Abstract
Purpose
Examine the oral absorption enhancement mechanism of cromolyn sodium by sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC) by evaluating the effect of SNAC on cromolyn sodium lipophilicity and changes in Caco-2 cell membrane fluidity.
Materials and Methods
Standard Shake-flask method was used to evaluate the effect of SNAC on the lipophilicity of cromolyn sodium. The measurements were carried out in three partitioning solvents with varying hydrogen-bonding properties. Steady state fluorescence emission anisotropy technique was used to evaluate the effect of SNAC with/without cromolyn sodium on Caco-2 cell membrane fluidity.
Results
The lipophilicity measurements showed that SNAC had no influence on the lipophilicity of cromolyn sodium in the three partitioning solvent systems. The findings of the steady-state fluorescence anisotropy showed that SNAC increases the membrane fluidity of the Caco-2 cells in a concentration dependent manner. The increase in fluidity with SNAC was seen in the presence and absence of cromolyn sodium and the presence of cromolyn sodium did not augment the effect of SNAC on membrane fluidity.
Conclusions
The increase in membrane fluidity by SNAC plays a pivotal role in the permeation enhancement mechanism of cromolyn sodium. Therefore, the increase in permeation is a result of changing Caco-2 cell membrane fluidity resulting in change in membrane integrity and not due to an increase in the lipophilicity of cromolyn sodium through its interaction with SNAC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
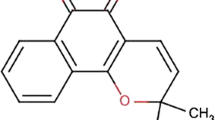
Cromolyn sodium is a disodium salt of 1,3-bis(2-carboxychromon-5-yloxy)-2-hydroxypropane (Fig. 1). It is a white-creamy powder with a molecular weight of 512 gm/mol and melting point of 241–242°C. It is freely soluble in water (100 mg/ml) at 20°C and practically insoluble in chloroform and alcohol (1). Cromolyn sodium is very hydrophilic, containing strong acidic twin carboxyl groups (pKa = 2.0) and has tremendous H-bonding capability (2). These structural features contribute to its high hydrophilicity (n-octanol/neutral aqueous buffer distribution coefficient less than 0.001) (3). Oral human bioavailability study of cromolyn sodium showed that it was poorly absorbed from the GI tract, less than 1% of the administered dose (500 mg) was absorbed in 12 volunteers (4). In 3 subjects 0.28–0.5% of the administered dose was recovered in the first 24 h in the urine. The mean urinary excretion of an administered dose over 24 h in the remaining 9 subjects was 0.45%. Cromolyn sodium prodrugs have been synthesized to improve the oral absorption. These compounds shows higher oral absorption and thereby higher oral bioavailability in rat and rabbits, however the absorption mechanism is still unclear and the toxicity of these prodrugs has not yet been fully investigated (3,5).
Emisphere Technologies Inc. has developed an oral formulation of cromolyn sodium that has shown an increase in the oral bioavailability of the drug by eightfold in rats (6). In the first human study, escalating single oral doses of cromolyn sodium combined with an Emisphere™ delivery agent sodium N-[8-(2-hydroxybenzoyl) amino] caprylate (SNAC) (Fig. 2) demonstrated effective oral delivery and absorption. Subsequently, a Phase I randomized, double-blind, placebo controlled multi-dose study in human allergy patients was conducted to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics following a 10 day treatment regimen. This study revealed that the oral delivery of cromolyn sodium, using SNAC, was effective in allergic patients. Absorption was rapid and no adverse events were reported during the study. In the same study cromolyn sodium when administered orally by itself also has an excellent safety profile (7,8).
SNAC belongs to a family of low molecular weight delivery agents with a CMC of 56 mM at 37°C in PBS at pH 7.4 (9). When co-administered with certain polar drugs, such as human growth hormone, insulin, calcitonin, heparin, and cromolyn sodium it can markedly enhance their oral bioavailability by increasing their oral absorption (10). Based on several mechanistic studies at the cellular and subcellular levels it has been concluded that SNAC increases the passive transcellular transport of these molecules across the epithelial membrane without clear damage to the GI tract’s absorption barrier in vivo and in vitro (6,9,11,12). In addition, it has been proposed that SNAC non-covalently interacts with drugs to improve their physicochemical properties, mainly lipophilicity for absorption (12–14). In this study we examine the hypotheses that SNAC increases the oral bioavailability of a model drug, cromolyn sodium either by increasing the lipophilicity of the drug by non-covalent interaction or by membrane fluidization. The effect of SNAC on cromolyn sodium lipophilicity was examined by evaluating the distribution of cromolyn sodium in three partitioning systems with varying hydrogen-bonding properties. The effect of SNAC with/without cromolyn sodium on Caco-2 cell membrane fluidity was examined by steady state fluorescence emission anisotropy technique.
MATERIALS AND METHODS
Materials
Cromolyn sodium was purchased from MP Biomedical, Inc. (Solon, OH, USA). SNAC was synthesized and kindly provided by Emisphere Technology, Inc. (Tarrytown, NY, USA). 1-octanol 99% HPLC grade and chloroform >99.9% were obtained from Sigma-Aldrich Chemical Co. (Milwaukee, WI, USA). Propylene glycol dipelargonate (PGDP) 98% was purchased from Noveon, Inc (Cleveland, OH, USA). DPH: (1,6 diphenyl-1,3,5-hexatrine);TMA-DPH (1-(4-Trimethyl ammonium phenyl)-6-phenyl-1,3,5-hexatrine), benzyl alcohol anhydrous 99.8% and dimethylformamide (DMF) for molecular biology 99% were purchased from Sigma-Aldrich Chemical Co. (Milwaukee, WI, USA). Tetrahydrofuran was purchased from Fisher Scientific (Fair Lawn, NJ, USA).
The Caco-2 cell line (passage 18) ATCC® number HTB-37, Eagle’s Minimum Essential medium with Earle’s BBS and 2 mM L-glutamine (EMEM) modified by ATCC to contain: 1.0 mM sodium pyruvate, 0.1 mM nonessential amino acids, 1.5 g/l sodium bicarbonate were obtained from American Tissue Culture Collection (Manassas, VA USA). Penicillin-Streptomycin liquid (100X), Fetal Bovine Serum Certified, and 0.25% Trypsin-EDTA were purchased from Invitrogen™ (Carlsbad, CA, USA). PBS 1X buffer sterile Cellgro® was purchased from Mediatech, Inc (Herndon, VA, USA). Krebs-Ringer Bicarbonate Buffer (KRB) with 1,800 mg/l glucose, without calcium chloride and sodium bicarbonate was obtained from Sigma-Aldrich Chemical Co. (Milwaukee, WI, USA).
Methods
Distribution Measurements
The standard shake flask method was used to determine distribution coefficients. A 1:1 mixture of deionized water with one of three organic solvents, 1-octanol, chloroform, or PGDP was prepared. Solvents were allowed to equilibrate for 24 h without any solutes prior to measurement to minimize errors in detected values (15). Solutions of 25 μM cromolyn sodium alone or mixed with 100 or 500 μM SNAC were prepared in saturated deionized water and the pH was adjusted to 7.2–7.4 at 25°C using 1 N NaOH and 1 N HCl.
For distribution measurements the ratio of organic to water phase was set at 60:1 to account for the low solubility of cromolyn sodium in organic solvents. The mixture was placed in a shaker and mixed at 20 rpm for 48 h at 25°C. After 48 h the aqueous layer was removed and the solution was centrifuged at 3,000 rpm for 10 min and filtered using a 13 mm 0.45 μm hydrophobic PTFE syringe filter and used directly for analysis. The organic layer was centrifuged at 3000 rpm for 10 min and evaporated completely under vacuum at 60°C. Due to the high boiling point of PGDP (426.1°C) we were unable to evaporate the organic layer and analysis was restricted to the aqueous layer. The residue was dissolved in 5 ml of deionized water. The solution was filtered and used for analysis.
High-Performance Liquid Chromatography (HPLC)
The HPLC system used for quantifying cromolyn sodium and SNAC was Agilent 1100 Series LC system composed of Agilent 1100 Series Quaternary Pump, Agilent 1100 Series High performance Autosampler SL, Agilent 1100 Series Thermostatted Column Compartment, Agilent 1100 Series DAD-SL UV-VIS detector. A 5 μl sample was injected into a Zorbax SB-C18 Rapid Resolution cartridge (2.1 × 30 mm, 3.5 μm, Agilent). The column temperature was maintained at ambient conditions throughout the run. Mobile phase consisted of a gradient mixture of deionized water and acetonitrile with 0.1% trifluoroacetic acid. An initial 5–95% water: acetonitrile gradient (0.5 ml/min) was adjusted to optimize the elution for each individual compound of interest. Detection was at 240 and 254 nm with a run time of 10 min. This method ensured reproducible and rapid separation of cromolyn sodium and SNAC. The retention times of these compounds were 0.3 and 5.5 min respectively and the detection limit of cromolyn sodium was 0.1 μM.
Fluorescence Anisotropy Measurements
Caco-2 cells were grown to confluence in 25 cm2 cell culture flasks. Confluent cells were washed three times with phosphate buffer (PBS, pH 7.4) and incubated with KRB solutions or positive controls or SNAC 67 and 83 mM with/without cromolyn sodium 10 mM for 0, 30, 60, and 120 min at 37°C. The cells were washed three times with PBS, trypsinized and resuspended in PBS and the final concentration was adjusted to 1 × 105 cells/ml. Caco-2 cell suspensions were labeled with DPH by adding 2.5 ml of 1 mM freshly prepared DPH stock solution in tetrahydrofuran to 2.5 ml of cell suspension. Suspensions were then incubated at room temperature in the dark for 30 min to complete labeling. Cells were labeled with TMA-DPH solubilized in dimethylformamide in a similar manner.
The fluorescence anisotropy of DPH and TMA-DPH labeled Caco-2 cells was measured with QuantaMaster™ Luminescence fluorometer L-format, excited with 355 nm vertically polarized light and measuring emission intensity at 428 nm in the direction parallel and perpendicular to the exciting light. The steady-state fluorescence emission anisotropy was calculated using the following equation:
Where r is anisotropy, I VV and I VH are the intensities measured in directions parallel and perpendicular to the polarized exciting light, respectively, and G was calculated as a ratio between I VH/I HH.
Statistical Analysis
The statistical program SigmaStat (Window version 3.11 Copyright© 2004 Systat software, Inc.) was used to analyze HPLC and emission anisotropy, r data. The areas obtained from the chromatograms were compared using one-way ANOVA at 5% significance level with three replicates for each data point (n = 3). The calculated anisotropy, r values were compared using one-way ANOVA at 5% significance level and five replicates were used per time point (n = 5).
RESULTS
Cromolyn Sodium Distribution Measurements
Distribution coefficient determination at pH 7.4 in 1-octonol: water system showed that in the aqueous layer no statistically significant difference in cromolyn sodium 25 μM concentration in the absence or presence of SNAC 100 and 500 μM was noted (data not shown for SNAC 100 μM) . No cromolyn sodium was detected in the 1-octanol layer after 48 h equilibration in the absence and presence of SNAC (Table I). However, SNAC alone was detected in the 1-octanol layer at a retention time of 5.608 min. Distribution coefficient determinations in chloroform-water system and PGDP-water systems similarly showed not statistically significant difference in cromolyn sodium concentration in the aqueous layer in the presence and absence of SNAC (Table I) (data not shown for SNAC 100 μM). SNAC was detected in the chloroform layer in the chloroform-water system at 48 h equilibration time at 5.503 min.
Membrane Fluidity Measurements
The effect of SNAC on the membrane bilayer was determined by membrane fluidity measurements using DPH and TMA-DPH fluorescence. Anisotropy is inversely related to membrane fluidity, therefore if there is a decrease in DPH fluorescence anisotropy upon exposure to SNAC it may be concluded that there is increase in the membrane fluidity within the core of the bilayer. The same may be concluded with TMA-DPH, except that the location of greater fluidity is at the surface of the bilayer. The overall consequence would be that the membrane structure has been altered which may lead to subsequent enhancement of drug absorption. The r value for the negative controls (no treatment) of DPH and TMA-DPH labeled Caco-2 cell membranes at the zero time point were 0.145 ± 0.016, and 0.282 ± 0.016, respectively. Tables II and III list the r values for the Caco-2 cells labeled with DPH and TMA-DPH respectively for the entire study. Incubation of Caco-2 cells with KRB buffer for 30, 60 and 120 min caused no statistically significant change compared to controls in the fluorescence anisotropy, r values of DPH and the TMA-DPH (Figs. 3 and 4). A similar trend was observed when cells were incubated with 10 mM cromolyn sodium alone for 30, 60 and 120 min, and no statistically significant change in the r value was observed as compared to the control.
Changes in Caco-2 cell bilayer hydrophobic core fluidity evaluated using DPH fluorescence anisotropy upon treatment with KRB, 30 mM benzyl alcohol w/o/w 10 mM Cromolyn sodium, 83 mM SNAC w/o/w 10 mM Cromolyn sodium (error bars indicate SD, n = 5, * denotes statistical significance at p value <0.05 compared to control KRB).
Changes in Caco-2 cell surface fluidity evaluated using TMA-DPH fluorescence anisotropy upon treatment with KRB, 30 mM benzyl alcohol w/o/w 10 mM Cromolyn sodium, 83 mM SNAC w/o/w 10 mM Cromolyn sodium (error bars indicate SD, n = 5, * denotes statistical significance at p value <0.05 compared to control KRB).
Benzyl alcohol is known to induce changes in bilayer member fluidity of the hydrophobic core and the surface regions of living cells (16,17). Therefore in this study we used 30 mM benzyl alcohol as a positive control. Caco-2 cells were treated with 30 mM benzyl alcohol and incubated at 30 and 60 min with no statistically significant difference in r values of DPH and TMA-DPH as compared to controls (Figs. 3 and 4). However incubation for 120 min with 30 mM benzyl alcohol showed a significant decrease in the r values of DPH 0.046 ± 0.009 (32% of the control) and TMA-DPH 0.066 ± 0.033 (24% of the control) (Figs. 3 and 4). A similar trend was observed when Caco-2 cells were co-incubated with 10 mM cromolyn sodium and 30 mM benzyl alcohol for 30, 60, and 120 min. The r value of DPH was 0.047 ± 0.019 (32% of the control) and TMA-DPH was 0.079 ± 0.012 (28% of the control) and a significant decrease was detected only upon 120 min incubation (Figs. 3 and 4).
When cells were incubated with 67 mM SNAC alone or mixed with 10 mM cromolyn sodium, no statistically significant difference in r values of DPH and TMA-DPH after the three incubation periods 30, 60, and 120 min was observed (Figs. 3 and 4). When cells were incubated for 30 min with 83 mM SNAC alone or mixed with 10 mM cromolyn sodium no statistically significant differences compared to controls were detected in the r values of DPH and TMA-DPH (Figs. 3 and 4). However upon incubation of Caco-2 cells for 60 min with 83 mM SNAC alone, the r values of DPH and TMA-DPH were 0.044 ± 0.008 (30% of the control) and 0.149 ± 0.013 (53% of the control) respectively which are significantly smaller compared to the untreated Caco-2 cells or cells incubated with KRB buffer. The same results were obtained when the cells were co-incubated for 60 min with 83 mM SNAC and 10 mM cromolyn sodium, the r values of DPH and TMA-DPH were 0.055 ± 0.028 (38% of the control) and 0.147 ± 0.021 (52% of the control) respectively (Figs. 3 and 4). When cells were incubated for 120 min with 83 mM SNAC alone a statistically significant decrease compared to control resulted in the r value of DPH of 0.047 ± 0.019 (32% of the control) and for TMA-DPH of 0.079 ± 0.012 (28% of control) (Figs. 3 and 4). In addition, co-incubation of the cells for 120 min with 83 mM SNAC and 10 mM cromolyn sodium resulted in a statistically significant decrease compared to control r values for both probes. The r value for DPH was 0.018 ± 0.016 (13% of the control) and for TMA-DPH was 0.072 ± 0.007 (26% of the control) (Figs. 3 and 4).
In summary the findings of the steady-state fluorescence emission anisotropy study are as follows:
-
1.
A 10-mM cromolyn sodium has no statistically significant effect compared to controls on the fluidity of Caco-2 cell membranes;
-
2.
A 67-mM SNAC alone or mixed with 10 mM cromolyn sodium has no statistically significant effect compared to controls on the fluidity of Caco-2 cell membranes;
-
3.
A 83-mM SNAC alone or mixed with 10 mM cromolyn sodium significantly increases the fluidity of the core hydrophobic region as well as the surface polar region of the phospholipid bilayer of Caco-2 cell membranes (Figs. 3 and 4);
-
4.
A 83-mM SNAC alone or mixed with 10 mM cromolyn sodium significantly increases the membrane fluidity upon incubation for 60 and 120 min. In contrast 30 mM benzyl alcohol alone (positive control) or mixed with 10 mM cromolyn sodium showed effect only upon incubation for 120 min (Figs. 3 and 4);
-
5.
SNAC effects the membrane fluidity in a concentration dependant manner above its CMC (46 mM) (9);
Ding et al. (14,18) showed that 67 mM SNAC does not significantly increase the permeability of 10 mM Cromolyn across the Caco-2 cell membrane but 83 mM SNAC does. This indicates that SNAC fluidizes the membrane in a concentration dependent manner allowing for cromolyn sodium permeation transcellularly upon fluidization. Li (12) and Wu (9) showed that 124 mM SNAC enhances the transcellular passive diffusion of human growth hormone (hGH) across the Caco-2 cell monolayer. However, the enhancement is concentration dependent and the minimal concentration of SNAC needed to trigger the transcellular passive diffusion of hGH across the Caco-2 monolayer was 124 mM. This conclusion can be supported by the findings of previous studies that confirm that the enhancement mechanism of SNAC and other amino acid derivatives of poorly permeable molecules is passive and mainly transcellular (9,12,19–21). Thus it can be seen that SNAC interacts with the membrane in a concentration dependent manner allowing for molecules of differing molecular weights to pass through the membrane. In the case of 83 mM SNAC, it is likely that the increase in the Caco-2 membrane fluidity contributes to the enhancement of cromolyn sodium permeation across the cell line and thus the transcellular route contributes to the transport of cromolyn sodium across the cell membrane.
DISCUSSION
Cromolyn sodium, an antiallergic medication, has poor oral bioavailability due to its low lipophilicity (3). SNAC enhanced the oral bioavailability of cromolyn sodium both in animals and human (6,8). This enhancement in oral bioavailability of cromolyn sodium in the presence of SNAC is due to an increase in cromolyn sodium’s permeation through passive transcellular pathway across the intestinal epithelium and also across the Caco-2 monolayer (6,14,18).
Two main approaches have been used to overcome the issue of the poor permeability of a drug in the GI tract, either by modifying the intestinal epithelium or modifying the drug. The first approach is by modifying the intestinal epithelium permeability thereby enhancing the uptake by transcellular or the paracellular pathways (8,22–25). The second approach is to increase the permeability of the drug molecule itself without changing the integrity of the intestinal epithelium. In this approach, the drug permeability is increased by forming a prodrug which is a substrate for one of the transporters in the GI tract epithelium or by increasing its lipophilicity thereby increasing its transcellular passive uptake. Increasing drug lipophilicity can be done by covalent (prodrug) or non-covalent modification of the drug. A type of non-covalent modification is by complexing the drug non-covalently with a complexing agent leading to enhanced drug lipophilicity and thereby permeability (26–30).
Previous studies have proposed that SNAC may interact non-covalently with drugs to improve their physicochemical properties, mainly lipophilicity for absorption (12–14). To examine this hypothesis we set two specific aims, the first being to see if a non-covalent interaction exists between SNAC and cromolyn sodium and the second if this interaction leads to increased cromolyn sodium lipophilicity. Given that the GI tract presents two types of absorption barriers, an aqueous and a lipophilic one, the interaction between SNAC and cromolyn sodium needed to be investigated in both types of environments. In a previous study the interaction between cromolyn sodium and SNAC was investigated using 1H NMR and 2D NOESY in two media, aqueous and lipophilic cosolvent systems (31,32). No interaction between SNAC and cromolyn sodium was detected in aqueous media. However, a weak interaction was detected between the two compounds in lipophilic medium. The interaction was weak and did not produce a large change in the chemical shifts of either compounds, therefore we were not able to calculate the association constant. The distribution of cromolyn sodium with/without SNAC was evaluated in three partitioning systems of varying hydrogen bonding capacities. However, due to the extreme hydrophilicity of cromolyn sodium it was not detected in any of the organic layers (n-octanol and chloroform) studied and no statistically significant decrease in the concentration of cromolyn sodium in the aqueous layer was observed for all systems. The limit of detection of cromolyn sodium of the HPLC method was 0.1 μM. The concentration of cromolyn sodium used in all the distribution studies was a 250-fold higher. Thus based on this method no increase in the lipophilicity of cromolyn sodium was detected in all three systems. Therefore, it is clear from the association and the partitioning studies that the interaction between SNAC and cromolyn sodium is weak and has no effect in increasing the lipophilicity of cromolyn sodium thereby increasing its permeability across the Caco-2 membrane. These findings showed that SNAC does not increase the permeability of cromolyn sodium by changing its physicochemical properties.
Thus the only other possibility for the permeation enhancement by passive transcellular route is that SNAC causes perturbation of the epithelial membrane resulting in an increase in cromolyn sodium permeation without causing obvious damage to the membrane. To test this hypothesis, we examined the changes in the membrane fluidity of Caco-2 cells in the presence of SNAC and/or cromolyn sodium using marker molecules. The findings of the steady-state fluorescence anisotropy showed that SNAC increases the membrane fluidity of the Caco-2 monolayer in a concentration-dependent manner. SNAC increases the fluidity with or without cromolyn sodium and the presence of cromolyn sodium did not augment the effect of SNAC on membrane fluidity. The increase in membrane fluidity due to SNAC results in changes in membrane integrity, thus playing a pivotal role in the permeation enhancement mechanism of cromolyn sodium.
Thus, based on our findings it seems that SNAC interacts with the membrane and perturbs it, allowing for molecules such as cromolyn sodium to permeate. This conclusion is further supported by previous studies where SNAC has been shown to release different cellular components after incubation with Caco-2 cells. Studies by Wu using SNAC 124 mM with/without 1 μg/ml human growth hormone showed perturbation to the cell membrane and released intracellular components. Incubation of Caco-2 cells for 120 min caused a statistically significant release of lactate dehydrogenase into bathing medium which is an indication of membrane disruption (9). SNAC 124 mM caused statistically significant decrease in Caco-2 cell mitochondrial dehydrogenase activity after 60 min incubation as measured by the MTT assay which is also indicative of perturbation to the cell membrane (9). Additional cytotoxicity studies were conducted by Li with two concentrations of SNAC 83 and 124 mM with or without 1 μg/ml human growth hormone (12). The Caco-2 cell membrane damage was evaluated by trypan blue exclusion test and by neutral red assay. When Caco-2 cells were treated for 60 min an increase in the trypan blue uptake was observed for both concentrations in treated cells, and the results were significantly different compared to the KRB negative control. The Caco-2 cell lysosomal compartment damage was evaluated using neutral red assay. The Caco-2 cells exhibited decreased biding of neutral red indicative of decreased cell viability.
In conclusion, intestinal cell membrane perturbation is essential to enhance the permeation of cromolyn sodium and it is not likely that the enhancement in cromolyn sodium permeation is a result of increasing its lipophilicity due to the non-covalent interaction with SNAC. However, the enhancement in permeability of macromolecules by SNAC is more complicated and non-covalent interactions may still play a role in the enhancement mechanisms but this hypothesis remains to be evaluated.
References
S. Budavari, M. J. O’Neil, A. Smith, and P. E. Heckelman. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (Merck Index), MERCK & CO., Inc., Rahway, NJ, 1989.
H. S. White. Histamine and antihistamine drugs. In Remington (ed.), The Science and Practice of Pharmacy, Williams & Wilkins, Philadelphia, 2000, pp. 1464–1476.
T. Mori, K. Nishimura, S. Tamaki, S. Nakamura, H. Tsuda, and N. Kakeya. Pro-drug for the oral delivery of disodium cromoglycate. Chem. Pharm. Bull. 36:338–344 (1988).
PDR®. Physician’s Desk Reference, Thomson PDR, 1992.
A. Yoshimi, H. Hashizume, S. Tamaki, H. Tsuda, F. Fukata, K. Nishimura, and N. Yata. Importance of hydrolysis amino acid moiety in water-soluble prodrug of disodium cromoglycate for increased oral bioavailibility. J. Pharmacobio-Dyn. 15:339–345 (1992).
A. Leone-Bay, H. Leipold, D. Sarubbi, B. Variano, T. Rivera, and R. A. Baughman. Oral delivery of sodium cromolyn: preliminary studies in vivo and in vitro. Pharm. Res. 13:222–226 (1996).
Emisphere Technology. Emisphere’s oral cromolyn sodium program, http://www.emisphere.com/pc_ocs.asp.
M. Goldbege. Oral macromolecule delivery: review of the large and expanding clinical database. Winter symposium & 11th International symposium on recent advanced drug delivery systems, Salt Lake city, Utah, 2003.
S.-J. Wu. Mechanistic studies on the Enhanced Mucosal Transport of Human Growth Hormone by Certain Amino Acid Derivatives, School of Pharmacy, University of Wisconsin, Madison, 1999.
B. N. Singh and S. Majuru. Oral delivery of therapeutic macromolecules: a prospective using the eligenTM technology. Drug Deliv. Technol. 3:53–62 (2003).
D. Brayden, E. Creed, A. O’Connell, H. Leiopold, R. Agarwal, and A. Leone-Bay. Heparin absorption across the intestine: Effect of Sodium N-[8-(2-Hydroxybenzoyl)amino]Caprylate in rat In situ Instillations and in Caco-2 monolayers. Pharm. Res. 14:1772–1778 (1997).
B. Li. Non-covalent Carrier Enhanced Protein Absorption-cellular and Subcellular Mechanistic Studies, School of Pharmacy, University of Wisconsin, Madison, 2001.
S.-J. Wu and J. R. Robinson. Transcellular and lipophilic complex-enhanced intestinal absorption of human growth hormone. Pharm. Res. V16:1266–1272 (1999).
X. Ding, P. Rath, R. Angelo, T. Stringfellow, E. Flanders, S. Dinh, I. Gomez-Orellana, and J. R. Robinson. Oral absorption enhancement of cromolyn sodium through noncovalent complexation. Pharm. Res. 21:2196–2206 (2004).
J. C. Dearden and G. M. Bresnen. The measurement of partition coefficients. Quant. Struct.-Act. Relatsh. 7:133–144 (1988).
B. D. Rege, J. P. Y. Kao, and J. E. Polli. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 16:237–246 (2002).
F. Giraud, M. Claret, K. R. Bruckdorfer, and B. Chailley. The effects of membrane lipid order and cholesterol on the internal and external cationic sites of the Na+–K+ pump in erythrocytes. Biochim. Biophys. Acta Biomembr. 647:249–258 (1981).
X. Ding. Oral Absorption Enhancement of Cromolyn Sodium Through Non-covalent Complexation, Pharmaceutical Sciences, University of Wisconsin-Madison, Madison, 2004, pp. 153.
A. Leone-Bay, D. R. Paton, J. Freeman, C. Lercara, D. O’Toole, D. Gschneidner, E. Wang, E. Harris, C. Rosado, T. Rivera, A. DeVincent, M. Tai, F. Mercogliano, R. Agarwal, H. Leiopold, and R. A. Baughman. Synthetic and evaluation of compounds that facilitate the gastrointestinal absorption of heparin. J. Med. Chem. 41:1163–1171 (1998).
A. Leone-Bay, C. McInnes, N. Wang, F. DeMorin, D. Achan, C. Lercara, D. Sarubbi, S. Hass, J. Press, E. Barantseich, B. O’Broin, S. Milstein, and D. Paton. Microsphere formation in a series of derivatized a-amino acids: Properties, molecular modeling, and oral delivery of Salmon Calcitonin. J. Med. Chem. 38:4257–4262 (1995).
A. Leone-Bay, N. Santiago, D. Achan, K. Chaudhary, F. DeMorin, L. Falzarano, S. Hass, S. Kalbag, D. Kaplan, H. Leiopold, C. Lercara, D. O’Toole, T.Rivera, C. Rosado, S. D, E. Vauocolo, N. Wang, S. Milstein, and R. A. Baughman. N-acelated-a-amino acids as novel oral delivery agents for proteins. J. Med. Chem. 38:4263–4269 (1995).
J. L. Madara. Regulation of the movement of solutes across tight junctions. Annu. Rev. Physiol. 60:143–159 (1998).
A. L. Daugherty and R. J. Mrsny. Transcellular uptake mechanisms of the intestinal epithelial barrier part one. Pharm. Sci. Technol. Today 2:144–151 (1999).
E. C. Swenson and W. J. Curatolo. Intestinal permeability enhancement for proteins, peptides and other polar drugs: Mechanisms and potential toxicity. Adv. Drug Deliv. Rev. 8:39–92 (1992).
E. S. Swenson, W. B. Milisen, and W. Curatolo. Intestinal permeability enhancement: efficacy, acute local toxicity, and reversibility. Pharm. Res. 11:1132–1142 (1994).
W. L. Hayton, D. E. Gutman, and G. Levy. Effect of complex formation on drug absorption XI: Complexation of prednisone and prednisolone with dialkylpropionamide and its effect on prednisone transfer through artificial lipoid barrier. J. Pharm. Sci. 61:356–361 (1972).
W. L. Hayton and G. Levy. Effect of complex formation on drug absorption XII: Enhancement of intestinal absorption of prednisone and prednisolone by dialkylpropionamide in rats. J. Pharm. Sci. 61:362–366 (1972).
W. L. Hayton and G. Levy. Effect of complex formation on drug absorption XV: Structural requirement for enhancement of intestinal absorption of steroids by N,N-Di-n-propylpropionamide. J. Pharm. Sci. 61:649–651 (1972).
W. L. Hayton and G. Levy. Effect of complex formation on drug absorption XIII: Effect of constant concentration of N,N-Di-n-propylpropionamide on prednisolone absorption from rate small intestine. J. Pharm. Sci. 61:367–371 (1972).
W. L. Hayton, G. Levy, and C. Regardh. Effect of complex formation on drug absorption XIV: effect of N,N-Di-n-propylpropionamide on intestinal absorption of certain nonsteroid drugs in the rat. J. Pharm. Sci. 61:473–474 (1972).
A. G. Alani and J. R. Robinson. Mechanistic understanding of oral drug absorption enhancement of cromolyn sodium by an amino acid derivative, Thirteenth International Symposium on Recent Advances in Drug Delivery Systems "Overcoming long-standing barriers", Salt Lake City, UT, 2007.
A. G. Alani and J. R. Robinson. Mechanistic Understanding of Oral Drug Absorption Enhancement of Cromolyn Sodium by an Amino Acid Derivative, School of Pharmacy, University of Wisconsin, Madison, 2007, p. 178.
Acknowledgments
The authors would like to thank the School of Pharmacy, University of Wisconsin-Madison for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alani, A.W.G., Robinson, J.R. Mechanistic Understanding of Oral Drug Absorption Enhancement of Cromolyn Sodium by an Amino Acid Derivative. Pharm Res 25, 48–54 (2008). https://doi.org/10.1007/s11095-007-9438-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9438-6