The ester of thioctic (α-lipoic) acid and 2-ethyl-6-methyl-3-hydroxypyridine was synthesized. The toxicity decreased while the antihypoxic activity simultaneously increased for an equimolar mixture of thioctic acid and 2-ethyl-6-methyl-3-hydroxypyridine after they were combined into 2-ethyl-6-methylpyridinol-3-yl thioctate (laboratory name thioxypine). The tolerance to asphyxia increased by 51%; to hypercapnic hypoxia, by 47% after intraperitoneal (i.p.) administration of thioxypine at a dose of 145 μmol/kg. Such a combination of effects corresponded to the formal criteria for classifying thioxypine as an antihypoxant. A fourfold reduction in the dosage of thioxypine (to 36.25 μmol/kg) reduced the resistance to histotoxic hypoxia by 32%. The synthesized ether did not affect the resistance to hemic hypoxia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Salts of 2-ethyl-6-methyl-3-hydroxypyridine are widely used as drugs with antihypoxic activity [1, 2]. The antihypoxic activity of these drugs is largely determined by their protective and therapeutic potential under extreme conditions and with various diseases. This applies mainly to the cerebral protective effect with disturbances of cerebral blood flow [3] and diabetic damage of the central nervous system (CNS) [4, 5]. The traditionally declared dependence of the antihypoxic potential of 2-ethyl-6-methyl-3-hydroxypyridine salts on their antioxidant activity [1] has not always been confirmed in experimental and clinical studies [6,7,8]. Furthermore, situations in which the corresponding drugs have varied effects on the tolerance to hypoxia of various etiologies are known [7]. This circumstance illustrates the advisability of searching for 2-ethyl-6-methyl-3-hydroxypyridine derivatives with the optimal antihypoxic activity profile. Esterification of 2-ethyl-6-methyl-3-hydroxypyrimidine by thioctic (α-lipoic) acid, which satisfies formal criteria of being an antioxidant, is a promising approach to solving this problem [7]. It is worth adding that the succinate salt of the alcohol part (of 2-ethyl-6-methyl-3-hydroxypyridine) of the proposed ester was considered a standard antihypoxic agent [9].
The present article was dedicated to a consideration of issues with the synthesis of 2-ethyl-6-methylpyridinol-3-yl thioctate, its toxicological evaluation, and the protective activity for acute hypoxia of various etiologies.
Experimental Chemical Part
Target compound 2-ethyl-6-methylpyridinol-3-yl thioctate was synthesized according to the scheme below.
The starting reagents were (R,S)-thioctic (α-lipoic) acid (NuSci, USA; CAS No. 1077-28-7) and 2-ethyl-6-methyl-3- hydroxypyridine, the base of which was obtained from the substance powder methylethylpyridinol hydrochloride (2-ethyl-6-methyl-3-hydroxypyridine hydrochloride; ZAO Obninsk Chemical Pharmaceutical Company, Russia). A saturated aqueous solution of 2-ethyl-6-methyl-3-hydroxypyridine hydrochloride was treated with an equivalent amount of NaOH. The resulting precipitate was filtered off cold, rinsed on the filter with cold H2O, and dried in a desiccator over solid KOH.

Thioctic (α-lipoic) acid (20.62 g, 0.100 mol), triethylenediamine (12.0 g, 0.107 mol), and 1,2-dichloroethane (DCE, 75 mL) were placed into a conical glass flask. The flask was hermetically sealed and tared on a technical balance. Then, the contents of the flask were stirred until the solids were completely dissolved. The flask was placed into an ice bath. The chilled solution in the flask was treated with trifluoroacetic anhydride (TFAA, 22.5 g, 0.107 mol, ~15 mL) that was previously chilled in an ice bath. The TFAA was added in portions of 2 – 3 mL (3 – 4.5 g) at 2 – 3 min intervals. The flask was hermetically sealed after addition of each portion of TFAA and vigorously stirred without allowing local heating. The amount of added TFAA was monitored by weighing the flask with the reaction mixture after addition of each portion of anhydride. When the TFAA addition was complete, the flask with the reaction mixture was placed into a water bath (40 – 43ºC) and held there for 10 – 12 min with periodic stirring. This synthetic step formed the mixed anhydride of thioctic and trifluoroacetic acids.
The flask with the synthesized anhydride was placed into an ice bath. After the solution was chilled, 2-ethyl-6-methyl- 3-hydroxypyridine (13.72 g, 0.100 mol) that was previously ground into a powder and dried in a vacuum desiccator was added to it. The flask with the reaction mixture was placed into a water bath (56 – 60°C) and vigorously stirred until the 2-ethyl-6-methyl-3-hydroxypyridine was completely dissolved. The resulting solution was held for another 20 min at the same temperature, cooled to room temperature, and poured into cold distilled H 2 O (3 – 5°C, 1.0 L). The mixture was vigorously stirred for 5 min and left to stand until the phases separated. The upper (aqueous) phase containing the triethylenediamine salt and trifluoroacetic acid was decanted. The lower phase containing a solution of the target product (2-ethyl-6-methylpyridinol-3-yl thioctate) in DCE was washed (3×) with distilled H2O (1.0 L) at room temperature. The solution of the resulting ester was washed with HCl solution (0.005 M) (2 × 1 L) and H2O (2×, 1.0 L).
The washed solution of the ester was transferred to a rotary evaporator flask. The solvent was removed at 25 – 35°C. The solution could be dried in a stream of air in a fume hood as an alternate approach. In both instances, the yield of target product was ≥85% of theoretical. The obtained product (2-ethyl-6-methylpyridinol-3-yl thioctate) was an oily viscous yellow liquid with a weak specific aroma. The ester could contain insignificant amounts of 2-ethyl-6-methyl-3- hydroxypyridine hydrochloride, thioctic acid, and triethylenediamine. The obtained ester was extracted with refluxing n-hexane to afford a chromatographically homogeneous target product. The target ester was isolated as a bright-yellow oil during cooling of the hexane extract. When the extraction was finished, the cold n-hexane was carefully decanted from the receiving flask. Traces of n-hexane were removed in a rotary evaporator (40 – 45°C). All synthesis and purification steps of the target compound were conducted in a fume hood.
The chemical purity of the obtained compound was evaluated by TLC on Sorbfil plates. Elution by n-heptane(i-PrOH (5:1) followed by detection by I2 vapor gave Rf = 0.61 for the target ester. The purification procedure was repeated if necessary because the product was not pure enough.
The structure of the product was analyzed using NMR, IR, and UV spectroscopy and cryometric determination of the molecular mass.
The PMR spectrum was recorded in CDCl3 solution with residual solvent protons as an internal standard on a Bruker Avance III 500 MHz spectrometer (Germany). The IR spectrum was taken on a Nicolet 380 FT-IR spectrometer (USA). The UV spectrum was obtained on an SF-2000 spectrophotometer (Russia). The molecular mass of the product was determined by a cryometric method using a Beckmann TL-1 differential thermometer (1/100°C, Russia).
PMR spectrum (500.13 MHz, , ppm): 1.27 (t, 3H, C(17)H3 , J 7.6 Hz), 1.61 (m, 2H, C(4)H2 , J 8.3 Hz), 1.78 (m, 2H, C(5)H2 , J 4.0 Hz), 1.87 (m, 2H, C(3)H2 , J 7.3 Hz), 1.97 (sext, 1H, C(7)H2 (2), J 7.3 Hz), 2.52 (quint, 1H, C(7)H2 (1), J 6.2 Hz), 2.57 (s, 3H, C(15)H3), 2.66 (t, 2H, C(2)H2 , J 7.5 Hz), 2.74 (quart, 2H, C(16)H2 , J 7.6 Hz), 3.17 (sext, 1H, C(8)H2 (1), J 5.0), 3.25 (sext, 1H, C(8)H2(2), J 4.7 Hz), 3.64 (quint, 1H, C(6)H, J 6.8 Hz), 7.06 (d, 1H, C(11)H, J 8.2 Hz), 7.28 (d, 1H, C(12)H, J 8.2 Hz).

IR spectrum (v, cm(1, KBr): 1761.4 sec (v C(1)=O), 1228.3 sec, 1153.5 sec, 1123.1 sec br ester band [symm and asymm v C(1)(O(C(13)].
UV spectrum (95% EtOH): λmax 332 nm (S(S(n→σ* transition.
According to cryometric analysis, the molecular mass of the target product dissolved in benzene was 324 ± 3 g/mol with a calculated value of 325.34 g/mol.
2-Ethyl-6-methylpyridinol-3-yl thioctate was a viscous bright-yellow oil liquid that solidified into a glassy transparent solid at low temperatures. The liquid compound had a refractive index n25 – 27 = 1.546 ± 0.001 (RL3 refractometer, Poland), was insoluble in H2O, and gradually hydrolyzed on contact with neutral, alkaline, and acidic solutions. The target compound was poorly soluble in aliphatic hydrocarbons, moderately soluble in benzene, and very soluble in CHCl3, DCE, DMSO, EtOH, i-PrOH, Me2CO, dioxane, and dilute solutions of strong acids (precipitated from solutions as an emulsion at pH > 1.65).
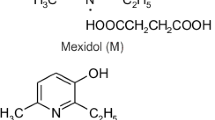
The obtained ester was given the laboratory name thioxypine, consisting of the prefix thio and the root xypine, denoting the acyl and alcohol components, respectively, of the target compound.
Experimental Biological Part
The experimental biological part of the work was organized and conducted in compliance with the principle of minimization of unavoidable distress of laboratory animals based on principles of domestic and international regulations [10, 11]. This part of the research included an acute toxicity study of thioxypine and its influence on the tolerance to acute hypoxia of various etiologies. Animals in all series of the biological part were administered thioxypine and a reference drug as emulsions in a medium consisting of aqueous NaCl solution (9 g/L) and Tween-80, the concentration of which in the toxicological part of the work (10.9 g/L) was substantially greater than the corresponding concentration in experiments evaluating the antihypoxic activity (0.84 g/L). This difference was due to the need to emulsify significantly higher concentrations of thioxypine used in the toxicological part (Table 1) as compared to the concentrations of the ester for evaluating its antihypoxic potential (Table 2). In all cases, the pH of the administered emulsions was within the range 7.2 – 7.6. The volume of administered thioxypine emulsion was 25 mL/kg in experiments with rats. The corresponding value in experiments with mice was 50 mL/kg. The studied doses of thioxypine were calculated from previously calculated mean therapeutic dose equivalents (MTDE = 72.5 μmol/g) of 2-ethyl-6-methyl-3-hydroxypyridine hydrochloride (emoxypine) [5]. Thioxypine was administered at doses equimolar to 4.77 – 25.5 MTDE of emoxypine for rats and 4.47 – 13.06 MTDE for mice in the toxicological studies. Thioxypine was used at doses equimolar to 0.5, 1.0, and 2.0 MTDE of emoxypine for mice in experiments on antihypoxic activity.
The toxicological part of the research was conducted according to recommendations for studying acute toxicity of chemical substances [10, 12, 13]. Acute toxicity of thioxypine was evaluated after a single i.p. injection followed by observation of the animals for four weeks. This part of the work was conducted on female laboratory rodents of two species (Wistar rats, 200 ± 20 g and white outbred mice, 30 ± 3 g). In both instances, the lethality of animals was recorded after administration of thioxypine at seven different doses, each of which was tested in 10 animals. Acute toxicity of a reference compound, i.e., a mixture of 2-ethyl- 6-methyl-3-hydroxypyridine hydrochloride and thioctic acid, administered at doses equimolar to the thioxypine doses was evaluated in parallel. The results were processed by probit analysis according to Finney [14] using an on-line calculator (http://probitsfinney.s3-website.us-east-2.amazonaws.com/). The results were expressed as LD 50 and its standard error (s) and the additionally calculated 95% confidence interval (95% CI) [12, 15]. Statistical comparison of the LD50 of thioxypine and the reference compound used the toxicological version of the t-criterion [12, 15]. A conclusion about the effect of esterification on the toxicity of the mixture of thioxypine components was made only if consistent statistically significant changes of LD50 were established in experiments on mice and rats.
The effect of thioxypine on tolerance to acute hypoxia was studied for hypoxic, hypercapnic, hemic, and histotoxic models of hypoxia. This part of the research was conducted on mice of both sexes. Each experimental group numbering 10 mice included five males and five females. The reference compounds were 2-ethyl-6-methyl-3-hydroxypyridine hydrochloride, thioctic acid, and their equimolar mixture. Thioxypine and the reference compounds at doses equimolar to thioxypine were administered i.p. 30 min before modeling acute hypoxia. Mice of the control group received an equal volume of medium (Tween-80, 0.84 g/L) in aqueous NaCl solution (9 g/L), which was used to emulsify thioxypine and the reference compounds. Acute hypoxic hypoxia was modeled using the asphyxia by drowning test [16]. Hypercapnic hypoxia was reproduced by placing mice into individual hermetically sealed 200-mL glass jars [10]. Hemic hypoxia was induced by a single injection of sodium nitrite at a dose of 200 mg/kg (20 mL/kg of 1% NaNO2 solution, subcutaneous) [10]. Histotoxic hypoxia was induced by a single injection of sodium nitroprusside at a dose of 20 mg/kg (20 mL/kg of a 0.1% solution, i.p.) [17]. In all instances, the criterion for tolerance to acute hypoxia was the time from induction to the death of the animals. Statistical analysis of this part was studied using the SPSS-17.0 applied software suite. Data were processed by descriptive statistics and presented as medians (Me) and the range between the lower (LQ, 25 percentile) and upper quartiles (UQ, 75 percentile). The significance of differences was assessed from the Mann(Whitney U-criterion. A conclusion about the antihypoxic action of the studied compounds was made for a statistically significant increase of the latency of death of mice established in at least two acute hypoxia models [10]. The significance of intergroup differences in lethality was evaluated using the Fisher exact criterion. All parts of the statistical analysis met criteria of the intersection-union test excluding the use of corrections for multiple comparisons [18]. The statistical hypotheses were checked at critical significance level p = 0.05.
Results and Discussion
Deaths of animals were noted only during the first two days from the time of administration of the test compounds in all series of the toxicological part of the research. The results allowed thioxypine to be classified as a marginally toxic compound [13]. This was indicated by the acute toxicity (in units of mg/kg) established in experiments in mice and rats (Table 1).
The LD50 values of thioxypine in both cases fell in the range 101 – 1000 mg/kg, which was characteristic of low toxicity of chemical substances upon i.p. injection [13]. It is noteworthy that an equimolar mixture of thioctic acid and emoxypine demonstrated higher toxicity than their esterification product. This manifested as a statistically significant decrease of the LD50 of the mixture of the free components of thioxypine as compared to the LD50 of their ester derivative. This trend was established in experiments on mice and rats (Table 1). In the first instance, the LD50 of thioxypine (in units of μmol/kg) exceeded the corresponding value of the mixture of its free components by 1.63 times; in the second, by 1.27 times.
The reduction of the toxicity of the mixture of thioctic acid and emoxypine after their esterification was associated with a relative increase of the antihypoxic activity of the synthesized ester. This was most evident in the acute hypoxic hypoxia model (asphyxia by drowning), the tolerance to which increased statistically significantly after administration of thioxypine at moderate (MTDE) and maximal doses (2 MTDE) (Table 2). The antihypoxic activity of thioxypine was observed to increase in a dose-dependent manner and reached the greatest strength (151% of the median of the control) upon using the maximum dose. The mixture of thioctic acid and emoxypine at a dose equimolar to 2 MTDE of thioxypine also caused a statistically significant antihypoxic effect, increasing the latency of death of mice up to 120% of the median control values (Table 2). However, thioxypine at the maximum dose considerably exceeded the mixture of its free components at an equimolar dose (p = 0.037) with respect to antihypoxic activity. Thioctic acid, administration of which at the minimum dose (1/2 MTDE) statistically significantly prolonged the life of mice up to 129% of the median control group, was another reference compound that exhibited a protective effect with hypoxic hypoxia (Table 2). This result agreed partially with previous research results that demonstrated antihypoxic activity of thioctic acid in NaCl solution (0.9%) not only at the minimum but also higher doses (MTDE and 2 MTDE) of the studied range [7].
Apparently, the substantial change of the dose dependence of the antihypoxic activity of thioctic acid could be a consequence of its interaction with the medium [Tween-80 (0.84 g/L) in NaCl solution (0.9%)], which was used to emulsify the tested compounds in the studies. The same applies to emoxypine, which was soluble in NaCl solution (0.9%) and protected the animals under asphyxia conditions [7] but did not exhibit antihypoxic activity when emulsified in a medium containing Tween-80 (Table 2). The antihypoxic activity of the synthesized ester was confirmed in the hypercapnic hypoxia model, the tolerance to which increased up to 143% of the control medium under the influence of thioxypine at the maximum dose (Table 2). None of the reference compounds had a significant effect on the lifespan of mice under hypercapnic conditions (p = 0.29 – 0.88). The increase in the latency of death of mice that was established in the two hypoxia models under the influence of thioxypine allowed it to be classified as a true antihypoxant [10].
The effect of thioxypine on the tolerance to histotoxic hypoxia differed qualitatively from its protector effects with hypoxic and hypercapnic hypoxia. This was manifested in the reduction of the latency of death of mice to 68% of the control median under the influence of thioxypine at a dose of 1/2 MTDE (Table 2). Thioctic acid caused an analogous effect at an equimolar dose. The results were comparable to those of previous research that demonstrated a reduction in the tolerance to histotoxic hypoxia after administration of thioctic acid dissolved in NaCl solution (0.9%) [7]. The alcohol component (emoxypine) diluted in isotonic saline had the same activity at a dose equimolar to 1/2 MTDE of thioxypine [7]. It is possible that the acyl and alcohol components of thioxypine administered at the minimum dose potentiated reductive stress caused by blockage of the distal part of the respiratory chain with histotoxic hypoxia [19]. This effect may have been nullified at higher doses because of concentration inversion of the antioxidant activity of emoxypine and an increase in the pro-oxidant activity of thioctic acid [6].
Reproduction of hemic hypoxia, in contrast to the other models of acute hypoxia, did not always lead to a fatal outcome. The lethality of the animals in the formed groups was recorded within 2 h from the time of NaNO2 administration and varied within 60 – 100% (Table 2). None of the tested compounds had a significant effect on the frequency of death of mice (p = 0.47 – 0.56) or on the lifespan of animals that died (p = 0.19 – 0.93).
In general, the results indicated that the toxicity decreased with a simultaneous increase of antihypoxic activity of an equimolar mixture of thioctic acid and 2-ethyl-6- methyl-3-hydroxypyridine after their combination into the ester 2-ethyl-6-methylpyridinol-3-yl thioctate (thioxypine). Administration (i.p.) of thioxypine at a dose of 145 μmol/kg increased the tolerance to asphyxia by 51% and to hypercapnic hypoxia by 43%. This combination of effects met the formal criteria allowing thioxypine to be classified as a true antihypoxant [10]. A fourfold decrease of the thioxypine dose (to 36.25 μmol/kg) decreased the tolerance to histotoxic hypoxia by 32%. The resulting ester did not affect the tolerance to hemic hypoxia.
References
T. A. Voronina, Zh. Nevrol. Psikhiatr. im. S. S. Korsakova, 112(12), 86 – 90 (2012).
V. E. Novikov and S. O. Losenkova, Obz. Klin. Farmakol. Lek. Ter., 3(1), 2 – 14 (2004).
E. I. Gusev and V. I. Skvortsova, Nervn. Bolezni, No. 1, 3 – 7 (2002).
I. A. Volchegorskii and N. V. Mester, Khin. Med., 85(2), 40 – 45 (2007).
I. A. Volchegorskii, L. M. Rassokhina, and I. Yu. Miroshnichenko, Eksp. Klin. Farmakol., 74(12), 27 – 32 (2011).
I. A. Volchegorskii, L. M. Rassokhina, I. Yu. Miroshnichenko, et al., Byull. Eksp. Biol. Med., 150(9), 295 – 301 (2010).
I. A. Volchegorskii, I. Yu. Miroshnichenko, L. M. Rassokhina, and M. P. Malkin, Ross. Fiziol. Zh. im. I. M. Sechenova, 105(3), 363 – 374 (2019).
I. A. Volchegorskii, M. G. Moskvicheva, and E. N. Chashchina, Klin. Med., 82(11), 31 – 35 (2004).
N. N. Karkishchenko, V. N. Karkishchenko, E. B. Shustov, et al., Biomedical (preclinical) study of antihypoxic activity of drugs. Methodical recommendations, Scientific Center for Biomedical Technologies, Federal Medical and Biological Agency, Moscow (2017).
A. N. Mironov (ed.), Handbook for Preclinical Drug Studies [in Russian], Grif i K, Moscow (2012).
S. Louhimies, Altern. Lab. Anim., 30(2) Suppl., 217 – 219 (2002).
M. L. Belen’kii, Elements of Quantitative Evaluation of Pharmacological Effects [in Russian], Meditsina, Leningrad (1963).
I. V. Berezovskaya, Khim.-farm. Zh., 37(3), 32 – 34 (2003).
D. J. Finney, Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve, Cambridge University Press, Cambridge (1952).
V. B. Prozorovskii, Psikhofarmakol. Biol. Narkol., 7(3 – 4), 2090 – 2120 (2007).
V. I. Kulinskii, I. A. Ol’khovskii, and A. N. Kovalevskii, Byull. Eksp. Biol. Med., 101(6), 669 – 671(1986).
A. I. Kurbanov, N. N. Samoilov, E. N. Stratienko, et al., Psikhofarmakol. Biol. Narkol., 6(1 – 2), 1164 – 1170 (2006).
M. Neuhauser, Fundam. Clin. Pharmacol., 20(6), 515 – 523 (2006).
M. V. Bilenko, Ischemic and Reperfusion Damage of Organs [in Russian], Meditsina, Moscow (1989).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 57, No. 3, pp. 14 – 19, March, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Volchegorskii, I.A., Grobovoi, S.I., Sinitskii, A.I. et al. Synthesis, Toxicological Evaluation, and Antihypoxic Effect of 2-Ethyl-6-Methylpyridinol-3-yl Thioctate. Pharm Chem J 57, 347–352 (2023). https://doi.org/10.1007/s11094-023-02888-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02888-z