al. 2019b). In a study undertaken by Serebrenik et al., an amorphous solid dispersion of genistein was evaluated, to determine its safety profile at different doses. Mild- to moderate toxicities were reported, and no observable adverse reactions were recorded, on doses up to 500 mg. Based on the study results, the maximum safe dosage in humans was identified to be 3000 mg (Serebrenik et al. 2023).
An experiment designed by Godschalk et al. evaluated the implication of genistein exposure during pregnancy in a mouse model, and it was observed that the offspring may be at an increased risk of oxidative stress. This might trigger testicular abnormalities, due to DNA damage, impacting reproductive health and functioning (Godschalk et al. 2022). Similar studies on a larger scale would be necessary to fully comprehend the impact of genistein supplementation on various organ systems, including the reproductive system. While there are limited studies to establish the threshold for the dosage of soy isoflavones, the US FDA has established a safety limit of 25 g/day, with no toxic effects observed upon consumption up to this level (Sharifi-Rad et al. 2021). However, more studies are needed to assess the overall safety profile of soy isoflavones, as well as specific members belonging to this class of compounds.
Conclusions and future perspectives
As a multifaceted and complex disease, cancer exhibits a variety of different characteristics, with the most significant being uncontrolled cell growth and evading apoptosis. As one of mankind’s most prevalent medical issues, chemopreventive approaches are a promising method to prevent the occurrence of cancer and death from it (Yang and Wang 2021; Liu et al. 2023). Despite having a number of drawbacks, such as non-specific targeting, an unfavorable pharmacokinetic profile of anti-cancer medications, low solubility and stability, sluggish metabolism, insufficient drug effectiveness, and inadequate biodistribution, conventional therapeutic modalities are still utilized to treat cancer. Therefore, it is crucial to create new anti-cancer medications that can handle the problems mentioned above and target tumors specifically without seriously impairing the functioning of healthy tissues. Next-generation anti-cancer medications should make use of specially designed nanoparticles to achieve the following qualities: increased solubility and stability, reduced protease degradation, longer half-life in the systemic circulation, site-specific targeting, enhanced biodistribution, sustained drug release, and delivery of multiple medications to reduce drug resistance.
A thorough review of the clinical and experimental studies on the potential proapoptotic function of genistein has been presented here. In addition, there is a comprehensive overview of its targets in the signaling transduction pathways. Genistein, as a natural compound, exhibits considerable variation in its therapeutic effects. Several in vitro and in vivo experiments have been carried out, but clinical studies are currently being performed using these agents at specific therapeutic doses. In addition, it is needed to perform clinical and pre-clinical experiments on genistein are to evaluate the therapeutic potential of this molecule. Despite extensive data collection, further research is necessary to determine the effectiveness of genistein as a pharmaceutical agent, based on the specific carriers of genistein for different clinical purposes.
Data availability
This document includes citations for all the data that were analyzed throughout the literature review.
References
Abdulridha MK, Al-Marzoqi AH, Al-Awsi GRL, Mubarak SM, Heidarifard M, Ghasemian A (2020) Anticancer effects of herbal medicine compounds and novel formulations: a literature review. J Gastrointest Cancer 51:765–773
Ahmad A, Biersack B, Li Y, et al (2013) Deregulation of PI3K/Akt/mTOR signaling pathways by isoflavones and its implication in cancer treatment. Anti-cancer agents in medicinal chemistry (formerly current medicinal chemistry-anti-cancer agents) 13:1014–1024
Akimoto T, Nonaka T, Ishikawa H, et al (2001) Genistein, a tyrosine kinase inhibitor, enhanced radiosensitivity in human esophageal cancer cell lines in vitro: possible involvement of inhibition of survival signal transduction pathways. International Journal of Radiation Oncology* Biology* Physics 50:195–201
Alorda-Clara M, Torrens-Mas M, Morla-Barcelo PM et al (2022) High concentrations of genistein decrease cell viability depending on oxidative stress and inflammation in colon cancer cell lines. Int J Mol Sci 23:7526
Arora L, Mohan CD, Yang MH et al (2021) Tris (dibenzylideneacetone) dipalladium (0)(Tris DBA) abrogates tumor progression in hepatocellular carcinoma and multiple myeloma preclinical models by regulating the STAT3 signaling pathway. Cancers 13:5479
Baburajeev C, Mohan CD, Patil GS et al (2016) Nano-cuprous oxide catalyzed one-pot synthesis of a carbazole-based STAT3 inhibitor: a facile approach via intramolecular C-N bond formation reactions. RSC Adv 6:36775–36785
Bi Y-l, Min M, Shen W, Liu Y (2018) Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 39:10–16
Bosland MC, Enk E, Schmoll J et al (2021) Soy protein supplementation in men following radical prostatectomy: a 2-year randomized, placebo-controlled clinical trial. Am J Clin Nutr 113:821–831
Bosland MC, Schmoll J, Watanabe H, Randolph C, Kato I (2022) Randomized, placebo-controlled six-month intervention study of soy protein isolate in men with biochemical recurrence after radical prostatectomy: a pilot study. Nutr Cancer 74:555–564
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X (1999) Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 15:269–290
Chan L, Pang Y, Wang Y et al (2022) Genistein-induced mitochondrial dysfunction and FOXO3a/PUMA expression in non-small lung cancer cells. Pharm Biol 60:1876–1883
Chen M, Wang J (2002) Initiator caspases in apoptosis signaling pathways. Apoptosis 7:313–319
Chen J, Duan Y, Zhang X, Ye Y, Ge B, Chen J (2015) Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct 6:995–1000
Chen L-R, Ko N-Y, Chen K-H (2019b) Isoflavone supplements for menopausal women: a systematic review. Nutrients 11:2649
Chen C, Wang Y, Chen S et al (2020) Genistein inhibits migration and invasion of cervical cancer HeLa cells by regulating FAK-paxillin and MAPK signaling pathways. Taiwan J Obstet Gynecol 59:403–408
Chen M, Samuel VP, Wu Y, et al (2019a) Nrf2/HO-1 mediated protective activity of genistein against doxorubicin-induced cardiac toxicity. Journal of Environmental Pathology, Toxicology and Oncology 38
Cheng W-X, Huang H, Chen J-H et al (2020) Genistein inhibits angiogenesis developed during rheumatoid arthritis through the IL-6/JAK2/STAT3/VEGF signalling pathway. Journal of Orthopaedic Translation 22:92–100
Chiawpanit C, Panwong S, Sawasdee N, Yenchitsomanus P-t, Panya A (2022) Genistein sensitizes human cholangiocarcinoma cell lines to be susceptible to natural killer cells. Biology 11:1098
Chinni SR, Alhasan SA, Multani AS, Pathak S, Sarkar FH (2003) Pleotropic effects of genistein on MCF-7 breast cancer cells. Int J Mol Med 12:29–34
Dai X, Zhang J, Arfuso F et al (2015) Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med 240:760–773
Dev A, Sardoiwala MN, Kushwaha AC, Karmakar S, Choudhury SR (2021) Genistein nanoformulation promotes selective apoptosis in oral squamous cell carcinoma through repression of 3PK-EZH2 signalling pathway. Phytomedicine 80:153386
Dutta S, Moses JA, Anandharamakrishnan C (2018) Encapsulation of nutraceutical ingredients in liposomes and their potential for cancer treatment. Nutr Cancer 70:1184–1198
Elmowafy M, Shalaby K, Elkomy M et al (2022) Impact of highly phospholipid-containing lipid nanocarriers on oral bioavailability and pharmacodynamics performance of genistein. Pharm Dev Technol 27:435–447
Fan Y, Liu B, Chen F et al (2021) Hepcidin upregulation in lung cancer: a potential therapeutic target associated with immune infiltration. Front Immunol 12:612144
Ferrado JB, Perez AA, Baravalle ME, Renna MS, Ortega HH, Santiago LG (2021) Genistein loaded in self-assembled bovine serum albumin nanovehicles and their effects on mouse mammary adenocarcinoma cells. Colloids Surf, B 204:111777
Ferrado JB, Perez AA, Menegon M et al (2023) PEGylation of genistein-loaded bovine serum albumin nanoparticles and its effect on in vitro cell viability and genotoxicity properties. Colloids Surf, B 222:113082
Gao J, Xia R, Chen J et al (2020) Inhibition of esophageal-carcinoma cell proliferation by genistein via suppression of JAK1/2-STAT3 and AKT/MDM2/p53 signaling pathways. Aging (albany NY) 12:6240
Garbiec E, Cielecka-Piontek J, Kowalówka M, Hołubiec M, Zalewski P (2022) Genistein—opportunities related to an interesting molecule of natural origin. Molecules 27:815
Ghasemi Goorbandi R, Mohammadi MR, Malekzadeh K (2020) Synthesizing efficacious genistein in conjugation with superparamagnetic Fe3O4 decorated with bio-compatible carboxymethylated chitosan against acute leukemia lymphoma. Biomater. Res. 24:1–13
Girisa S, Shabnam B, Monisha J et al (2019) Potential of zerumbone as an anti-cancer agent. Molecules 24:734
Godschalk RW, Janssen MC, Vanhees K, van Doorn SBvW, van Schooten F-J (2022) Maternal exposure to genistein during pregnancy and oxidative DNA damage in testes of male mouse offspring. Frontiers in nutrition 9
Gong L, Li Y, Nedeljkovic-Kurepa A, Sarkar FH (2003) Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 22:4702–4709
Hakami T, Mahmoud M, de Juan E, Cooney M (2021) Pharmacokinetics of genistein distribution in blood and retinas of diabetic and non-diabetic rats. Drug Metab Pharmacokinet 39:100404
Hazafa A, Rehman K-U-, Jahan N, Jabeen Z. (2020) The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr Cancer 72:386–397
He H, Chen L, Zhai M, Chen JZ (2009) Genistein down-regulates the constitutive activation of nuclear factor-κB in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Phytotherapy Research: an International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 23:868–873
Hsiao YC, Peng SF, Lai KC et al (2019) Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ Toxicol 34:443–456
Hsiao YC, Chueh FS, Ma YS et al (2021) Genistein enhances the effects of L-asparaginase on inducing cell apoptosis in human leukemia cancer HL-60 cells. Environ Toxicol 36:764–772
Hsieh P-L, Liao Y-W, Hsieh C-W, Chen P-N, Yu C-C (2020) Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating miR-34a/RTCB axis. Nutrients 12:1924
Hu C-D, Choo R, Huang J (2015) Neuroendocrine differentiation in prostate cancer: a mechanism of radioresistance and treatment failure. Front Oncol 5:90
Hu Q-p, Yan H-x, Peng F et al (2021) Genistein protects epilepsy-induced brain injury through regulating the JAK2/STAT3 and Keap1/Nrf2 signaling pathways in the developing rats. Eur J Pharmacol 912:174620
Islam A, Islam MS, Uddin MN, Hasan MMI, Akanda MR (2020) The potential health benefits of the isoflavone glycoside genistin. Arch Pharmacal Res 43:395–408
Jeong J-W, Lee HH, Han MH, Kim G-Y, Kim W-J, Choi YH (2014) Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem Biol Interact 212:30–39
Ji Z, Huo C, Yang P (2020) Genistein inhibited the proliferation of kidney cancer cells via CDKN2a hypomethylation: role of abnormal apoptosis. Int Urol Nephrol 52:1049–1055
Jiang H, Fan J, Cheng L, Hu P, Liu R (2018) The anticancer activity of genistein is increased in estrogen receptor beta 1-positive breast cancer cells. OncoTargets Therapy: 8153–8163
Jin C-Y, Park C, Cheong J et al (2007) Genistein sensitizes TRAIL-resistant human gastric adenocarcinoma AGS cells through activation of caspase-3. Cancer Lett 257:56–64
Jin C-Y, Park C, Moon S-K et al (2009a) Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anticancer Drugs 20:713–722
Jin C-Y, Park C, Kim G-Y, Lee S-J, Kim W-J, Choi YH (2009b) Genistein enhances TRAIL-induced apoptosis through inhibition of p38 MAPK signaling in human hepatocellular carcinoma Hep3B cells. Chem Biol Interact 180:143–150
Jin C-Y, Park C, Park S-E, Hong S-H, Choi Y-H (2011) Enhancement of TRAIL-mediated apoptosis by genistein in human hepatocellular carcinoma Hep3B cells: roles of p38 MAPK signaling pathway. J. Life Sci. 21:1549–1557
Joshi H, Malik A, Aggarwal S, et al (2019) In-vitro detection of phytopathogenic fungal cell wall by polyclonal sera raised against trimethyl chitosan nanoparticles. International Journal of Nanomedicine: 10023–10033
Joshi H, Kumar G, Tuli HS, Mittal S (2023) Inhibition of cancer cell metastasis by nanotherapeutics: current achievements and future trends. Nanotherapeutics in Cancer. Jenny Stanford Publishing, pp. 161–209
Julián-Serrano S, Yuan F, Wheeler W et al (2021) Hepcidin-regulating iron metabolism genes and pancreatic ductal adenocarcinoma: a pathway analysis of genome-wide association studies. Am J Clin Nutr 114:1408–1417
Kamel NM, Helmy MW, Abdelfattah E-Z et al (2019) Inhalable dual-targeted hybrid lipid nanocore–protein shell composites for combined delivery of genistein and all-trans retinoic acid to lung cancer cells. ACS Biomater Sci Eng 6:71–87
Kaushik S, Shyam H, Agarwal S et al (2019) Genistein potentiates centchroman induced antineoplasticity in breast cancer via PI3K/Akt deactivation and ROS dependent induction of apoptosis. Life Sci 239:117073
Kim I-S (2021) Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 10:1064
Kim I-S, Kim C-H, Yang W-S (2021) Physiologically active molecules and functional properties of soybeans in human health—a current perspective. Int J Mol Sci 22:4054
Křížová L, Dadáková K, Kašparovská J, Kašparovský T (2019) Isoflavones Molecules 24:1076
Landauer MR, Harvey AJ, Kaytor MD, Day RM (2019) Mechanism and therapeutic window of a genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. J Radiat Res 60:308–317
Lavigne JA, Takahashi Y, Chandramouli GV et al (2008) Concentration-dependent effects of genistein on global gene expression in MCF-7 breast cancer cells: an oligo microarray study. Breast Cancer Res Treat 110:85–98
Lee SR, Kwon SW, Lee YH et al (2019a) Dietary intake of genistein suppresses hepatocellular carcinoma through AMPK-mediated apoptosis and anti-inflammation. BMC Cancer 19:1–12
Lee JH, Mohan CD, Basappa S et al (2019b) The IκB kinase inhibitor ACHP targets the STAT3 signaling pathway in human non-small cell lung carcinoma cells. Biomolecules 9:875
Lee JH, Rangappa S, Mohan CD et al (2019c) Brusatol, a Nrf2 inhibitor targets STAT3 signaling cascade in head and neck squamous cell carcinoma. Biomolecules 9:550
Lee JH, Mohan CD, Shanmugam MK et al (2020a) Vitexin abrogates invasion and survival of hepatocellular carcinoma cells through targeting STAT3 signaling pathway. Biochimie 175:58–68
Lee JH, Mohan CD, Deivasigamani A et al (2020b) Brusatol suppresses STAT3-driven metastasis by downregulating epithelial-mesenchymal transition in hepatocellular carcinoma. J Adv Res 26:83–94
Lee D, Kim J-Y, Kim H-W, Yoo J-E, Kang KS (2021) Combined beneficial effect of genistein and atorvastatin on adipogenesis in 3t3-L1 adipocytes. Biomolecules 11:1052
Li Y, Sarkar FH (2002) Inhibition of nuclear factor κB activation in PC3 cells by genistein is mediated via Akt signaling pathway. Clin Cancer Res 8:2369–2377
Li Y, Bhuiyan M, Sarkar FH (1999a) Induction of apoptosis and inhibition of c-erbB-2 in MDA-MB-435 cells by genistein. Int J Oncol 15:525–558
Li Y, Upadhyay S, Bhuiyan M, Sarkar FH (1999b) Induction of apoptosis in breast cancer cells MDA-MB-231 by genistein. Oncogene 18:3166–3172
Li Z, Li J, Mo B et al (2008) Genistein induces cell apoptosis in MDA-MB-231 breast cancer cells via the mitogen-activated protein kinase pathway. Toxicol in Vitro 22:1749–1753
Li K, Hong S, Lin S, Chen K (2020) Genistein inhibits the proliferation, migration and invasion of the squamous cell carcinoma cells via inhibition of MEK/ERK and JNK signalling pathways. J BU ON 25:1172–1177
Li J, Li J, Yue Y, et al (2014) Genistein suppresses tumor necrosis factor α-induced inflammation via modulating reactive oxygen species/Akt/nuclear factor κB and adenosine monophosphate-activated protein kinase signal pathways in human synoviocyte MH7A cells. Drug Design, Development Therapy:315–323
Lian F, Li Y, Bhuiyan M, Sarkar FH (1999) p53-independent apoptosis induced by genistein in lung cancer cells. Nutr Cancer 33:125–131
Lian JP, Word B, Taylor S, Hammons GJ, Lyn-Cook BD (2004) Modulation of the constitutive activated STAT3 transcription factor in pancreatic cancer prevention: effects of indole-3-carbinol (I3C) and genistein. Anticancer Res 24:133–138
Lian F, Bhuiyan M, Li YW, Wall N, Kraut M, Sarkar FH (1998) Genistein‐induced G2‐M arrest, p21WAF1 upregulation, and apoptosis in a non‐small‐cell lung cancer cell line
Liu X, Sun C, Jin X et al (2013) Genistein enhances the radiosensitivity of breast cancer cells via G2/M cell cycle arrest and apoptosis. Molecules 18:13200–13217
Liu S, Xu X, Ye J et al (2023) Metal-coordinated nanodrugs based on natural products for cancer theranostics. Chem Eng J 456:140892
Lu L-JW, Chen N-W, Brunder DG et al (2022) Soy isoflavones decrease fibroglandular breast tissue measured by magnetic resonance imaging in premenopausal women: a 2-year randomized double-blind placebo controlled clinical trial. Clinical Nutrition ESPEN 52:158–168
Luo Y, Wang S-x, Zhou Z-q et al (2014) Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-κB) pathway. Tumor Biology 35:11483–11488
Ma C-h, Zhang Y-x, Tang L-h et al (2018) MicroRNA-1469, a p53-responsive microRNA promotes genistein induced apoptosis by targeting Mcl1 in human laryngeal cancer cells. Biomed Pharmacother 106:665–671
Malojirao VH, Girimanchanaika SS, Shanmugam MK et al (2020) Novel 1, 3, 4-oxadiazole targets STAT3 signaling to induce antitumor effect in lung cancer. Biomedicines 8:368
Mesmar F, Dai B, Ibrahim A et al (2019) Clinical candidate and genistein analogue AXP107-11 has chemoenhancing functions in pancreatic adenocarcinoma through G protein-coupled estrogen receptor signaling. Cancer Med 8:7705–7719
Mittal P, Vrdhan H, Ajmal G, Bonde G, Kapoor R, Mishra B (2019) Formulation and characterization of genistein-loaded nanostructured lipid carriers: pharmacokinetic, biodistribution and in vitro cytotoxicity studies. Curr Drug Deliv 16:215–225
Mohammad RM, Al-Katib A, Aboukameel A, Doerge DR, Sarkar F, Kucuk O (2003) Genistein sensitizes diffuse large cell lymphoma to CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy. Mol Cancer Ther 2:1361–1368
Mohan CD, Bharathkumar H, Bulusu KC et al (2014) Development of a novel azaspirane that targets the Janus kinase-signal transducer and activator of transcription (STAT) pathway in hepatocellular carcinoma in vitro and in vivo. J Biol Chem 289:34296–34307
Mohan CD, Yang MH, Rangappa S et al (2021a) 3-Formylchromone counteracts STAT3 signaling pathway by elevating SHP-2 expression in hepatocellular carcinoma. Biology 11:29
Mohan CD, Rangappa S, Nayak SC, Sethi G, Rangappa KS (2021b) Paradoxical functions of long noncoding RNAs in modulating STAT3 signaling pathway in hepatocellular carcinoma. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer 1876:188574
Mohan CD, Rangappa S, Preetham HD, et al (2022) Targeting STAT3 signaling pathway in cancer by agents derived from Mother Nature. Seminars in cancer biology. Elsevier, pp. 157–182
Naujokat C, McKee DL (2021) The “big five” phytochemicals targeting cancer stem cells: curcumin, EGCG, sulforaphane, resveratrol and genistein. Curr Med Chem 28:4321–4342
Nazim UM, Park S-Y (2015) Genistein enhances TRAIL-induced cancer cell death via inactivation of autophagic flux. Oncol Rep 34:2692–2698
Nozawa F, Itami A, Saruc M et al (2004) The combination of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL/Apo2L) and genistein is effective in inhibiting pancreatic cancer growth. Pancreas 29:45–52
Obinu A, Burrai GP, Cavalli R et al (2021) Transmucosal solid lipid nanoparticles to improve genistein absorption via intestinal lymphatic transport. Pharmaceutics 13:267
Oishi M, Iizumi Y, Taniguchi T, Goi W, Miki T, Sakai T (2013) Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 8:e55922
Ouyang G, Yao L, Ruan K, Song G, Mao Y, Bao S (2009) Genistein induces G2/M cell cycle arrest and apoptosis of human ovarian cancer cells via activation of DNA damage checkpoint pathways. Cell Biol Int 33:1237–1244
Parajuli B, Shin S-J, Kwon S-H et al (2013) The synergistic apoptotic interaction of indole-3-carbinol and genistein with TRAIL on endometrial cancer cells. J Korean Med Sci 28:527–533
Park C, Cha H-J, Lee H et al (2019) Induction of G2/M cell cycle arrest and apoptosis by genistein in human bladder cancer T24 cells through inhibition of the ROS-dependent PI3k/Akt signal transduction pathway. Antioxidants 8:327
Patra A, Satpathy S, Naik PK, Kazi M, Hussain MD (2022) Folate receptor-targeted PLGA-PEG nanoparticles for enhancing the activity of genistein in ovarian cancer. Artificial Cells, Nanomedicine, and Biotechnology 50:228–239
Paul B, Li Y, Tollefsbol TO (2018) The effects of combinatorial genistein and sulforaphane in breast tumor inhibition: role in epigenetic regulation. Int J Mol Sci 19:1754
Peffley DM, Sharma C, Hentosh P, Buechler RD (2007) Perillyl alcohol and genistein differentially regulate PKB/Akt and 4E-BP1 phosphorylation as well as eIF4E/eIF4G interactions in human tumor cells. Arch Biochem Biophys 465:266–273
Petak I, Houghton JA (2001) Shared pathways: death receptors and cytotoxic drugs in cancer therapy. Pathol Oncol Res 7:95–106
Pinski J, Wang Q, Quek ML et al (2006) Genistein-induced neuroendocrine differentiation of prostate cancer cells. Prostate 66:1136–1143
Pintova S, Dharmupari S, Moshier E, Zubizarreta N, Ang C, Holcombe RF (2019) Genistein combined with FOLFOX or FOLFOX–Bevacizumab for the treatment of metastatic colorectal cancer: phase I/II pilot study. Cancer Chemother Pharmacol 84:591–598
Pool H, Campos-Vega R, Herrera-Hernández MG et al (2018) Development of genistein-PEGylated silica hybrid nanomaterials with enhanced antioxidant and antiproliferative properties on HT29 human colon cancer cells. Am. J. Transl. Res. 10:2306
Qi W, Weber CR, Wasland K, Savkovic SD (2011) Genistein inhibits proliferation of colon cancer cells by attenuating a negative effect of epidermal growth factor on tumor suppressor FOXO3 activity. BMC Cancer 11:1–9
Qin J, Teng J, Zhu Z, Chen J, Huang W-J (2016) Genistein induces activation of the mitochondrial apoptosis pathway by inhibiting phosphorylation of Akt in colorectal cancer cells. Pharm Biol 54:74–79
Rasheed S, Rehman K, Shahid M, Suhail S, Akash MSH (2022) Therapeutic potentials of genistein: new insights and perspectives. J Food Biochem 46:e14228
Rendón JP, Cañas AI, Correa E et al (2022) Evaluation of the effects of genistein in vitro as a chemopreventive agent for colorectal cancer-strategy to improve its efficiency when administered orally. Molecules 27:7042
Sacko K, Thangavel K, Shoyele SA (2019) Codelivery of genistein and miRNA-29b to A549 cells using aptamer-hybrid nanoparticle bioconjugates. Nanomaterials 9:1052
Safa AR (2013) Roles of c-FLIP in apoptosis, necroptosis, and autophagy. Journal of carcinogenesis & mutagenesis
Sajith AM, Narasimhamurthy KH, Shanmugam MK et al (2021) Pyrimidine-2, 4-dione targets STAT3 signaling pathway to induce cytotoxicity in hepatocellular carcinoma cells. Bioorg Med Chem Lett 50:128332
Salem AM, Jackson IL, Gibbs A et al (2022) Interspecies comparison and radiation effect on pharmacokinetics of BIO 300, a nanosuspension of genistein, after different routes of administration in mice and non-human primates. Radiat Res 197:447–458
Schneider LS, Hernandez G, Zhao L et al (2019) Safety and feasibility of estrogen receptor β targeted phytoSERM formulation for menopausal symptoms: phase 1b/2a randomized clinical trial. Menopause (new York, NY) 26:874
Serebrenik AA, Verduyn CW, Kaytor MD (2023) Safety, pharmacokinetics, and biomarkers of an amorphous solid dispersion of genistein, a radioprotectant, in healthy volunteers. Clin. Pharmacol. Drug Dev. 12:190–201
Shafiee G, Saidijam M, Tavilani H, Ghasemkhani N, Khodadadi I (2016) Genistein induces apoptosis and inhibits proliferation of HT29 colon cancer cells. Int J Mol Cell Med 5:178
Shafiee G, Saidijam M, Tayebinia H, Khodadadi I (2022) Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch Physiol Biochem 128:694–702
Sharifi-Rad J, Quispe C, Mukazhanova Z et al (2021) Resveratrol-based nanoformulations as an emerging therapeutic strategy for cancer. Front Mol Biosci 8:649395
Sharma S, Malhotra L, Yadav P, Mishra V, Sharma RS, Samath EA (2022) Genistein: a novel inhibitor of IL-6/IL-6R interface of the interleukin-6–mediated STAT3 dependent pathway of carcinogenesis. J Mol Struct 1258:132668
Shen H, He D, Wang S, Ding P, Wang J, Ju J (2018) Preparation, characterization, and pharmacokinetics study of a novel genistein-loaded mixed micelles system. Drug Dev Ind Pharm 44:1536–1542
Shim H-Y, Park J-H, Paik H-D, Nah S-Y, Kim DS, Han YS (2007) Genistein-induced apoptosis of human breast cancer MCF-7 cells involves calpain-caspase and apoptosis signaling kinase 1–p38 mitogen-activated protein kinase activation cascades. Anticancer Drugs 18:649–657
Shukla RP, Dewangan J, Urandur S et al (2020) Multifunctional hybrid nanoconstructs facilitate intracellular localization of doxorubicin and genistein to enhance apoptotic and anti-angiogenic efficacy in breast adenocarcinoma. Biomaterials Science 8:1298–1315
Shushan A, Ben-Bassat H, Mishani E, Laufer N, Klein BY, Rojansky N (2007) Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil Steril 87:127–135
Siegelin MD, Siegelin Y, Habel A, Gaiser T (2009) Genistein enhances proteasomal degradation of the short isoform of FLIP in malignant glioma cells and thereby augments TRAIL-mediated apoptosis. Neurosci Lett 453:92–97
Slee EA, Harte MT, Kluck RM et al (1999) Ordering the cytochrome c-initiated caspase cascade: hierarchical activation of caspases-2,-3,-6,-7,-8, and-10 in a caspase-9-dependent manner. J Cell Biol 144:281–292
Smeriglio A, Calderaro A, Denaro M, Laganà G, Bellocco E (2019) Effects of isolated isoflavones intake on health. Curr Med Chem 26:5094–5107
Sohel M, Biswas P, Al Amin M et al (2022) Genistein, a potential phytochemical against breast cancer treatment-insight into the molecular mechanisms. Processes 10:415
Soleimanpour M, Tamaddon AM, Kadivar M, Abolmaali SS, Shekarchizadeh H (2020) Fabrication of nanostructured mesoporous starch encapsulating soy-derived phytoestrogen (genistein) by well-tuned solvent exchange method. Int J Biol Macromol 159:1031–1047
Suksri K, Semprasert N, Limjindaporn T, Yenchitsomanus P-t, Kooptiwoot S, Kooptiwut S (2022) Cytoprotective effect of genistein against dexamethasone-induced pancreatic β-cell apoptosis. Sci Rep 12:12950
Sutrisno S, Aprina H, Simanungkalit HM et al (2018) Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. Tradit. Complement. Med. 8:278–281
Szliszka E, Czuba ZP, Jernas K, Król W (2008) Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Int J Mol Sci 9:56–64
Tang Q, Ma J, Sun J et al (2018) Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol Rep 40:579–588
Tian J, Guo F, Chen Y, Li Y, Yu B, Li Y (2019) Nanoliposomal formulation encapsulating celecoxib and genistein inhibiting COX-2 pathway and Glut-1 receptors to prevent prostate cancer cell proliferation. Cancer Lett 448:1–10
Tuli HS, Tuorkey MJ, Thakral F et al (2019) Molecular mechanisms of action of genistein in cancer: recent advances. Front Pharmacol 10:1336
Tuli HS, Joshi H, Vashishth K, et al (2023) Chemopreventive mechanisms of amentoflavone: recent trends and advancements. Naunyn-Schmiedeberg's Archives of Pharmacology
Vodnik VV, Mojić M, Stamenović U et al (2021) Development of genistein-loaded gold nanoparticles and their antitumor potential against prostate cancer cell lines. Mater Sci Eng, C 124:112078
Wang G, Zhang D, Yang S, Wang Y, Tang Z, Fu X (2018) Co-administration of genistein with doxorubicin-loaded polypeptide nanoparticles weakens the metastasis of malignant prostate cancer by amplifying oxidative damage. Biomater. Sci. 6:827–835
Wang Z, Yang L, Li Y et al (2022) An activatable, carrier-free, triple-combination nanomedicine for ALK/EGFR-mutant non-small cell lung cancer highly permeable targeted chemotherapy. New J Chem 46:17673–17677
Xu J, Loo G (2001) Different effects of genistein on molecular markers related to apoptosis in two phenotypically dissimilar breast cancer cell lines. J Cell Biochem 82:78–88
Xu Y, Zhang D, Yang H et al (2021) Hepatoprotective effect of genistein against dimethylnitrosamine-induced liver fibrosis in rats by regulating macrophage functional properties and inhibiting the JAK2/STAT3/SOCS3 signaling pathway. Frontiers in Bioscience-Landmark 26:1572–1584
Xu H, Ma H, Zha L, Li Q, Pan H, Zhang L (2022) Genistein promotes apoptosis of lung cancer cells through the IMPDH2/AKT1 pathway. Am. J. Transl. Res. 14:7040
Xue J-P, Wang G, Zhao Z-B, Wang Q, Shi Y (2014) Synergistic cytotoxic effect of genistein and doxorubicin on drug-resistant human breast cancer MCF-7/Adr cells. Oncol Rep 32:1647–1653
Yan H, Jiang J, Du A, Gao J, Zhang D, Song L (2020) Genistein enhances radiosensitivity of human hepatocellular carcinoma cells by inducing G2/M arrest and apoptosis. Radiat Res 193:286–300
Yang Y-Y, Tsai T-H (2019) Enterohepatic circulation and pharmacokinetics of genistin and genistein in rats. ACS Omega 4:18428–18433
Yang L, Wang Z (2021) Natural products, alone or in combination with FDA-approved drugs, to treat COVID-19 and lung cancer. Biomedicines 9:689
Yang Y, Zang A, Jia Y et al (2016a) Genistein inhibits A549 human lung cancer cell proliferation via miR-27a and MET signaling. Oncol Lett 12:2189–2193
Yang Y, Yang Y, Dai W, Li X, Ma J, Tang L (2016b) Genistein-induced apoptosis is mediated by endoplasmic reticulum stress in cervical cancer cells. Eur Rev Med Pharmacol Sci 20:3292–3296
Yang MH, Jung SH, Sethi G, Ahn KS (2019) Pleiotropic pharmacological actions of capsazepine, a synthetic analogue of capsaicin, against various cancers and inflammatory diseases. Molecules 24:995
Yeh T-C, Chiang P-C, Li T-K et al (2007) Genistein induces apoptosis in human hepatocellular carcinomas via interaction of endoplasmic reticulum stress and mitochondrial insult. Biochem Pharmacol 73:782–792
Yu L, Rios E, Castro L, Liu J, Yan Y, Dixon D (2021) Genistein: dual role in women’s health. Nutrients 13:3048
Zhang Q, Cao WS, Wang XQ et al (2019a) Genistein inhibits nasopharyngeal cancer stem cells through sonic hedgehog signaling. Phytother Res 33:2783–2791
Zhang Q, Bao J, Yang J (2019b) Genistein-triggered anticancer activity against liver cancer cell line HepG2 involves ROS generation, mitochondrial apoptosis, G2/M cell cycle arrest and inhibition of cell migrationand inhibition of cell migration. Arch Med Sci 15:1001–1009
Zhen AW, Nguyen NH, Gibert Y et al (2013) The small molecule, genistein, increases hepcidin expression in human hepatocytes. Hepatology 58:1315–1325
Zhou P, Wang C, Hu Z, Chen W, Qi W, Li A (2017) Genistein induces apoptosis of colon cancer cells by reversal of epithelial-to-mesenchymal via a Notch1/NF-κB/slug/E-cadherin pathway. BMC Cancer 17:1–10
Zhu Y, Zheng F, Xiao C, Liu X, Yao X, Zeng W (2022) Synthesis and bio-evaluation of 2-alkyl substituted fluorinated genistein analogues against breast cancer. Med Chem 18:589–601
Zou J-P, Zhang Z, Lv J-Y et al (2023) Design, synthesis and anti-cancer evaluation of genistein-1, 3, 5-triazine derivatives. Tetrahedron 134:133293
Author information
Authors and Affiliations
Contributions
HJ, HST, DSG, GK, NKA, CDM, JK, SR, IR, and DA conceived the conceptualization, methodology, validation, and writing, a review. MG and HSA performed the formal analysis and resources. HST did the data curation and editing. All authors have read and agreed to the published version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors have their consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Joshi, H., Gupta, D.S., Abjani, N.K. et al. Genistein: a promising modulator of apoptosis and survival signaling in cancer. Naunyn-Schmiedeberg's Arch Pharmacol 396, 2893–2910 (2023). https://doi.org/10.1007/s00210-023-02550-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02550-1