Abstract
Eco-friendly plasma technology is appeared as promising for the improvement of crop yield and hence it was applied to study the paddy plant growth and yield. Applications of plasma technology in this study are two-fold: (1) paddy seeds (Oryza sativa L.) were treated with low pressure (100 torr) glow air discharge (LPGAD) plasma for duration of 30, 60, 90, 120 and 150 s for finding the highest germination rate for field application, and (2) plasma activated waters (PAWs) were prepared and applied as foliar spray to the plants grown from the treated seeds to investigate the combined effects on plant growth, yield and total soluble protein and sugar concentrations in the produced paddy grains. Seedlings grown from the 90 s LPGAD plasma treated seeds, depending on the results obtained from seed germination test, were transplanted in the field and PAWs were applied 1–5 times during vegetative growth stage of the paddy plants. The results reveal that (a) the maximum paddy seed germination rate of ~ 7% with respect to control was obtained from 90 s treatment duration, out of five treatment durations, with LPGAD plasma, (b) plants growth parameters were enhanced due to the combined effects of plasma seed treatment along with PAW application, (c) defense mechanisms of plants were improved through enhancement of enzymatic activities, (d) concentrations of total soluble protein and sugar were enhanced in the paddy grains, and (e) finally yield was increased by ~ 16.67%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Food requirement is growing up gradually in order to meet the demand of increasing population growth in highly populated countries mainly in south-east Asian and African countries. Therefore, FAO (sustainable development goal of the United Nations Food and Agricultural Organization) [1] is emphasizing to the improvement of crops yields for the fulfillment of sustainable development goal (SDG). The cultivable land is reducing due to rapid industrialization, urbanization, river erosion, and climate change in south-east Asian regions. Application of plasma technology seems to be promising for the improvement of crop yield that can also lessen the use of harmful chemical pesticides and fertilizers. Enhancement of food production is facing challenges as provided by the reduction of farming area as well as by climate vulnerability due to salinity, flood and draught, mainly in Bangladesh. In spite of flood, draught, salinity and reduction in cropped area, food security in Bangladesh is likely to be addressed by implementing new eco-friendly technology in agriculture.
In recent times, application of plasma technology [2] is fascinating researchers because of its prospective use in agriculture. A plenty of researchers have investigated [3] the applications of plasma technology in agriculture and found that the seed germination rate [4,5,6,7], plant growth rate [8,9,10,11] and crop yield [10, 11] were increased. Ling et al. [12] have studied the effectiveness of seed treatment on stress tolerance by atmospheric pressure cold (APC) plasma and have found improved stress tolerance. Further, Kabir et al. [13] have investigated the application of APC plasmas to stress tolerance of crops to detoxification and noted that the plants grown from APC plasma treated seeds can tolerate more stresses than that of control plants. Furthermore, it has found [14, 15] that the APC plasma treatment disinfects [16] seeds from bacteria and fungi. Plasma technology has applied so far in agriculture in the following ways: (1) direct treatment of seeds, where plasma species interact with the seed coat and subsequently modify the surface morphology of the seed coat [10, 11]; and (2) preparation of plasma activated water (PAW) [17, 18], where different species can be produced in water through plasma-water interactions.
PAWs can be used [19] in the field of agriculture for the enhancement of seed germination rate [9, 13, 20], seed decontamination [21,22,23], plants growth [24] and development [25]. PAWs can also be used in antifungal or antimicrobial activities [26] for biomedical applications [27]. Production of plasma species [28] in the media depend on power supply used, type of discharges, gas used etc. In the field of agriculture, reactive oxygen (ROS) and nitrogen species (RNS) are considered as growth enhancer and stimulator [25] through production of different signaling pathways. On the other hand, the excess amounts ROS may cause cell death through membrane damage by oxidative stress [29, 30] in biological tissues. Generally, the following ROS and RNS (RONS) are produced [31] by air discharge plasmas in water [22]: hydrogen peroxide (\({\text{H}}_{2} {\text{O}}_{2}\)), nitrites (\({\text{NO}}_{2}^{ - }\)), nitrates (\({\text{NO}}_{3}^{ - }\)) and ozone (\({\text{O}}_{3}\)), and short-lived species, such as hydroxyl radical (\(^{ \cdot } {\text{OH}}\)), nitric oxide (\({\text{NO}}^{ \cdot }\)), superoxide (\({\text{O}}_{2}^{ - }\)), peroxynitrate (\({\text{OONO}}_{2}^{ - }\)) and peroxynitrite (\({\text{OONO}}^{ - }\)). Due to the production of different active species, properties of water (\(pH\), electrical conductivity, \(EC\) and concentration of dissolve oxygen, \(DO\)) are likely to be different.
Applications of PAWs were investigated by Takahata et al. [25, 32] on the growth rate of spinach, radish and strawberry and found that the applications PAWs produced the faster growth of spinach and radish, whereas the higher sugar concentration obtained in strawberry. Combined (PAWs were applied to the plants grown from the plasma treated seeds) effects on seed germination and growth of radish, tomato and sweet pepper were studied by Sivachandiran and Khacef [33]. They have noted in their findings that the stem length of the plants was increased by ~ 60% watered with 15 min treated PAW with respect to control, but the higher PAW treatment duration showed negative effects.
Maniruzzaman et al. noted [34] that \({\text{NO}}_{3}^{ - }\) is one of the most essential nutrients delivering composition to the plants among the nitrogen containing varieties. Proteins, amino acids, and chlorophyll along with other cellular components and metabolites contain \({\text{NO}}_{3}^{ - }\) that contribute to the growth and development of the plants. Furthermore, the optimum concentrations of \({\text{H}}_{2} {\text{O}}_{2}\) in PAW create [9, 24, 25, 35] signals in triggering proteins or genes that participates in the growth and development of the plants. In addition, it is also well-known [26, 36] that optimum concentrations of \({\text{H}}_{2} {\text{O}}_{2}\) act as pesticides or fungicides instead of conventional ones.
In this report, it was intended to investigate the effects of (1) low pressure (100 torr) [37] glow air discharge (LPGAD) plasma seed treatment duration on paddy seed germination rate, and (2) application of PAWs as foliar spray [38] on paddy plants (grown from both the untreated and plasma treated seeds) growth and yield in field condition. So far to the best of our knowledge, this is the first report where the combined, foliar spray of PAWs were applied to the paddy plants grown from the plasma treated seed, effects on plant growth rate, yield of paddy and a few nutritional quality of paddy grains are studied in field condition. Materials and methods are discussed in “Materials and methods” section, wherein, seed treatment reactor, preparation of PAW, measurement of PAW properties, field experiment, determination of seed germination rate, plant growth and yield parameters, estimation of antioxidant enzymes in plant tissues, total soluble protein and sugar concentrations in plant tissues and paddy grains, and statistical analyses are furnished. Results and discussion are presented in “Results" and “Discussion” sections, respectively and conclusion is drawn in “Conclusion” section.
Materials and Methods
Paddy seeds (Oryza sativa L., cv. Variety BRRIdhan 28), were collected from the Regional Rice Research Institute, Shampur, Rajshahi. A brief description concerning the paddy seed treatment reactor and the preparation PAW will be provided below.
Seed Treatment Reactor
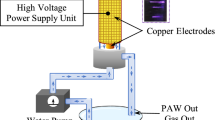
The paddy seeds were treated in a low pressure (100 Torr) glow air discharge LPGAD plasma reactor. The LPGAD seed treatment reactor, as shown schematically in Fig. 1a, was made of a pyrex glass pipe (length 9 cm, diameter 4.5 cm). One end of the glass pipe was sealed with a stainless steel (SS) circular disk and used as power electrode, while the other end was covered with another SS circular disk that can move axially and be opened during filling up the reactor with seeds to be treated, and used as grounded electrode. A servo-motor was installed to rotate the reactor horizontally in order to rummage the seeds for uniform seed coat treatment. The reactor along with the servo-motor was then placed inside a bell jar and a rotary pump was used for reducing inside pressure of the bell jar. The inside pressure of the bell jar was maintained at 100 Torr during seed treatments. A sinusoidal bipolar variable power supply (variable voltage range: 1–10 kV, variable frequency range: 0.5–10 kHz) was used for the production of GAD plasma. Figure 1b shows the photograph of the reactor filled with paddy seeds under treatment. Voltage-current (V–I) characteristics were measured with a digital oscilloscope (Rigol Technologies, Model: DS1104Z Plus) in combination with a high voltage probe and current probe. The V–I characteristics, under seed treatment condition, are presented in Fig. 1c. The absorbed power was ~ 9 W of the LPGAD plasma determined by integrating the discharge voltage and current over a period. Figure 1d shows the optical emission spectra (OES) captured by two spectrophotometers (Ocean Optics: USB 2000 + XR1: resolution 1.7 nm, slit width 25 μm, grating 500 lines/mm and wavelength range 200–1100 nm and AvaSpec-2048, slit: 10 μm, grating: 2400 lines/mm, resolution 0.07 nm and wavelength range: 300–500 nm). The major electronic transitions observed in the OES are: nitrogen second positive system \({\text{N}}_{2} \left( {C^{3} \Pi_{u} - B^{3} \Pi_{g} } \right)\) in the range 294–380 nm, first negative system \({\text{N}}_{2}^{ + } \left( {B^{2} \sum_{u}^{ + } - X^{2} \sum_{g}^{ + } } \right)\) in the range 391–405 nm, \({\text{N}}^{ + }\) at 517.52 nm and 755.905 nm are observed and they are considered as RNS, whereas the \({\text{OH}}\left( {A^{2} \sum^{ + } \left( {v^{\prime\prime} = 0} \right) \to X^{2} \Pi \left( {v^{\prime} = 0} \right)} \right)\) band transition at 305–320 nm and \({\text{O}}\left( {4s(^{3} D} \right)4d \to 4p(^{3} P^{0} ))\) at 777 nm are visible and they are considered as ROS. The rotational temperature, is considered as gas temperature, was determined [31] by fitting the first negative system \({\text{N}}_{2}^{ + } \left( {B^{2} \sum_{u}^{ + } - X^{2} \sum_{g}^{ + } } \right)\) using LIFBASE software [39] and it was ~ 335 K.
a Schematic of the low pressure (100 torr) glow air discharge (LPGAD) plasma production setup for the treatment of paddy seeds, b image of the seed treatment reactor under treatment of seeds, c voltage-current characteristics of the LPGAD plasma seed treatment reactor measured under loaded with seeds, and d optical emission spectrum (OES) acquired from the LPGAD plasma seed treatment reactor under loaded with seeds
Preparation Plasma Activated Water
Detail construction of the water treatment reactor for the preparation of PAW has been communicated [40]. A plasma jet was used for the preparation of PAW. A tungsten wire was inserted into the jet tube and used as power electrode. A grounded wire was dipped into the water. The plasma jet was submerged into water. A volume of 600 ml pyrex glass bottle was used as water container. A 1–10kV, 1–10kHz monopolar pulsed variable power supply was used for operating the air discharge plasma jet. Air was used as working gas for the production of underwater plasma jet. Air flow within the jet was controlled with a gas flow controller. Air was flown at a rate of 1 lpm into the jet. 250 ml distilled water was treated at a time for the treatment duration of 10 min. Maximum PAW treatment duration was selected depending on the results obtained by Sivachandiran and Khacef [33] in order to avoid negative impacts on paddy plants growth and development. The amount of power absorbed by the atmospheric pressure underwater air discharge plasma was ~ 16 W.
Test of Water Imbibition, Germination and Surface Morphology
The paddy seeds treated with LPGAD plasmas under different durations were immersed in tap water for the maximum period of 24 h in different petri dishes. Weights of the seeds were registered at 1, 2, 4, 8, 12, 16 and 24 h. The water imbibition (\(w_{a}\)) of wet seeds were calculated using the following formula [11, 41, 42]
where \(w_{0}\) and \(w_{1}\) are the weights of dry and wet seeds, respectively. The result of water imbibition of the seeds is presented in Fig. 2a.
Effects of low pressure (100 Torr) glow air discharge (LPGAD) plasma treatment of paddy seeds on a wettability and b germination percentage. The mean of the three replications and capped lines indicate the standard error. Bars indicate standard errors of three replications. Letters represent statistically significant differences (\(p < 0.05\))
For the determination of seed germination rate, paddy seeds (\(3\,replications\) \(\times\,5\,treatment\,durations\) \( \times\,100\,seeds = 1500\)) were selected randomly and divided into six groups (five treatment duration and control) following the instructions [43] of the International Seed Testing Association 2018 (\(ISTA\)). Five groups of seeds were treated with LPGAD plasmas in the seed treatment reactor for the treatment durations of 30, 60, 90, 120 and 150 s at an internal reactor pressure of 100 torr. The treated seeds were kept in separate petri dishes, as per treatment conditions, covering with double-layer moistened filter papers and the petri dishes were incubated in a dark incubator for the period of \(3 d\). Temperature and relative humidity were maintained at 25 °C and 75%, respectively, inside the incubator. The filter papers were moistened daily with tap water in order to provide sufficient water as required by the seeds for germination. The seeds germinated were counted at 12 h duration which had radicle length half of the seed length. The rate of germination (\(g_{r}\)) is estimated [44] employing the following equation
where \(N_{3d}\) and \(N_{t}\) are the number of seeds germinated within 3 days and the total number of seeds considered for germination test, respectively. The results of germination percentage of paddy seeds are presented in Fig. 2b. The surface morphology of the untreated and treated paddy seeds were investigated by taking scanning electron microscopic [SEM] picture as shown in Fig. 3a–d.
Estimation of PAW Properties (pH, O3, H2O2, \({\text{NO}}_{2}^{ - }\) and \({\text{NO}}_{3}^{ - }\))
pH and the concentrations of O3, H2O2, \({\text{NO}}_{2}^{ - }\) and \({\text{NO}}_{3}^{ - }\) of the 10 min treated distilled water were measured. Distilled waters were treated with atmospheric pressure underwater air discharge plasma jet. \(pH\) was measured using a pH meter (Model: HI 2002–02, \(pH\) range: \(- 2.00\) to \(16.00\), resolution: 0.01, accuracy: \(\pm 0.01\), Hanna Instrument, USA). \({\text{O}}_{3}\) (Test kit model: HI93757-0, Hanna Instrument, USA), \({\text{H}}_{2} {\text{O}}_{2}\) (Test kit model: HI3844-0, Hanna Instrument, USA), \({\text{NO}}_{2}^{ - }\) (Test kit model: HI3873-0, Hanna Instrument, USA) and \({\text{NO}}_{3}^{ - }\) (Test kit model: HI3874-0, Hanna Instrument, USA) were used for the development of colors. The absorbances of O3, H2O2, \({\text{NO}}_{2}^{ - }\) and \({\text{NO}}_{3}^{ - }\) were measured (following the instructions provided by Hanna Instrument, USA) at 352 nm, 390 nm, 548 nm and 527.2 nm, respectively using a UV–Vis spectrophotometer (Model: UV-1900i, Shimadzu Corporation, Japan) and their corresponding concentrations were determined and are presented in Table 1.
Field Experiment
Depending on the performance of the paddy seed germination test, 90 s seed treatment duration was selected for field investigation. The 90 s treated paddy seeds were immersed in tap water for 24 h. The seeds were withdrawn from water and enclosed with gunny bags. The seeds were began germinating after 48 h and were sown after 72 h in the previously prepared muddled nursery bed. After 35 days of sowing, the seedlings were uprooted and then transplanted in the well muddled plots according to the experimental design.
The field experiment was carried out from the mid-February to May 2020 in a plot of the Agricultural Project area of the University of Rajshahi, Bangladesh. Subplots were organized following the randomized complete block (RCB) method to accomplish the experiment. Firstly, seedlings, grown from the untreated and plasma treated seeds, were transplanted in different subplots. It is to be mentioned that the standard amount (as per instruction of the Bangladesh Rice Research Institute, BRRI) of urea (90 g) urea, triple super phosphate (24 g) and murate of potash (18 g) were provided to the subplot of area 4 m2 each. Secondly, one to five times of foliar spray of PAWs (500 ml was applied at a time to each subplot) were applied to different subplots depending on the experimental conditions. Urea was applied thrice: 50% was applied during the preparation of muddled field, 25% was applied at 20 DAT, and the rest 25% was applied at 50 DAT. PAWs were sprayed within the vegetative growth stage between 20-50 DAT.
Measurement of Plant Growth and Yield Parameters
The roots and shoots of the plants were collected at 70 DAT from the study field and washed with tap water. Their lengths were measured with a digital slide caliper and the data were recorded. Dry weights of roots and shoots were taken with an analytical balance (Shinko Denshi Co. Ltd, Japan) after drying for 72 h at 70 °C. For the determination of chlorophyll concentration, fresh leaves were collected (at 70 DAT) randomly, weighted and grounded with a mortar-pestle in 90% methanol, and the homogenized mixture was then centrifuged at 5000 rpm for the period of 5 min. The absorbances of the collected supernatants were measured at 666 nm and 653 nm, for chlorophyll a and b respectively, using a spectrophotometer (-1900i, Shimadzu Corporation, Japan) and the chlorophyll concentrations were estimated from the absorbance data using standard methods [45]. Total carotene concentration was estimated by measuring the absorbances at 666 nm, 653 nm and 470 nm using the method described in [46, 47]. Yield contributing parameters, length of panicle, number of grain per panicle were measured from the randomly collected panicles and finally yield was estimated.
Concentration Measurements of Antioxidant Enzymes in Plant Tissues
Concentrations of antioxidant catalase (\(CAT\)), superoxide dismutase (\(SOD\)) and ascorbate peroxidase (\(APX\)) were estimated in the tissues of roots and leaves of the paddy plants. Methods concerning the determinations of their concentrations will be mentioned briefly. 100 mg tissue (collected at 70 DAT) from the plants [48, 49] was grounded using a mortar-pestle with 5 ml of phosphate buffer (100 mM, pH 7.0). The mixture was homogenized and centrifuged at 8000 rpm for the duration of 10 min. The supernatant was collected in several eppendrof tubes. For the estimation of \(CAT\) activity, 1.5 ml reaction mixture was prepared by mixing with 100 μl tissue extract, 400 μl H2O2 (6%) and 100 mM potassium phosphate buffer (pH7.0). The same spectrophotometer was used to measure the optical density at 240 nm of the reaction mixture for 1 min at an interval of 30 s. Concentration of \(CAT\) was estimated from the recorded data (extinction coefficient 0.03 mM−1 cm−1).
To estimate [50] the concentration of \(SOD\), plant tissue extract of 100 μl was mixed with 0.1 mM ethylene di-amine tetra acetic acid (EDTA), 50 mM sodium carbonate/bicarbonate buffer (\(pH 7.0\)) and 0.6 mM epinephrine enzyme. Data regarding the formation of adrenochrome for \(4 min\) was registered with the spectrophotometer at 475 nm and thereby the concentration of \(SOD\) was estimated per unit activity of \(SOD\) in which the amount of enzyme required for the inhibition of 50% epinephrine oxidation.
In the estimation of \(APX\) activity, reaction mixture was prepared [51] by taking 0.10 mM EDTA, 0.50 mM ascorbic acid, 50 mM potassium phosphate buffer (\(pH 7.0\)), 0.10 mM H2O2 and 0.10 ml tissue extract. The optical absorbance of the reaction mixture was measured at 290 nm using the spectrophotometer. The \(APX\) activity was calculated incorporating the extinction coefficient (2.80 mM−1 cm−1) depending on absorbance.
Estimation of Total Soluble Protein and Sugar in Plant Tissues and Paddy Grains
Concentrations of total soluble protein (\(TSP\)) and total soluble sugar (\(TSS\)) in roots, leaves and paddy grains were determined [52] employing spectroscopic methods. In brief, the collected (at 70 DAT) fresh leaves and roots of paddy plants were washed, weighed and grounded in a ice-cold mortar-pestle in combination with assay buffer containing 50 mM Tris–HCl (pH 7.5), 2 mM EDTA and \(0.04\% \left( {v/v} \right)\) 2-mercaptoethanol for isolation of protein. The homogenized mixture was centrifuged for 10 min at 7000 rpm at a temperature of 25 °C. The extracted supernatant was mixed with 1 ml coomassie brilliant blue (CBB) in glass cuvettes. The optical absorbance of solution was measured with a spectrophotometer at 595 nm and the data were recorded. The concentration of \(TSP\) was estimated comparing the recorded data with the standard curve of bovine serum albumin (BSA). Same procedure was employed for the determination of \(TSP\) in grains.
\(TSS\) in leaves, roots and paddy grains were estimated [53] by spectroscopic method. Homogenized solutions were prepared from the roots, leaves and paddy grains using hot aqueous ethanol (\(v/v80\%\)) and centrifuged at 5000 rpm for the period of 5 min, and then collected in glass test tube. A hot water bath was used for incubation for 8 min and kept on ice. Optical absorbance at 620 nm was recorded from the ice-cold samples and compared with the standard curve of BSA in order to estimate the concentration of \(TSS\).
Statistical analyses
Three independent replications were considered for all investigations. Each group of data was analyzed statistically for the level of significance at \(p \le 0.05\) by one-way ANOVA. The analyses were carried out under Duncan’s Multiple Test Range (DMRT) using SPSS statistics 20 software. The figures presented in this article were prepared using Microcal Origin 6.0 software.
Results
Results obtained in this experiment will be provided in the following subsections.
Effects of Plasma Seed Treatment on Water Imbibition and Germination Rate
Figure 2a shows the effects of seed treatments with LPGAD plasma on wettability and germination rate. The water imbibition results show that the amount of water absorption was increased with the increase of seed treatment durations. The germination rate was increased with treatment duration up to 90 s, while it is decreased above 120 s. 90 s treatment duration showed the best result which is ~ 7% higher with respect to untreated seeds. The SEM (Zeiss EVO-18, USA) images, as shown in Fig. 3, reveal that a significant number of trichomes (hair-like structure) from the seed coat are reduced due to plasma etching but not completely removed. The husks contain lemma and lines of tubercules that are partially modified due to plasma treatments. The roughness of the seed coat is also reduced by plasma treatment.
Effects of Plasma Seed Treatment and PAW Foliar Spray on Plant Growth
Figure 4a–d show the combined effects of LPGAD plasma paddy seed treatment as well as foliar spray of PAWs on plant height (PH), stem diameter (SD), dry weight (DW), total chlorophyll and total carotene (TC) concentrations, respectively. It is observed from Fig. 4a that the plants grown from the untreated (control) seeds, plasma treated seeds (\(P + W_{0}\)) and plasma treated seeds along with five times PAW foliar spray (\(P + W_{5}\)) are produced the plant heights of 37.16, 38.49 and 43.74 cm, respectively, measured at 30 DAT. The plants heights are found to increase accordingly at 50 and 70 DAT. The highest plant heights are produced by \(P + W_{5}\) with respect to other treatment conditions. The maximum stem diameters, shown in Fig. 4b, of 7.25, 9.22 and 10.62 mm, respectively, are produced by \(P + W_{5}\) measured at 30, 50 and 70 DAT. Similarly, the maximum dry weights, shown in Fig. 4c, of 7.74, 10.59 and 14.92 g, respectively, are obtained by \(P + W_{5}\) measured at 30, 50 and 70 DAT. On the other hand, the maximum total chlorophyll and total carotene concentrations, shown in Fig. 4d, of 4.89 and ~ 110 mg g−1, respectively, are produced by \(P + W_{5}\) measured at 70 DAT.
Effects of low pressure (100 torr) glow air discharge (LPGAD) plasma paddy seed treatment and foliar spray of plasma activated water (PAW) on plant a height (PH), b stem diameter (SD), c dry weight (DW) (measured at 30, 50 and 70 days after transplant (DAT)), and d chlorophyll and carotene concentrations (measured at 70 DAT). (\(P\) and \(W_{x}\), represent plasma treated seeds, and \(x\) times of PAWs were applied, respectively). Error bars indicate standard errors of three replications. Letters represent statistically significant differences (\(p < 0.05\))
Effects of Plasma Seed Treatment and PAW Foliar Spray on Plant Enzymatic Activities
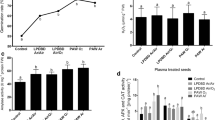
Figure 5a–c display the combined effects of LPGAD plasma seed treatment and the foliar spray of PAW on antioxidant enzymes APX, CAT and SOD, respectively, extracted (at 70 DAT) from the plants grown under treatment conditions considered herein. It is seen from Fig. 5a that the APX concentrations \(19.70\) and \(16.76\;{\text{nmol}}\;{\text{min}}^{ - 1} \left( {{\text{mg}}\;{\text{protein}}} \right)^{ - 1}\) in untreated, and \(17.62\) and \(20.00\;{\text{nmol}}\;{\text{min}}^{ - 1} \left( {{\text{mg}}\;{\text{protein}}} \right)^{ - 1}\) in \(P + W_{5}\) treated plants are found in the roots and leaves, respectively. APX concentrations are found maximum in the roots of the plants grown from the untreated seeds, whereas it is seen the maximum in the leaves of the plants grown from the \(P + W_{5}\) treated plants. The CAT concentrations \(30.55\) and \(9.72\) \({\text{nmol}}\;{\text{min}}^{ - 1} \left( {{\text{mg}}\;{\text{protein}}} \right)^{ - 1}\) are found, as can be seen from Fig. 5b, in the roots and leaves, respectively, of the plants grown from the untreated seeds which is the maximum; whereas, it is the minimum 11.11 and \(1.39\) \({\text{nmol}}\;{\text{min}}^{ - 1} \left( {{\text{mg}}\;{\text{protein}}} \right)^{ - 1}\) in the roots and leaves, respectively, of the \(P + W_{5}\) treated plants. The concentrations in SOD are found the maximum \(24.20\) and \(11.55\) \({\text{nmol}}\;{\text{min}}^{ - 1} \left( {{\text{mg}}\;{\text{protein}}} \right)^{ - 1}\) in the roots and leaves as shown in Fig. 5c, respectively, in the plants grown from the untreated seeds. Besides, the maximum SOD concentrations \(35.75\) and \(26.95\) \({\text{nmol}}\;{\text{min}}^{ - 1} \left( {{\text{mg}}\;{\text{protein}}} \right)^{ - 1}\) are produced in the roots and leaves, respectively, of the plants grown from the untreated seeds (control), whereas it is \(24.20\) and \(11.55\) \({\text{nmol}}\;{\text{min}}^{ - 1} \left( {{\text{mg}}\;{\text{protein}}} \right)^{ - 1}\) are found minimum in the roots and leaves, respectively, of the plants grown from the \(P + W_{5}\) treated seeds.
Effects of low pressure(100 torr) glow air discharge (LPGAD) plasma treatments of paddy seed and applications of plasma activated waters (PAWs) as foliar spray on the concentrations of antioxidant enzymes: a ascorbate peroxidase (APX), b catalase (CAT) and c superoxide dismutase (SOD) of roots and leaves of the rice plants (measured at 70 DAT). (\(P\), and \(W_{x}\), represent plasma treated seeds and \(x\) times of PAWs were applied, respectively). Bars indicate standard errors of three replications. Letters represent statistically significant differences (\(p < 0.05\))
Effects Plasma Seed Treatment and PAW Foliar Spray on TSP and TSS
Figure 6a, b show the effects of LPGAD plasma paddy seed treatment and foliar spray of PAWs on TSS and TSP in roots, leaves (collected at 70 DAT) and grains, respectively. In roots, the maximum and minimum TSS concentrations \(234\) and \(152\) mg g−1 FW are found from the untreated and \(P + W_{5}\) treated plants, respectively, as can be seen from Fig. 6a. But in case of leaves, the maximum and minimum concentrations \(363\) and \(213\) mg g−1 FW of TSS, respectively, are found from \(P + W_{5}\) treated and untreated plants. While in case of grains, the maximum and minimum concentrations of it \(240\) and \(201\) mg g−1 FW are produced in the plants grown from the \(P + W_{5}\) treated and untreated plants, respectively. Therefore, the concentrations of TSS are increasing in leaves and grains with the increase of PAW applications. On the other hand, it is seen from Fig. 6b that the maximum and minimum TSP concentrations of \(312\) and \(218\) mg g−1 FW are produced in the roots grown from the untreated and \(P + W_{5}\) treated plants, respectively. That is, TSP concentration in roots is decreasing with increasing PAW application. But in case of leaves, the maximum and minimum concentrations of \(335\) and \(206\) mg g−1 FW of TSP are found grown from \(P + W_{5}\) treated and untreated plants, respectively. While in case of grains, the maximum and minimum concentrations of it \(462\) and \(291\) mg g−1 FW are produced grown from \(P + W_{5}\) and untreated plants, respectively. Like TSS, the concentrations of TSP are also increasing in leaves and grains with increasing the application of PAW foliar spray.
Effects of low pressure(100 torr) glow air discharge (LPGAD) plasma treatment of paddy seeds and the applications of plasma activated water (PAW) as foliar spray on the concentrations of a total soluble sugar (TSS) in roots and leaves of rice plants measured at 70 DAT and in grains, and b total soluble protein (TSP) and (\(P\), and \(W_{x}\), represent plasma treated seeds, and \(x\) times of PAWs were applied, respectively). Bars indicate standard errors of three replications. Letters represent statistically significant differences (\(p < 0.05\))
Effects of Plasma Seed Treatment and PAW Foliar Spray on Yield
Figure 7a, b represent the effects of plasma seed treatment and foliar spray of PAWs on yield related characters and yield, respectively. Figure 7a shows the length of panicle and the number of grains per panicle. This figure reveals that the maximum and minimum, panicle length of ~ 24.00 and 20.50 cm, and number of grains per panicle of 124 and 90, are produced grown from \(P + W_{5}\) and untreated plants, respectively. Whereas, Fig. 7b shows the 1000-grain weight and yield of paddy. The maximum and minimum 1000-grain weights of 22.18 and 18.43 g, respectively, are obtained from \(P + W_{5}\) and untreated plants, respectively. But the maximum and minimum yields of 7.78 and 6.17 MT ha−1, respectively, are found from \(P + W_{5}\) and untreated plants.
Effects of low pressure(100 torr) glow air discharge (LPGAD) plasma treatment of paddy seeds and applications of plasma activated water (PAW) as foliar spry on a length of panicle (PL) and grain per panicle (GPP), and b 1000-grain weight (GW) and yield of rice (\(P\), and \(W_{x}\), represent plasma treated seeds, and \(x\) times of PAWs were applied, respectively). Error bars indicate standard errors of three replications. Letters represent statistically significant differences (\(p < 0.05\))
Discussion
The combined roles of LPGAD plasma paddy seed treatments along with the application of foliar spray [54, 55] of PAWs are investigated to reveal the new insights pertaining to the improvement of seed germination rate, plant growth rate, yield contributing parameters and yield. Discussion on the results obtained of this experiment will be provided below.
The plasma species produced (Fig. 1d) in LPGAD plasmas within the seed treatment reactor interact with seed coat (husk) and partially the roughness as well as thickness of the seed coat are reduced by plasma etching. Besides, the micropyles of the seed coat become widen due to plasma etching. The SEM images (Fig. 3) reveal that a significant number of trichomes (hair-like structure) from the seed coat are reduced due to plasma treatments. The husks contain lemma and lines of tubercules are modified to some extent due to plasma treatments. The water imbibition results show (Fig. 2a) that the amount of water absorption is increased with the increase of seed treatment durations. Water imbibitions of seeds are complex mechanism, however, the increased water imbibition of paddy seeds due to plasma treatment may be attributed by the phenomena [56, 57] (1) adsorption, (2) diffusion and (3) trapping of RONS to the seed coat as they produced (Fig. 1d) in the air plasmas. RONS can be adsorbed on surface of the seed coat, be diffused through the comparatively thin seed coat and be trapped within lemmas, tubercules and trichomes. Therefore, it is reasonable to expect higher RONS concentrations in the seeds due to plasma treatments. Seeds with high RNS concentrations absorb water faster [44] than the seeds with lower RNS concentrations. Therefore, one can infer that the water imbibitions can be increased due to adsorption, diffusion and trapping of RONS in the seed coat. Water plays an essential role for the initiation of paddy seed germination. High RNS content [44] in the seeds plays roles in the enhancement of seed germination rate. Thus, one may draw conclusion taking into account the surface morphology of seed coat, enhanced RNS content in the seeds or seed coat and water imbibitions of seeds that the paddy seed treatment by plasma can cause higher water imbibitions as well as higher seed germination rate.
The growth parameters PH, SD, DW as well as total chlorophyll and carotene concentrations of plants are investigated (Fig. 4). The first three plant growth parameters reveal the significance of plasma seed treatments and application of PAWs. PH, SD and DW are produced higher by \(\sim16\%\), \(\sim17\%\) and \(\sim19\%\), respectively, of the plants where the combined \(P + W_{x}\) treatments (LPGAD plasma seed treatments and application of PAWs) were applied with respect to the control plants. Total chlorophyll and total carotene concentrations are also produced higher by \(\sim48\%\) and \(\sim114\%\) where \(P + W_{5}\) were applied as compared to control. The growth parameters are continuously increased with the increased application of PAWs. The enhanced plant growth can be ascribed as follows. Firstly, the plant growth parameters are enhanced due to the LPGAD plasma seed treatment. Because, RNS can be incorporated through adsorption, diffusion and trapping [56] in the paddy seed during plasma seed treatments. As a consequence, RNS content in the seeds can be increased. Secondly, PAWs contain a significantly higher concentrations (Table 1) of RNS (\({\text{NO}}_{2}^{ - }\), \({\text{NO}}_{3}^{ - }\)) with respect to ROS (\({\text{H}}_{2} {\text{O}}_{2} , {\text{O}}_{3}\)). These high concentrations of RNS can likely be transferred from PAW to plants. Therefore, one may infer from the above facts that these higher concentrations of RNS can contribute [44] to the enhanced plant growth. Because, the nitrogen related radicals (\({\text{NO}}_{2}^{ - }\), \({\text{NO}}_{3}^{ - }\)) absorbs as amino acid in the roots and leaves through enzymatic processes. Therefore, \({\text{NO}}_{3}^{ - }\) plays crucial roles for biochemical and physiological processes through regulating signals for plant metabolism and developments [34]. On the other hand, there are no negative impacts are found in the plants growth and development parameters by the applications PAWs due to containing the properties of PAWs as noted in Table 1. This result also indicates that probably the paddy plants be capable of accepting higher concentrations of \({\text{NO}}_{2}^{ - }\) and \({\text{NO}}_{3}^{ - }\) to that of mentioned in Table 1 that may produce higher plants growth parameters.
Antioxidant enzymes [58, 59] provide defense against environmental stresses to plants. The oxidative stress can be produced through biotic and abiotic stresses. Production of ROS (mainly H2O2) can be increased in the plant cells during both biotic and abiotic stresses, but the excess concentrations of ROS can cause oxidative damage of the cells. On the other hand, foliar spray of PAWs is also responsible for the increased H2O2 concentration in leaves. The enzyme APX plays [60] key role as catalyzer to detoxify ROS through conversion of H2O2 into H2O using ascorbate as electron donor [61]. One can see from Fig. 5a that the concentrations of APX are decreasing in the roots, while it is increasing in the leaves, with increasing the number of PAW foliar spray. It is to be mentioned that the PAWs were applied during the vegetative stages (from 20 to 50 DAT) of paddy plant growth cycle [62]. The enhanced concentration of APX in the leaves may occur to mitigate the excess ROS because of enhanced application PAWs as it contains (Table 1) certain amount of ROS along with the much higher concentrations of RNS. Plants need more nitrogen supply during their vegetative stage for growth and development. PAWs, contain RNS in the form of \({\text{NO}}_{2}^{ - }\) and \({\text{NO}}_{3}^{ - }\), able to supply more nitrogen through foliar spray to plants. Therefore, it is reasonable to consider that the concentrations of photosynthetic pigments (total chlorophyll and carotene, Fig. 4d) and APX (in leaves, Fig. 5a) are increased through enhanced applications of PAWs to the plants.
One of the most pertinent enzymes is the CAT accountable [63,64,65] for seed germination in the early stage [61] and afterward peroxidase plays supplementary important function. The CAT activities are decreased (Fig. 5b) both in roots and leaves with increasing PAWs applications. This result is consistent with the findings of Kucerova et al. [62]. The higher concentration of CAT is present in the control plants with respect to that of PAW treated plants, where deficiency of nitrogen was more prominent, i.e. in the control plants. It is to be mentioned that the mechanisms [66] of conversion of H2O2 within the plant cells occurs in different ways. APX isoforms as prevailed in chloroplasts, mitochondria, peroxisome and cytosol. H2O2 converts into H2O using ascorbate as donor of particular electron. Whereas, CAT, occurs in peroxisomes [67], catalyzes through dismutation reaction without using any reductant.
SOD is also reducing both in roots and leaves with the increased (Fig. 5c) applications of PAWs. SOD performs as the key mediator that offers defense against oxidative stress produced by ROS in the plants. Besides, SOD serves as metalloenzyme that dismutases superoxide radicals through conversion of H2O2 and oxygen. The SOD activities are found higher in roots than those found in leaves. This result is consistent with the findings of Kucerova et al. [62]. Both CAT and SOD are decreasing may be due to the presence of higher concentrations of nitrate and nitrite through increased applications of PAWs with respect to control. The similar results have also been devised byManiruzzaman et al. [34]. This result is likely to indicate that the higher concentrations of RNS produce better defense against oxidative stresses.
Concentration of TSS is slightly decreased in the roots while it is sharply increased (Fig. 6a) in the leaves with the enhanced application of PAWs compared to the control plants. TSS provides [68] adaptive response against stresses that include draught, pathogen attack, low temperature, anoxic injury and surplus excitation energy. The level of exogenous sugar concentration is increased to a maximum by \(P + W_{5}\) treatment with respect to control. On the contrary, leaves carry higher TSS concentration compared to roots. It is interesting to note that the highest TSS concentration is produced in the paddy grains by \(P + W_{5}\) treatment where minimum urea was used. 2009_Ozaki et al. [68] have studied the effects of H2O2 on the enrichment of sugar content in melon. They noted that the 20 mM H2O2 concentration was most effective for the highest TSS concentration in the melon leaves and TSS is then translocated from leaves to fruits. Therefore, depending on this finding one may consider that the concentration of TSS is increased in the rice grains due to the enhanced applications of PAWs as it contains certain amount of H2O2. The bulk reserves [69, 70] of paddy grains are considered to contain carbohydrates, protein and lipids. Nitrogen rich grains, due to plasma seed treatment, can be correlated [71, 72] with the content of free amino acid instead of protein. As a result, concentration of amino acid can likely be increased in the grains from the nitrogen enriched plants.
The maximum concentrations of TSP [73] are generated in roots, leaves and grains in the plants where both plasma seed treatment along with maximum PAWs were applied (Fig. 6b) with respect to the control plants. Concentration of TSP is decreased in the roots while it is increased in the leaves with increased application of PAWs. Comparison between the control and \(P + W_{5}\) treated plants for the concentration TSP reveals that the plasma seed treatment and PAW application become effective in the generation of higher TSP concentration with respect to control. Since, nitrogen is one of the most necessary elements for the production of protein. On the other hand, \({\text{NO}}_{3}^{ - }\) is one of the key varieties of nitrogen which the plants can assimilate. Most probably, the high concentration of TSP in leaves is transconducted to grains during grain filling stage of plants. Tang et al. [74] have studied the consequences of externally introduced sucrose on rice plants because sucrose converts into starch catalyzed by a series of enzymes in the rice grain filling process. Sucrose synthase [75] is assumed as a key enzyme that catalyzes the conversion of sucrose into starch. They have found that the higher concentration of sucrose in paddy plants produced higher grain filling rates, sucrose and protein concentrations and starch accumulation. Therefore, one may conclude that the increased PAW application causes enhancement of sucrose and subsequently rice grains become enriched with higher concentrations of sugar and protein.
The maximum panicle length and grain/panicle are produced by \(P + W_{5}\) treatment. This can likely be occurred due to adequate nitrogen content in the seeds incorporated through plasma seed treatment and by PAW application. There is a great scarcity of data regarding yield of paddy through plasma technology. However, our previous results [10, 11] regarding the yield of wheat using plasma technology can be considered here. In those wheat experiments, 18–20% increased yield was obtained. But in case of paddy, the yield of paddy is increased by ~ 16.67% where \(P + W_{5}\) treatment was applied with respect to control.
Conclusion
The combined effects of seed treatment on germination rate and water imbibition by LPGD air plasma and foliar spray of PAWs on plant growth and yield of paddy have been investigated. Water imbibition of the paddy seed was improved due to treatment of seeds with low pressure GD air plasma. Seed germination rate was found to increase because of the enhancement of nitrogen contents in the seeds through adsorption, diffusion and trapping of RNS produced in plasmas. The combined effects of plasma seed treatment along with the application of PAWs were increased PH, SD, DW, total chlorophyll and carotene concentrations. The antioxidant enzymatic activities reveal that the plant defense mechanisms are found to improve due to plasma seed treatments along with the application of PAW. Better adaptive response against stresses is produced in the plants because of enhanced TSS concentrations as produced by plasma treatments. The highest TSS and TSP concentrations are found in the paddy grains where combined LPGAD plasma seed treatment and PAWs were applied. This indicates that the food value of grain is likely to improve as a consequence of plasma seed treatment and PAW application. Finally, the germination rate of ~ 7% and yield of ~ 16.67% of paddy are increased with respect to control, due to the combined effects of plasma applications.
References
Food and Agriculture Organization of the United Nations, FAO, http://www.fao.org/faostat/en/. Accessed 03 Dec 2020
Štěpánová V, Slavíček P, Kelar J, Prášil J, Smékal M, Stupavská M, Jurmanová J, Černák M (2017) Plasma Process Polym:e1700076
Mošovská S, Medvecká V, Halászová N, Ďurina P, Valíka Ľ, Mikulajová A, Zahoranová A (2018) Food Res Int 106:862–869
Šerá B, Šerý M, Štrañák V, Špatenka P (2009) Plasma Sci Technol 11:750–754
Los A, Ziuzina D, Boehm D, Cullen PJ, Bourke P (2019) Plasma Process Polym e1800148:1–12
Zhou R, Zhou R, Zhang X, Zhuang J, Yang S, Bazaka K, Ostrikov K (2016) Sci Rep 6:32603
Ling L, Jiafeng J, Jiangang L, Minchong S, Xin H et al (2014) Sci Rep 4:5859
Junior CA, Vitoriano JO, Da Silva DLS, Farias MFL, Dantas NBL (2016) Sci Rep 6:33722
Rahman MM, Sajib SA, Sifat MS, Tahura S, Roy NC, Parvez S, Reza MA, Talukder MR, Kabir AH (2018) Sci Rep 8:10498
Roy NC, Hasan MM, Talukder MR, Hossain MD, Chowdhury AN (2018) Plasma Chem Plasma Process 38:13–28
Roy NC, Hasan MM, Kabir AH, Reza MA, Talukder MR, Chowdhury AN (2018) Plasma Sci Technol 20:115501
Ling L, Jiangang L, Minchong S, Chunlei Z, Yuanhua D (2015) Sci Rep 4:5859
Kabir AH, Rahman MM, Das U, Sarkar U, Roy NC, Reza MA, Talukder MR, Uddin MA (2019) PLoS ONE 14(4):e0214509
Mitra A, Li YF, Klämp TG, Shimizu T, Jeon J et al (2014) Food Bioprocess Technol 7:645–653
Zhou R, Zhou R, Prasad K, Fang Z, Speight R, Bazaka K, Ostrikov K (2018) Green Chem 20:5276–5284
Ochi A, Konishi H, Ando S, Sato K, Yokoyama K, Tsushima S, Yoshida S, Morikawa T, Kaneko T, Takahashi H (2017) Plant Pathol 66:67–76
Thirumdas R, Kothakota A, Annapure U, Siliveru K, Blundell R, Gatt R, Valdramidis VP (2018) Trends Food Sci Technol 77:21–31
Takashima K, Hu Y, Goto T, Sasaki S, Kaneko T (2020) J Phys D Appl Phys 53:354004
Park DP, Davis K, Gilani S, Alonzo CA, Dobrynin D, Friedman G, Fridman A, Rabinovich A, Fridman G (2013) Curr Appl Phys 13:S19–S29
Penado KNM, Mahinay CLS, Culaba IB (2018) Jpn J Appl Phys 57:01AG08
Swiecimska M, Tulik M, Šerá B, Golinska P, Tomeková J, Medvecká V, Bujdáková H, Oszako T, Zahoranová A, Šerý M (2020) Forests 11:837
Zhao YM, Ojha S, Burgess CM, Sun DW, Tiwari BK (2020) J Appl Microbiol 129:1248–1260
Liao X, Bai Y, Muhammad AI, Liu D, Hu Y, Ding T (2020) J Phys D Appl Phys 53:064003
Islam S, Omar FB, Sajib SA, Roy NC, Reza MA, Hasan M, Talukder MR, Kabir AH (2019) Gesunde Pflanzen 71:175–185
Sajib SA, Billah M, Mahmud S, Miah M, Hossain F, Omar FB, Roy NC, Hoque KMF, Talukder MR, Kabir AH, Reza MA (2020) Plasma Chem Plasma Process 40:119–143
Jo YK, Cho J, Tsai TC, Staack D, Kang MH, Roh JH, Shin DB, Cromwell W, Gross D (2014) Crop Sci 54:796–803
Kaushik NK, Ghimire B, Li Y, Adhikari M, Veerana M, Kaushik N, Jha N, Adhikari B, Lee SJ, Masur K, von Woedtke T, Weltmann KD, Choi EH (2018) Biol Chem 400(1):39–62
Gorbanev Y, Maldonado AP, Bogaerts A (2018) Anal Chem 90(22):13151–13158
Muller K, Linkies A, Vreeburg RAM, Fry SC, Krieger-Liszkay A, Luebner-Metzger G (2009) Plant Physiol 150:1855–1865
Dien DC, Mochizuki T, Yamakawa T (2019) Plant Prod Sci 22(4):530–545
Roy NC, Talukder MR (2018) Phys Plasmas 25:093502–093508
Takahata J, Takaki K, Satta N, Takahashi K, Fujio T, Sasaki Y (2015) Jpn J Appl Phys 54:01AG07-6
Sivachandiran L, Khacef A (2017) RSC Adv 7:1822–1832
Maniruzzaman M, Sinclair AJ, Cahill DM, Wang X, Dai XJ (2017) Plasma Chem Plasma Process 37:1393–1404
Yayci A, Baraibar AG, Krewing M, Fueyo EF, Hollmann F, Alcalde M, Kourist R, Bandow JE (2020) Chem Sus Chem 13:2072–2079
Zhou R, Zhou R, Wang P, Xian Y, Mai-Prochnow A, Lu X, Cullen PJ, Ostrikov K, Bazaka K (2020) J Phys D Appl Phys 53:303001–303027
Filatova I, Lyushkevich V, Goncharik S, Zhukovsky A, Krupenko N, Kalatskaja J (2020) J Phys D Appl Phys 53:244001–244009
Yadav S, Kanwar RS (2018) Plant Pathol J 17(1):33–38
LIFBASE simulation software. http://www.sri.com/cem/lifbase
Rashid MM, Rashid M, Hasan MM, Talukder MR (communicated)
Bormashenko E, Grynyov R, Bormashenko Y, Drori E (2012) Sci Rep 2:741
Bormashenko E, Shapira Y, Grynyov R, Whyman G, Bormashenko Y, Drori E (2015) J Exp Bot 66:4013–4021
International Seed Testing Association (ISTA), Zurichstr. 50, CH-8303, Bassersdorf, Switzerland, 2018
Hara Y (1999) Plant Prod Sci 2:129–135
Su S, Zhou Y, Qin JG, Yao W, Ma Z (2010) J Freshw Ecol 25:531–538
Lichtenthaler HK (1987) Methods Enzymol 148:350–382
Wellburn AR (1994) J Plant Physiol 144:307–313
Giannopolitis CN, Ries SK (1977) Plant Physiol 59:309–314
Chance B, Maehly AC (1955) Methods Enzymol 2:764–775
Sun M, Zigman S (1978) Anal Biochem 90:81–89
Almeselmani M, Deshmukh P, Sairam R, Kushwaha S, Singh T (2006) Plant Sci 171:382–388
Bradford MM (1976) Anal Biochem 72:248–254
Zheng YH, Jia A, Ning T, Xu J, Li Z, Jiang G (2008) J Plant Physiol 165:1455–1465
Álvarez EPP, Cerdán TG, Escudero EG, Vidaurre JMM (2017) J Sci Food Agric 97:2524–2532
Mondal AB, Mamun AA (2011) Front Agric China 5(3):372–374
Billah M, Sajib SA, Roy NC, Rashid MM, Reza MA, Hasan MM, Talukder MR (2020) Arch Biochem Biophys 681:108253–108310
Arima Y, Iwata H (2007) Biomaterials 28:3074–3082
Misra NN, Pankaj SK, Segat A, Ishikawa K (2016) Trends Food Sci Technol 55:39–47
Zargarchi S, Saremnezhad S (2019) Food Sci Technol 102:291–294
Ishikawa T, Shigeoka S (2008) Biosci Biotechnol Biochem 72(5):1143–1154
Puač N, Škoro N, Spasić K, Živković S, Milutinović M, Malović G (2017) Petrović Z Lj. Plasma Process Polym 15:e1700082–e1700112
Kučerová K, Henselová M, Slováková Ľ, Hensel K (2018) Plasma Process Polym 16:e1800131–e1800214
Tanida M (1996) Breed Sci 46:23–27
Itoh J, Nonomura K, Ikeda K, Yamaki S, Inukai Y, Yamagishi H, Kitano H, Nagato Y (2005) Plant Cell Physiol 46(1):23–47
Poli Y, Nallamothu V, Balakrishnan D, Ramesh P, Desiraju S, Mangrauthia SK, Voleti SR, Neelamraju S (2018) Front Plant Sci 9:1543–1614
Caverzan A, Passaia G, Rosa SB, Ribeiro CW, Lazzarotto F, Margis-Pinheiro M (2012) Genet Mol Biol 35(4):1011–1019
Anjum NA, Sharma P, Gill SS, Hasanuzzaman M, Khan EA, Kachhap K et al (2016) Environ Sci Pollut Res 23:19002–21929
Ozaki K, Uchida A, Takabe T, Shinagawa F, Tanaka Y, Takabe T, Hayashi Hattori T, Rai AK, Takabe T (2009) J Plant Physiol 166:569–578
Yang Y, Rao Y, Xu J, Shao G, Leng Y, Huang L, Wang L, Dai L, Zhang G, Hu J, Zhu L, Li C, Gao Z, Guo L, Qian Q, Zeng D (2014) S African J Bot 93:137–141
Soriano D, Huante P, Buen AG, Segovia AO (2013) Plant Ecol 214:1361–1375
Soriano D, López S, Sánchez EZ, Segovia AO, Buen AG (2015) S African J Bot 97:149–153
Couée I, Sulmon C, Gouesbet G, Amrani AE (2006) J Exp Bot 57:449–459
Jiang YH, Cheng JH, Sun DW (2020) Trends Food Sci Technol 98:129–139
Tang T, Xie H, Wang Y, Lu B, Liang J (2009) J Exp Bot 60(9):2641–2652
Kumari M, Asthir B (2016) Rice Sci 23(5):255–265
Acknowledgements
M. R. Talukder would like to acknowledge Ministry of Education (Grant No. LS2017544), Government of the People’s Republic of Bangladesh, and the University of Rajshahi (Grant No. 62/5/52/RU/Engg-05/2020-2021), for their partial financial supports to carry out this work. The author also would like to thank Mizanur Rahman, Lab Technician, Plasma Science and Technology Lab, Department of Electrical and Electronic Engineering, University of Rajshahi for providing time in the Lab and research field.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rashid, M., Rashid, M.M., Reza, M.A. et al. Combined Effects of Air Plasma Seed Treatment and Foliar Application of Plasma Activated Water on Enhanced Paddy Plant Growth and Yield. Plasma Chem Plasma Process 41, 1081–1099 (2021). https://doi.org/10.1007/s11090-021-10179-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-021-10179-2