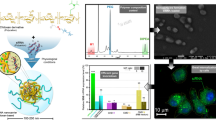
Abstract
Purpose
A novel siRNA carrier was formulated between chitosan (CS) and thiamine pyrophosphate (TPP). Their ability to deliver siRNA were evaluated in stable and constitutive EGFP-expressing HepG2 cells.
Methods
CS-TPP was prepared by dissolving CS in TPP solution at a CS:TPP molar ratio of 1.5:1. Complexes of CS-TPP/siRNA were formed at varying weight ratios and characterized using gel electrophoresis. Their morphologies and particle sizes were evaluated, and the transfection efficiency and cytotoxicity of CS-TPP/siRNA complexes were examined in stable and constitutive EGFP-expressing HepG2 cells.
Results
Gel electrophoresis results indicated that binding of CS-TPP and siRNA depended on the molecular weight (MW) and weight ratio of CS, and the particle sizes of CS-TPP/siRNA complexes were in nano-size. The CS-TPP-mediated siRNA silencing of the endogenous EGFP gene occurred maximally with 70–73% efficiency. The CS-TPP/siRNA complex with the lowest MW of CS (20 kDa) at a weight ratio of 80 showed the strongest inhibition of gene expression, which was higher than Lipofectamine 2000™. Over 90% the average cell viabilities of the complexes were observed by MTT assay.
Conclusions
This study suggests that CS-TPP is straightforward to prepare, safe and exhibits significantly improved siRNA delivery potential in vitro.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
RNA interference (RNAi) represents a powerful tool for the silencing of specific genes. This process is mediated via small interfering double-stranded RNA molecules (siRNAs) that sequence-specifically trigger the cleavage and subsequent degradation of target mRNA. It is a posttranscriptional gene silencing process mediated by a 21–23-mer duplex siRNA that has a sequence homologous to the silenced gene (1). As a consequence, the possibility of silencing genes implicated in disease by siRNA has led to a rapidly evolving area in drug discovery. For therapeutic applications, siRNA technology promises greater advantages over drugs that are currently on the market by offering new types of drugs that are easy to design and have very high target selectivity. With this, it is possible to inhibit specific gene expression in the cytoplasm and expect a decrease in toxicity due to metabolism of natural nucleotide components by the endogenous cell systems (2–3). Therapeutics using siRNA, however, are hindered by poor intracellular uptake and limited blood stability of siRNA and the delivery methods currently used to the target cells in vivo. When siRNA is administered into the blood, it is readily digested by nuclease and eliminated from the renal glomeruli before reaching the diseased tissues. In order to achieve the therapeutic effect of siRNA at the target site and knockdown specific genes in vivo, it is important to enhance the stability of siRNA in the blood circulation and to allow targeted delivery to the diseased area. Many delivery systems of siRNA based on physical and pharmaceutical approaches have been proposed. Viral vectors are promising delivery vehicles; however, safety issues in their usage are currently a major concern. Additional delivery systems, therefore, need to be developed. Cationic polymer- and lipid-based siRNA vectors have been shown to enter cells and mediate specific RNA interference in vitro and in vivo (4–7). There are two types of such vectors, natural polymers including chitosan (CS), and atelocollagen or synthetic polymers including poly-l-lysine (PLL), polyethyleneimine (PEI) and dendrimers. Among these polymers, CS, atelocollagen and PEI have been investigated in terms of the delivery of siRNA in animals.
Chitosan [(1→4) 2-amino-2-deoxy-β-d-glucan] is a copolymer of N-acetyl-d-glucosamine (GlcNAc) and d-glucosamine (GlcN) that is produced by the alkaline deacetylation of chitin. It is a weak base with a pK a value of the d-glucosamine residue of approximately 6.2–7.0, and is therefore insoluble at neutral and alkaline pH values, but soluble in an acidic medium such as acetic acid, citric acid, glutamic acid, aspartic acid, hydrochloric acid and lactic acid. Chitosan has been used in drug delivery as an absorption enhancer (8) as well as a vector for gene delivery. In addition, CS is biocompatible, biodegradable and non-toxic, and has therefore been proposed as a safer alternative to other non-viral vectors, such as cationic lipids and cationic polymers (9–13). At acidic pH levels below that of pK a the primary amines in the chitosan backbone become positively charged. These protonated amines enable CS to bind to negatively charged DNA and condense DNA into particles. Moreover, CS has shown the capability to protect DNA from DNase I&II degradation and to transfect into different cell types (14). Formulation parameters, such as molecular weight (MW), degree of deacetylation (DD), N/P ratio (ratio of positively charged chitosan to negatively charged DNA), and pH of the transfection medium were found to affect the transfection efficiency of CS/DNA complexes (15–16). In recent years, CS-based carriers are one of the non-viral vectors that have gained increasing interest as a safe and cost-effective delivery system for gene materials including plasmids, oligonucleotides and proteins and peptides. Although CS has been studied as a gene vector for more than a decade, there are few studies regarding the use of CS to deliver siRNA in vitro and in vivo (17–21). Chitosan salts (HCl, glutamate, lactate, etc.) have been used for drug delivery, protein delivery and transfection of DNA (11–12, 16, 22).
Thiamine pyrophosphate (TPP), or thiamine diphosphate, is a thiamine derivative. It plays an essential role as a cofactor in key reactions in carbohydrate metabolism. It is also involved in the metabolism of branched-chain amino acids and may have non-coenzyme (i.e., non-cofactor) roles in excitable cells, and is therefore vital to the cell’s energy supply (23). It is a water-soluble vitamin, and as such, it is least likely to reach toxic levels. The chemical structure of TPP is shown in Fig. 1. Due to the phosphate groups of TPP, the molecule can form salt with amine groups of CS, helping improve CS water solubility. The amine groups of TPP, especially at N of thiazolium (which is always positive even at physiological pH), increase the binding to negatively charged molecules (e.g., DNA or RNA) and condense them into complexes via charge interaction, thereby enhancing the transfection efficiency.
In this study, the chitosan salt formed between different molecular weights of chitosan (20, 45, 200 and 460 kDa) and TPP were evaluated for their ability to form a complex with siRNA. The transfection efficiency of CS-TPP/siRNA complexes was examined in HepG2 cells, a human hepatocarcinoma cell line, stably expressing green fluorescent protein (EGFP). Particle size and zeta potential of CS-TPP/siRNA complexes were evaluated. In addition, the cytotoxicity of CS-TPP/siRNA complexes was investigated in HepG2 cells.
MATERIALS AND METHODS
Materials
Chitosan was purchased from Seafresh Chitosan Lab., Thailand with MWs of 20, 45, 200, and 460 kDa and 85% deacetylation. Dimethylsulfoxide (DMSO) was purchased from Fisher Scientific (Fairlawn, NJ, USA). Lipofectamine2000™ was purchased from Invitrogen (NY, USA). Thiamine pyrophosphate (TPP), agarose, diethylpyrocarbonate (DEPC) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO, USA). Modified Eagle's medium (MEM), fetal bovine serum (FBS), Trypsin-EDTA and penicillin-streptomycin were purchased from Gibco BRL (Rockville, MD, USA). siRNA-EGFP(+) and siRNA-EGFP(-) were synthesized by using Ambion’s Silencer™ siRNA Construction Kit (Ambion, USA). HepG2 cells, a human hepatocarcinoma cell line, were obtained from American Type Culture Collection (ATCC, Rockville, MD, USA). All other chemicals were of cell culture and molecular biology quality.
Preparation of Chitosan Thiamine Pyrophosphate (CS-TPP)
An aqueous CS-TPP solution was prepared at a CS:TPP molar ratio of 1.5:1. Briefly, TPP (17.6 mg, 0.038 mmol) was dissolved in 10 ml distilled water, and CS (10 mg, 0.062 mmol) was added to the TPP solution and vigorously stirred with a magnetic stirrer at ambient temperature until the solution became clear.
Preparation of siRNA
siRNA-EGFP(+) and siRNA-EGFP(-) were synthesized by using Ambion’s SilencerTM siRNA Construction Kit (Ambion, USA). The duplex siRNA-EGFP (+) contains sense 5′-aag cug acc cug aag uuc auc-3′ and antisense 5′-aag aug aac uuc agg guc agc-3′. The siRNA-EGFP(-) contains sense 5′-aag cac cgc uua cgu gau acu-3′ and antisense 5′-aaa gua uca cgu aag cgg ugc-3′.
Complex Formation between CS-TPP and siRNA
Complexes of CS-TPP/siRNA were formulated at weight ratios of 0.4, 20, 40 and 80 by adding the siRNA solution to the CS-TPP solution and diluting with 0.1% DEPC water. The mixture was gently pipetted and vortexed for 3–5 sec to initiate the complex formation and left at room temperature for 30 min to complete the process. Complex formation was confirmed by electrophoresis. Agarose gels were prepared with 2% agarose solution in Tris-borate-EDTA buffer with ethidium bromide (0.5 μg/ml). The electrophoresis was carried out for 20 min at 100 V. The volume of the sample loaded in the well was 25 μl of CS-TPP/siRNA complex containing 4 pmol of siRNA.
Size and Zeta-Potential Measurement
The particle sizes and surface charge of the CS-TPP/siRNA complexes were measured by using the Zetasizer Nano ZS (Malvern Instruments Ltd., Malvern, UK) at room temperature. The complexes were diluted with 0.1% DEPC water that was passed through 0.22 μm membrane filter prior to use. All samples were measured in triplicate.
Morphology
The morphology of the CS-TPP/siRNA complexes was determined by Atomic Force Microscope (AFM) using tapping-mode AFM in air. The complexes were diluted with 0.1% DEPC water that was passed through 0.22 μm membrane filter prior to use. These samples were dropped immediately onto freshly cleaved mica and air-dried.
HepG2 Cells Stably Expressing Enhanced Green Fluorescent Protein (EGFP)
HepG2 cells with stable, constitutive expression of EGFP were generated. Briefly, HepG2 cells were transfected with a pEGFP-C2 Plasmid DNA (Clontech, USA) using Lipofectamine2000™ (Invitrogen, USA) according to manufacturer’s instructions. Briefly, 6 μg of pEGFP and 15 μl of Lipofectamine2000™ were diluted in each of 250 μl of serum-free MEM medium, mixed together and incubate at room temperature for 30 min. Prior to transfection, the cells were trypsinized and suspended in 2 ml complete medium and mixed with lipofectamine/pEGFP complex before seeding into six-well plates. The cells were incubated for 24 h at 37°C under 5% CO2 atmosphere. The medium was replaced with 2 ml of complete medium supplemented with G418. Stably transfected cell populations were generated by selection in MEM with 10% fetal bovine serum (FBS) supplemented with high dose of 1 mg/ml of G418 for the first two subpassages and reduced to 0.5 μg/ml to get single clones of stable cells. The maintenance dose of G418 for HepG2 cell was 0.1 μg/ml.
In Vitro Transfection
The HepG2 cells stably expressing EGFP were cultivated in modified Eagle's medium (MEM), supplemented with 10% FBS in a humidified atmosphere (5% CO2, 95% air, 37°C). On the day of transfection, the trypsinized cells were suspended in MEM at a concentration of 2.5 × 105 cell/ml. The CS-TPP/siRNA (EGFP-specific or EGFP-mismatch) complexes at various weight ratios were formed for 30 min by mixing equal volumes of siRNA and chitosan diluted in 0.1% DEPC water. A volume of 100 μl of cell suspension was mixed with 20 μl of CS-TPP/siRNA complexes (20 pmol/well siRNA) in a sterile Eppendorf tube. A volume of 120 μl of cell suspension was then transferred into black clear-bottom 96-well plates (Corning, USA) at the density of 25,000 cells/well. After two hours of transfection, the cells were adhered to the well. For the initial fluorescent intensity used to calculate % seeding variation, measurement of EGFP was performed using a fluorescence microplate reader (Universal Microplate Analyzer, Model AOPUS01 and AI53601, Packard BioScience, CT, USA) with excitation/emission at 485/530 nm. To ensure the same number of cells in each well, we first calculated % seeding variation (Eq. 1) and then used the values to adjust the fluorescent intensity obtained from each well by Eq. 2. The variation of the fluorescence intensity of each well was less than 10%. After 24 h of transfection, the medium was removed and the cells were washed with PBS. Following this, the media were replaced with fresh medium. Plates were incubated for five days at 37°C and 5% CO2, measuring the fluorescence intensity daily. Percentage of inhibition of green fluorescence was calculated by Eq. 3.
where I average,day0 is the average fluorescent intensity of all wells at 2 h after transfection (day 0), I n,day0 is the fluorescent intensity of individual well at day 0, I n,d1–5 is the fluorescent intensity at days one through five of each well, I ad is adjusted fluorescent intensity, I ad,siRNA(+) is adjusted fluorescent intensity of the cell with CS/siRNA (+) complexes, and I ad,siRNA(−) is adjusted fluorescent intensity of the cell with CS/siRNA (−) complexes.
Cytotoxicity
Evaluation of cytotoxicity of CS-TPP/siRNA complexes was performed by MTT assay (24). The HepG2 cells stably expressing EGFP were seeded and transfected using the same method as the in vitro transfection. After 24 h of transfection, the medium was removed and the cells were washed with PBS. Following this, 20 μl of MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl-tetrazolium bromide, 5 mg/ml) was added to each well and the incubation was continued for 4 h. The medium was then removed, the cells were rinsed with PBS of pH 7.4, and formazan crystals that had formed in the living cells were dissolved in 100 μl of DMSO per well. Relative viability (%) was calculated based on absorbance at 550 nm using a microplate reader (Universal Microplate Analyzer, Model AOPUS01 and AI53601, Packard BioScience, CT, USA). Viability of non-treated control cells was arbitrarily defined as 100%.
Statistical Analysis
The statistical significance of differences in transfection efficiency and cell viability was examined using one-way analysis of variance (ANOVA) followed by an LSD post hoc test. The significance level was set at p < 0.05.
RESULTS AND DISCUSSION
Characterization of CS-TPP/siRNA complexes
In order to investigate the optimal conditions in forming CS-TPP/siRNA complexes, it is necessary to evaluate the degree of binding between CS-TPP and siRNA at different MW of CS and weight ratios. The formation of complexes between CS and the siRNA was observed by the modified method of Grayson et. al. (25) based on gel retardation on 2% agarose. Figure 2 shows the naked siRNA and CS-TPP/siRNA complexes at weight ratios of 0.4, 20, 40 and 80 when different MW of CS was used. The results showed that siRNA binding to CS-TPP was dependent on both the MW of CS and the weight ratio. At a weight ratio of 0.4 (Fig. 2, Lanes 2, 6, 10 and 14), the migration behavior was almost the same as the siRNA control (Fig. 2, Lane 1). The gradual retardation effect on the migration of siRNA was observed when the weight ratio increased. When the MW of CS increased, a greater retardation effect on the migration of siRNA was observed. The complete binding of siRNA to CS-TPP, however, could not be observed in all of the CS-TPP tested. These data suggest that CS with greater charge density (i.e., a high weight ratio and MW) showed retarded siRNA migration verifying the necessity of high charge for complexes. These results were concomitant with the previous study which reported that the interaction between siRNA and CS by simple mixing was weak and did not yield complete complexes. In contrast, it has been reported that CS formed complete complexes with plasmid DNA even at a lower N/P ratio indicating the stronger interactive forces of large polyanionic species in comparison to siRNA (20). X. Liu et al. (18), using a polyanion displacement method, revealed that siRNA was rapidly displaced from the CS/siRNA complexes upon addition of polyanion (poly-l-aspartic acid); PAA).
Electrophoresis of CS-TPP/siRNA complexes on 2% agarose gel. Lane 1, siRNA-EGFP (4 pmol); lane 2–5, CS MW 20 kDa and weight ratio=0.4, 20, 40, and 80, respectively. Lane 6–9, CS MW 45 kDa and weight ratio=0.4, 20, 40, and 80, respectively. Lane 10–13, CS MW 200 kDa and weight ratio=0.4, 20, 40, and 80, respectively. Lane 14–17, CS MW 460 kDa and weight ratio=0.4, 20, 40, and 80, respectively.
The physical properties such as size and surface charge, that influenced cellular interactions and nanoparticles distribution were determined. The measured effective z-average diameters, which were determined to be the mean size using cumulant analysis, and the zeta potential of complexes formed from siRNA that was designed to inhibit the EGFP gene (siRNA-EGFP) and CS-TPP at a pH of 7.2 are shown in Fig. 3. The particle size of the complexes prepared from CS MWs 20, 45 and 200 kDa increased with increasing weight ratios from 0.4 to 20, and decreased to the range of 130 to 400 nm after a weight ratio of 40. At the weight ratio of 20, these complexes showed the largest particle size. In contrast, the complex prepared from CS MW 460 kDa showed similar sizes in the range of 360 to 600 nm at all weight ratios. Of all CS tested, the zeta potential values of CS-TPP/siRNA complexes were negative at a weight ratio of 0.4 and became positive at the weight ratio of 20. At weight ratios greater than 20, the zeta potential increased from approximately+5 to+15 mV. The comparative positive value of the surface charge of the CS-TPP/siRNA complexes increased with the increasing weight ratio. The increment was due to the increase in the number of positive charges of the CS. The influence of MW in complex formation can be attributed to a chain entanglement effect. Chain entanglement contributes less to complex formation as the MW of CS decreases since an increase in the number of CS chains is required to achieve the same weight ratio when compared to higher MWs of CS. Longer CS chains in high MW CS entangles better, trapping free siRNA once the initial electrostatic interaction has occurred. The need for more CS chains with low MW CS-TPP/siRNA binding may not be energetically favorable for complex formation (15).
The morphological examination of the CS-TPP/siRNA complexes formulated with CS-TPP MW 20 kDa at weight ratio of 80 was performed by AFM. The AFM images (Fig. 4) revealed that the complexes were spherical and nano-size.
In vitro siRNA transfection
The ability of CS to deliver siRNA both in vitro and in vivo was recently reported (18–21). No data, however, is available for CS-TPP salt. The HepG2 cells that stably express EGFP were used as a model system. Lipofectamine 2000™ was used as a positive control. Figure 5 shows the reduction in EGFP expression in stable constitutive EGFP-HepG2 cells treated with CS-TPP/siRNA complexes after one to five days post-transfection. The concentration of siRNA was kept constant at 20 pmol/well and the weight ratio was varied from 0.4 to 80. A negligible gene silencing effect was observed for naked siRNA. Complexes of CS-TPP/siRNA containing EGFP-mismatch siRNA (siRNA-EGFP(−)) showed no EGFP inhibition, confirming knockdown specificity. The effect of post-transfection time on the gene silencing efficiency is shown in Fig. 5. In CS-TPP/siRNA complexes, the stable silencing efficiency was observed at the post-transfection time of three days. The post-transfection time of three days was therefore chosen for comparison of the gene silencing efficiency of Lipofectamine 2000™/siRNA (positive control), CS-TPP/siRNA and conventional CS lactate/siRNA complexes at different weight ratios and pHs of transfection medium (Fig. 6). The silencing efficiencies were significantly influenced by weight ratios, which gradually increased as the weight ratio increased. The highest gene silencing level (70–73% inhibition) was reached at a weight ratio of 80 and a CS MW of 20 and 45 KDa. The complexes prepared at these conditions were the smallest in size (Fig. 3), suggesting the possibility that small complexes are more effective than larger ones in reducing gene expression. At a weight ratio of 40 and 80, the gene silencing efficiency of CS-TPP had a tendency to decrease as the MW increased. At different MWs, the maximum gene silencing efficiency was found at a CS MW of 20 and 45 KDa. These results indicated that both the MW of chitosan and the weight ratio of CS/siRNA complexes are the important formulation parameters that affect siRNA delivery. This could be due to an optimal association and dissociation between chitosan and siRNA in the CS/siRNA complexes with an optimal MW of chitosan and weight ratio of CS/siRNA complexes that resulted in high gene silencing efficiency. This event was inconsistent with results found in the previous studies with pDNA (22, 27–28). In pDNA, a high MW chitosan gave higher transfection efficiency at a low N/P ratio, and a low MW chitosan required a higher N/P ratio. This could be due to the different interaction between CS and siRNA, which were apparently weak, as CS and siRNA are easily dissociated from each other compared to CS associated with plasmid DNA at the same weight ratio.
Effect of weight ratio and molecular weight of chitosan on gene silencing. Percentage of EGFP gene knockdown by CS-TPP/siDNA complexes at various weight ratios (a)=0.4, (b)=20, (c)=40 and (d)=80 in HepG2 cells stably expressing EGFP after 1 to 5 days post-transfection. CS MW 20 kDa (filled circle), 45 kDa (filled triangle), 200 kDa (filled diamond) and 460 kDa (filled square). Data are plotted in mean ± SD of eight wells. Asterisks Differences values were statistically significant (P < 0.05).
Comparison of the percentage of EGFP gene knockdown by (a) Lipofectamine 2000 TM/siRNA (positive control;) and CS-TPP/siRNA (b) CS lactate/siRNA complexes at various weight ratios in HepG2 cells stably expressing EGFP after 3 days post-transfection at pH 6.6 (white bars) and pH 7.2 (black bars). Data are plotted in mean ± SD of eight wells. Asterisks Differences values were statistically significant (P < 0.05).
Transfections performed with CS-TPP prepared from 20-kDa CS at different weight ratios revealed that the gene silencing effect of such CS-TPP/siRNA complexes had a comparable effect to Lipofectamine 2000 ™ (Fig. 6). The gene silencing efficiency of Lipofectamine 2000 ™/siRNA complexes, according to manufacturer’s instructions, was 61% inhibition of EGFP gene expression. The CS-TPP complex gave a high gene silencing effect (70–73%), which was higher than that of chitosan lactate (CS-lac) (40–50%). Chitosan at an acidic pH has shown promise in protecting DNA from DNase I&II degradation (14) and in transfecting into different cell types. At an acidic pH below that of pKa, the primary amines in the chitosan backbone become positively charged. These protonated amines enable chitosan to bind to negatively charged DNA or siRNA and condense it into particles. With a pK a value of the d-glucosamine residue of approximately 6.2–7.0, CS is a weak base and is insoluble under neutral and alkaline conditions. The preparation of CS-TPP salt therefore offered a benefit in enhancing the CS solubility. In addition, previous studies reported that the transfection efficiency of CS was dependent on pH. Chitosan-mediated high gene transfection was observed at the medium pH values below 6.5 (28). As shown in Fig. 6 b, pH dramatically affected the inhibition of gene expression mediated by CS-Lac/siRNA. Increasing pH values from 6.6 to 7.2 led to the decrease of the gene silencing efficiency of CS-Lac/siRNA. The gene silencing of CS-TPP/siRNA (Fig. 6 b),however, was slightly affected by the increasing pH. This may be due to the amine groups of TPP, especially at the N-terminus of thiazolium (which is always positively charged, independent on pH) that continue to bind with negatively charged siRNA and therefore maintain the high transfection efficiency even under the neutral conditions.
Cytotoxicity
A required characteristic of a gene or siRNA delivery system is that it is not cytotoxic. The cytotoxicity of CS/DNA complexes was found to be low compared to other cationic complexes (16, 22). To investigate the potential cytotoxicity of CS-TPP/siRNA complexes, the cell viability was determined by MTT assay after 24 h incubation with CS-TPP/siRNA complexes. The results showed that CS-TPP/siRNA complexes formulated with various MW of CS (20, 45, 200 and 460 kDa) at various weight ratios of 0.4, 20, 40 and 80 were not significantly different from the untreated cells. Average cell viability was over 90% (Fig. 7). It was clear that CS-TPP/siRNA complexes formulated with CS-TPP were safe.
CONCLUSIONS
Successful siRNA delivery was observed in stable HepG2 cells by using a novel water-soluble chitosan salt (chitosan thiamine pyrophosphate, CS-TPP). The ability of this novel chitosan salt to transfer functionally active siRNA into cell culture is dependent upon the weight ratio and MW of chitosan. This is the first novel chitosan salt to show high gene silencing efficiency occurring without influencing pH and requiring no complicated chemical synthesis. This study suggests that CS-TPP is simple to prepare, safe and exhibits significantly improved siRNA delivery potential in vitro.
References
P. A. Sharp. RNA interference. Genes Dev. 15:485–490 (2001) doi:10.1101/gad.880001.
Y. Dorsett, and T. Tuschl. siRNAs: applications in functional genomics and potential as therapeutics. Nat. Rev. Drug Discov. 3:318–329 (2004) doi:10.1038/nrd1345.
R. C. Ryther, A. S. Flynt, J. A. Phillips, and J. G. Patton. siRNA therapeutics big potential from small RNAs. Gene Ther. 1:25–11 (2005).
B. Urban-Klein, S. Werth, S. Abuharbeid, F. Czubayko, and A. Aigner. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther. 12:461–466 (2005) doi:10.1038/sj.gt.3302425.
F. Takeshita, Y. Minakuchi, S. Nagahara, K. Honma, H. Sasaki, K. Hirai, T. Teratani, N. Namatame, Y. Yamamoto, K. Hanai, T. Kato, A. Sano, and T. Ochiya. Efficient delivery of small interfering RNA to bone-metastatic tumors by using atelocollagen in vivo. Proc. Natl. Acad. Sci. U. S. A. 102:12177–12182 (2005) doi:10.1073/pnas.0501753102.
C. N. Landen Jr., A. Chavez-Reyes, C. Bucana, R. Schmandt, M. T. Deavers, L.opez-G. Berestein, and A.K. Sood. Therapeutic EphA2 gene targeting in vivo using neutral liposomal small interfering RNA delivery. Cancer Res. 65:6910–6918 (2005) doi:10.1158/0008-5472.CAN-05-0530.
A. Hassan, Y. Tian, W. Zheng, H. Ji, K. Sandberg, and J. G. Verbalis. Small interfering RNA-mediated functional silencing of vasopressin V2 receptors in the mouse kidney. Physiol. Genomics. 21:382–388 (2005) doi:10.1152/physiolgenomics.00147.2004.
B. I. Florea, M. Thanou, H. E. Junginger, and G. Borchard. Enhancement of bronchial octreotide absorption by chitosan and N-trimethyl chitosan shows linear in vitro/in vivo correlation. J. Control. Release. 110:353–361 (2006) doi:10.1016/j.jconrel.2005.10.001.
M. Lee, J.W. Nah, Y. Kwon, J. J. Koh, K. S. Ko, and S. W. Kim. Water-soluble and low molecular weight chitosan-based plasmid DNA delivery. Pharm. Res. 18:427–431 (2001) doi:10.1023/A:1011037807261.
T. Ishii, Y. Okahata, and T. Sato. Mechanism of cell transfection with plasmid/chitosan complexes. Biochim. Biophys. Acta. 1514:51–64 (2001) doi:10.1016/S0005-2736(01)00362-5.
W. Weecharangsan, P. Opanasopit, T. Ngawhirunpat, T. Rojanarata, and A. Apirakaramwong. Chitosan lactate as a nonviral gene delivery vector in COS-1 cells. AAPS PharmSciTech. 7:E1–E6 (2006) doi:10.1208/pt070366.
X. Zhao, S. B. Yu, F. L. Wu, Z. B. Mao, and C. L. Yu. Transfection of primary chondrocytes using chitosan-pEEGFP nanoparticles. J. Control. Release. 112:223–228 (2006) doi:10.1016/j.jconrel.2006.01.016.
F. C. MacLaughlin, R.J. Mumper, J. Wang, J.M. Tagliaferri, I. Gill, M. HinCHyiffe, and A.P. Rolland. Chitosan and depolymerized chitosan oligomers as condensing carriers for in vivo plasmid delivery. J. Control. Release. 56:259–272 (1998) doi:10.1016/S0168-3659(98)00097-2.
M. Huang, C. W. Fong, E. Khorc, and L. Y. Lim. Transfection efficiency of chitosan vectors: effect of polymer molecular weight and degree of deacetylation. J. Control. Release. 106:391–406 (2005) doi:10.1016/j.jconrel.2005.05.004.
T. Kiang, J. Wen, H. W. Lim, and K. W. Leong. The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials. 25:5293–5301 (2004) doi:10.1016/j.biomaterials.2003.12.036.
M. Lavertu, S. Méthot, N. Tran-Khanh, and M.D. Buschmann. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials. 27:4815–4824 (2006) doi:10.1016/j.biomaterials.2006.04.029.
W. B. Tan, S. Jiang, and Y. Zhang. Quantum-dot based nanoparticles for targeted silencing of HER2/neu gene via RNA interference. Biomaterials. 28:1565–1571 (2007) doi:10.1016/j.biomaterials.2006.11.018.
X. Liu, K. A. Howard, M. Dong, M. O. Andersen, U. L. Rahbek, M. G. Johnsen, O. C. Hansen, F. Besenbacher, and J. Kjems. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials. 28:1280–1288 (2007) doi:10.1016/j.biomaterials.2006.11.004.
J. Y. Pille, H. Li, E. Blot, J. R. Bertrand, L. L. Pritchard, P. Opolon, A. Maksimenko, H. Lu, J. P. Vannier, J. Soria, C. Malvy, and C. Soria. Intravenous delivery of anti-RhoA small interfering RNA loaded in nanoparticles of chitosan in mice: safety and efficacy in xenografted aggressive breast cancer. Hum. Gene Ther. 17:1019–1026 (2006) doi:10.1089/hum.2006.17.1019.
H. Katas, and H. O. Alpar. Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release. 115:216–225 (2006).
K. A. Howard, U. L. Rahbek, X. Liu, C. K. Damgaard, S. Z. Glud, M. O. Andersen, M. B. Hovgaard, A. Schmitz, J. R. Nyengaard, F. Besenbacher, and J. Kjems. RNA interference in vitro and in vivo using a novel chitosan/siRNA nanoparticle system. Mol. Ther. 14:476–484 (2006) doi:10.1016/j.ymthe.2006.04.010.
W. Weecharangsan, P. Opanasopit, T. Ngawhirunpat, A. Apirakaramwong, T. Rojanarata, U. Ruktanonchai, and R. J. Lee. Evaluation of chitosan salts as non-viral gene vectors in CHO-K1 cells. Int. J. Pharm. 348:161–168 (2008) doi:10.1016/j.ijpharm.2007.07.011.
P. C. Engel. Enzyme cofactors. In P. C. Engel (ed.), Enzymology LabFax, Academic, California, 1996, pp. 244–245.
M. D. Smith, J. C. Barbenel, J. M. Courtney, and M. H. Grant. Novel quantitative methods for the determination of biomaterial cytotoxicity. Int. J. Artif. Organs. 15:191–184 (1992).
A. C. Grayson, A. M. Doody, and D. Putnam. Biophysical and structural characterization of polyethylenimine-mediated siRNA delivery in vitro. Pharm. Res. 23:1868–1876 (2006) doi:10.1007/s11095-006-9009-2.
M. O. Andersen, K. A. Howard, S. R. Paludan, F. Besenbacher, and J. Kjems. Delivery of siRNA from lyophilized polymeric surfaces. Biomaterials. 29:506–512 (2008) doi:10.1016/j.biomaterials.2007.10.003.
K. Romóren, S. Pedersen, G. Smistad, O. Evensen, and B.J. Thu. The influence of formulation variables on in vitro transfection efficiency and physicochemical properties of chitosan-based polyplexes. Int. J. Pharm. 261:115–127 (2003) doi:10.1016/S0378-5173(03)00301-6.
T. Sato, T. Ishii, and Y. Okahata. In vitro gene delivery mediated by chitosan: effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials. 22:2075–2080 (2001) doi:10.1016/S0142-9612(00)00385-9.
Acknowledgements
The authors are grateful for financial support by Genetic Engineering and Biotechnology (BIOTEC), Thailand (grant number: BT-B-01-MG-16–4812), the Commission of Higher Education (Thailand), the Thailand Research Funds and National Research Council of Thailand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rojanarata, T., Opanasopit, P., Techaarpornkul, S. et al. Chitosan-Thiamine Pyrophosphate as a Novel Carrier for siRNA Delivery. Pharm Res 25, 2807–2814 (2008). https://doi.org/10.1007/s11095-008-9648-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9648-6