It is established that succinic acid is capable of potentiating (synergism) the antihypoxic activity of 3-hydroxypyridine derivatives such as the succinate (Ia) and hydrochloride (Ib) of 3-(N,N-dimethylcarbamoyloxy)- 2-ethyl-6-methylpyridine. This effect makes it expedient to create new drugs containing a mixture of Ia or Ib and succinic acid derivatives that would possess antihypoxic, antiamnestic, and anticonvulsant activity. The antihypoxic activity of 3-hydroxypyridine drugs increases in the order emoxypin < mexidol < proxypin < Ia + succinic acid < Ib + succinic acid.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Despite recent successes in creating drugs for treating neurological diseases associated with brain ischemia and accompanied by mnestic disruptions, most existing drugs (according to multi-center clinical trials) do not reduce mortality from such diseases although they do improve the neurological outcome [1].
Drugs of the 3-hydroxypyridine series such as emoxypin and mexidol, which exhibit antioxidant and antihypoxic activity, play an important role in complex therapy of ischemic brain disease [2].
A new structural analog of mexidol is 2-ethyl-3- (N,N-dimethylcarbamoyloxy)-6-methylpyridine succinate [3], which has higher antihypoxic activity than emoxypin and mexidol. This compound under the name proxypin (Ia) was entered into clinical trials as a neuroprotectant for ischemic brain disease.

We found during an in-depth study of the pharmacology of proxypin and its analog as the hydrochloride (Ib) that succinic acid enhances the antihypoxic activity of these compounds. It should be emphasized that this effect is synergistic in nature (potentiation) but not additive (Table 1). Thus, a basis arose for creating combined antihypoxic drugs consisting of 2-ethyl-3-(N,N-dimethylcarbamoyloxy)-6-methylpyridine hydrochloride (Ib) or the corresponding succinate (Ia) and succinic acid.
The role of succinic acid in the manifestation of the synergism upon its combination with 3-hydroxypyridine derivatives is apparently related to the involvement of succinic acid in reductive processes occurring in organs and tissues, including with various pathologies [5]. Succinic acid itself is practically not used as a drug. Only the diagnostic limontar, which is a composition of citric and succinic acids that is used to study the stomach secretory function, is registered in Russia. Furthermore, its use for the prevention and treatment of alcohol intoxication has been reported [6]. In general, succinic acid is rather widely used in pharmaceutics as a salt-forming component of several drugs such as mexidol, metoprolol, etc. that ensures the active ingredients of these preparations are water-soluble.
Experimental chemical part
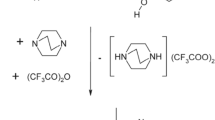
2-Ethyl-3-(N,N-dimethylcarbamoyloxy)-6-methylpyridine succinate (Ia, proxypin). A suspension of 2-ethyl-3- hydroxy-6-methylpyridine (109.74 g, 0.8 mol) in 1,2-dichloroethane (240 mL) was stirred at 22 ± 2°C, treated gradually with Et3N (105.24 g, 1.04 mol) and N,N-dimethylcarbamoylchloride (111.85 g, 1.04 mol). The suspension was heated to boiling, refluxed for 4 h, cooled to 22 ± 2°C, treated with H2O (120 mL), and stirred for 10 min. The aqueous layer was separated. The organic layer was washed with H2O (2× 60 mL) and dried over anhydrous Na2SO4. The desiccant was filtered off. The filtrate was evaporated in vacuo. The solid was treated with a hot solution of succinic acid (47.24 g, 0.4 mol) in isopropanol (300 mL). The resulting solution was heated to boiling, refluxed with activated carbon for 10 min, filtered, and left at 5 – 10°C for 10 – 15 h. The resulting precipitate was filtered off and dried at 50 – 60°C to afford 2-ethyl-3-(N,N-dimethylcarbamoyloxy)- 6-methylpyridine succinate (Ia) (151.8 g) as a white crystalline powder that was very soluble in water, alcohols, and acetone. Removal of solvent from the mother liquor and recrystallization of the solid from isopropanol afforded additional product (23.7 g). The overall yield of Ia was 175.5 g (82%), mp 84 – 86°C (isopropanol), C26H38N4O8. PMR spectrum (ppm): 1.20 (3H, t, J = 7.5 Hz, CH3 -ethyl), 2.50 (3H, s, 6-CH3), 2.56 (2H, s, CH2 -succinic acid), 2.72 (2H, q, J = 7.5 Hz, CH2-ethyl), 3.00 (3H, s, CH3-N), 3.15 (3H, s, CH3-N), 7.14 (1H, g, J = 8.3 Hz, H-5), 7.41 (1H, g, J = 8.3 Hz, H-4).
2-Ethyl-3-(N,N-dimethylcarbamoyloxy)-6-methylpyridine hydrochloride (Ib). The solid remaining after distillation of the dichloroethane that was obtained from 2-ethyl-6-methyl-3-hydroxypyridine (109.74 g, 0.8 mol) and was described in the preceding example, was treated with EtOAc (850 mL). The resulting solution was treated with activated carbon at 22 ± 2°C and filtered. The filtrate was treated with alcoholic HCl until the pH was 3 to afford 3-(N,N-dimethylcarbamoyloxy)-2-ethyl-6-methylpyridine hydrochloride (Ib) as a white crystalline powder that was very soluble in H2O and alcohols. Additional product (33.1 g) was obtained after evaporation of the mother liquor and treatment of the residue with EtOAc (180 mL). The overall yield of Ib was 168.4 g (86%), mp 181 – 183°C (isopropanol), C11H17ClN2O2. PMR spectrum (CD3OD, ppm): 1.35 (t, CH3 ,CH2), 3.02 [q, (CH3CH2)], 2.79 (br.s, 6-CH3), 3.04 and 3.19 (s, NMe2), 7.83 (br.d, C5H, JC5H C6H = 8.7 Hz), 8.30 (d, C4H).
Experimental pharmacological part
Considering the biological characteristics of succinic acid, including its antihypoxic properties [7], and the spectrum of pharmacological activity of 2-ethyl-3-(N,N-dimethylcarbamoyloxy)- 6-methylpyridine, it seemed interesting to study the antihypoxic, antiamnestic, and anticonvulsant activity of proxypin (Ia) and its analog Ib as compared with the aforementioned types of pharmacological activity of combined preparations consisting of proxypin (Ia) and succinic acid and the hydrochloride (Ib) with succinic acid.
1. Study of antihypoxic activity
1.1. Study for a mouse hypobaric hypoxia model
Hypobaric hypoxia was modeled in a 3-L flow—exhaust barochamber that was fabricated using a vacuum pump to rarefy the air. Mice (20 – 22 g) (one from the control group and two from the test group) were “elevated” simultaneously to a height of 11,000 m (corresponding to atmospheric pressure 185 – 190 mm Hg) at a “rise” rate of 40 m/s (“rise” time was 4 min 30 sec) [8]. The test compounds were administered i.p. or perorally into the stomach using a probe) separately or together. Control (that received 0.2 – 0.3 mL of 0.9% NaCl solution) and test animals were placed into the barochamber 1 h after receiving the test drugs. Antihypoxic activity of the drugs was estimated from the increase of life span of mice (beginning from the moment of reaching the “lethal area” until the appearance of the last agonal exhalation) compared with the control.
1.2. Study for a mouse normobaric (with hypercapnia) hypoxia model
Mice (20 – 22 g) (one) were placed into hermetically sealed 2-L jars 1 h after a single i.p. or peroral administration of the drugs separately or together [8]. Animals of the control group received peroral NaCl solution (0.9%, 0.2 – 0.3 mL). Antihypoxic activity of the test compounds was determined from the increase of life span of animals compared with the control.
2. Study of antiamnestic activity
2.1. Effect on mnestic functions in a mouse passive avoidance conditioned response test
The mouse passive avoidance conditioned response based on the genetic preference of rodents for dark places was used [9]. Mice were placed in the lighted section of a two-compartment chamber. The animals could for 3 min freely travel through both chamber sections. Then, they received in the dark section an unavoidable painful stimulus (0.6 mA alternating current electrical stimulus through the cage floor). Immediately after conditioning, the animals were administered i.p. or perorally compounds Ia, Ib, and succinic acid separately or together; the control animals, NaCl solution (0.9%, 0.2 – 0.3 mL). Amnesia was induced by the maximum electrical shock convulsion. The electrical shock convulsion was applied through electrodes placed on the eye cornea (50 mA, 60 Hz, 0.2 sec) using an ECT UNIT constant pulse generator (Ugo Basile, Italy). Mice were tested 24 h after conditioning and administering drugs by placing them into the lighted section and recording the latent period of staying in it. The antiamnestic effect of the drugs was determined from the increase of the latent period for mice to move from the lighted section into the dark one.
2.2. Effect on amnestic activity of scopolamine in a rat active avoidance conditioned response test
Rats developed an active avoidance conditioned response in a darkened chamber with an electrode floor (25 × 30 × 30 cm) and with a shelf at a height of 23 cm from the floor (safe place). Rats were placed on the shelf that was turned over every 30 sec and fell to the floor where after 5 sec they received a current pulse. In order to avoid it, rats had to jump again onto the shelf within 5 sec. The conditioning session continued for 5 min, during which each rat could complete 10 avoidances. The number of avoidances per learning session was recorded [9]. Scopolamine administered at a dose of 2 mg/kg s.c. 1 h before conditioning once per day for 5 d separately and in combination with the test compounds that were administered separately or in combination was used as the amnestic stimulus in these tests.
3. Study of anticonvulsant activity
Anticonvulsant activity of the drugs was studied using a clonic-tonic convulsion model induced by corazole (135 mg/kg s.c.) [10, 11]. The number of animals in which clonic-tonic convulsions and death were noted was recorded.
Statistical data obtained in the experiment were processed using the Biostat computer program.
Results and Discussion
Succinic acid at doses of 7, 14, and 28 mg/kg perorally, just like Ia and Ib at a dose of 25 mg/kg, exhibited moderate antihypoxic activity (Table 1). Statistically significant differences in the effects of Ia and Ib were not found. Enhancement (potentiation) of the antihypoxic activity of joint administration of Ia at a dose of 25 mg/kg and succinic acid at doses of 14, 28, and 56 mg/kg was noted when compared with the effect observed for their separate administration, just like when compared with the effect of mexidol at a dose of 100 mg/kg. Analogous but more apparent results were obtained for i.p. administration of Ia and Ib together with succinic acid. It should be noted that the effect of a combination of Ia and succinic acid was less evident (570%) than that of a combination of Ib and succinic acid (676%).
The study of the effects of Ia and Ib and their combinations with succinic acid on the life span of animals for the hypoxic hypoxia model with hypercapnia (sealed chamber) found that Ia only at a high dose (150 mg/kg) exhibited moderate antihypoxic activity (Table 2). Succinic acid itself at a dose of 168 mg/kg was ineffective. However, it in combination with Ia enhanced (potentiated) the antihypoxic activity of the latter. It should also be noted that the combination of Ia and succinic acid was also more effective than mexidol.
It was shown that administration of scopolamine (60 min before conditioning) for 5 d disrupted substantially the process of remembering information (Table 3). Compounds Ia and Ib at doses of 25 mg/kg, just like succinic acid at a dose of 28 mg/kg, did not influence memory destruction induced by scopolamine upon separate peroral administration whereas combined administration of Ib and succinic acid, but not on the first but the fifth day of the study, potentiated its protective action. It should be noted that mexidol at a dose of 25 mg/kg was ineffective in this model.
It was found that compounds Ia and Ib at doses of 100 mg/kg and succinic acid at a dose of 25 mg/kg perorally upon separate or combined administration to untreated animals did not have a substantial effect on their conditioning (left column of Table 4). Mice that received after conditioning an electrical shock (without administration of the test drugs) showed significant degradation of conditioned response activity. This was seen as a four-fold decrease of the latent period for passage into the dark section of the chamber (right column of Table 4). Administration of Ia at a dose of 100 mg/kg peroral and at doses of 2.5, 5, 10, and 25 mg/kg i.p., just like succinic acid at a dose of 25 mg/kg i.p., caused a slight (statistically insignificant) weakening of the amnestic effect of the electrical shock, which for their combined administration had a tendency toward more pronounced weakening of the amnesia (right column of Table 4).
Compound Ia at a dose of 25 mg/kg had practically no effect on the convulsive effect of corazole whereas Ib at the same dose increased the latent periods of tetanus and death (Table 5). Under analogous conditions succinic acid itself did not substantially change the effect of corazole whereas its administration in combination with Ia and Ib increased the latent periods for the onset of tonic extension and death (to a greater extent in combination with Ib).
Thus, we observed and studied the ability of succinic acid to potentiate the antihypoxic activity of succinate Ia and 2-ethyl-3-(N,N-dimethylcarbamoyloxy)-6-methylpyridine hydrochloride (Ib). This provided the impetus to develop potential combined drugs consisting of Ia or Ib and succinic acid that had both high antihypoxic activity and antiamnestic and anticonvulsive activity. These drugs could find application in neurology, cardiology, and geriatrics for treating various ischemic pathologies such as injury, infarct, Alzheimer’s disease, Parkinson disease, etc.
A comparison of the pharmacology of 3-hydroxypyridine drugs found that their antihypoxic activity increases in the order emoxypin < mexidol < proxypin < Ia + succinic acid < Ib + succinic acid.
References
M. Fisher and V. Shebitts, Zh. Nevrol. Psikh., 1, 21 – 23 (2001).
Federal Handbook on the Use of Drugs [in Russian], Moscow (2005), Issue 6, p. 316.
R. G. Glushkov, L. N. Dronova, M. D. Mashkovskii, et al., RF Pat. No. 2,095,350; Byull. Izobret., No. 31 (1997)
R. G. Glushkov, L. N. Dronova, L. A. Nikolaeva, et al., USSR Pat. No. 1,587,866 (1990).
Comprehensive Medical Encyclopedia [in Russian], Soviet Encyclopedia, Moscow (1986), pp. 526 – 527.
M. D. Mashkovskii, Drugs [in Russian], Novaya Volna, Moscow (2005), p. 1024.
L. D. Luk??yanova, Vestn. Ross. Akad. Med. Nauk, No. 9, 3 (2000).
M. V. Korablev and P. I. Lukienko, Antihypoxants [in Russian], Minsk, Belarus (1976), p. 40.
Ya. Buresh, O. Bureshova, and D. P. Houston, Methods and Basic Experiments for the Study of the Brain and Behavior [in Russian], Vysshaya Shkola, Moscow (1991).
G. Chen, B. Bohner, and C. Ensor, Proc. Soc. Exp. Biol. (N. Y.), 87, 334 (1954).
G. Everett and R. C. Richard, J. Pharmacol. Exp. Ther., 106, 303 (1952).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 45, No. 3, pp. 3–8, March, 2011.
Rights and permissions
About this article
Cite this article
Glushkov, R.G., Yuzhakov, S.D., Alekseev, M.V. et al. New potential antihypoxants based on 2-ethyl-3-(N,N-dimethylcarbamoyloxy)-6-methylpyridine. Pharm Chem J 45, 131–136 (2011). https://doi.org/10.1007/s11094-011-0575-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-011-0575-1