Abstract
The study deals with the effect of low pressure dielectric barrier discharge (LPDBD) plasma and plasma activated water (PAW) produced with Ar, O2 and Air on germination and growth in rapeseed. Although H2O2 concentration showed no significant changes, α‑amylase activity (germination inducer) significantly increased in seeds due to LPDBD plasma. In addition, the activity of SOD and CAT was significantly induced in seeds of rapeseed treated with PAW. Rapeseed plants grown from the LPDBD plasma treated seeds showed significant improvements in shoot characteristics, chlorophyll synthesis, total soluble protein and sugar concentration compared to controls. Interestingly, plasma treated plants also showed no significant variations of H2O2 in tissue(s) which is supported by the biochemical and molecular evidence(s) of antioxidant enzymes. Plants exhibited a significant increase in tissue APX and CAT activities along with BnAPX and BnCAT expression(s) in roots when seeds were treated with LPDBD Air/O2 and PAW O2. This suggests that LPDBD plasma might have been involved with elevated level of reactive oxygen species, which was tightly controlled through the upregulation of APX and CAT activities and thus trigger the growth and development in rapeseed plants. These findings reveal the role and mechanisms of LPDBD technique facilitating germination and growth in rapeseed plants.
Zusammenfassung
Die Studie beschäftigt sich mit der Wirkung von Niederdruckplasma mit dielektrischer Barriereentladung (engl. low pressure dielectric barrier discharge plasma, LPDBD plasma) und plasmaaktiviertem Wasser (PAW), die mit Ar, O2 und Luft hergestellt werden, auf die Keimung und das Wachstum von Raps. Obwohl die H2O2-Konzentration keine signifikanten Veränderungen zeigte, stieg die α‑Amylase-Aktivität (Keimungsinduktor) im Saatgut durch das LPDBD-Plasma signifikant an. Darüber hinaus wurde die Aktivität von SOD und CAT in Rapssamen, die mit PAW behandelt wurden, signifikant induziert. Rapspflanzen aus dem mit LPDBD-Plasma behandelten Saatgut zeigten signifikante Verbesserungen der Sprosseigenschaften, der Chlorophyllsynthese, der Gesamtprotein- und Zuckerkonzentration im Vergleich zur Kontrolle. Interessanterweise zeigten plasmabehandelte Pflanzen auch keine signifikanten Variationen von H2O2 in Gewebe(n), was durch die biochemischen und molekularen Nachweise von antioxidativen Enzymen bestätigt wird. Pflanzen zeigten einen signifikanten Anstieg der Gewebe-APX- und CAT-Aktivitäten sowie der BnAPX- und BnCAT-Expression(en) in den Wurzeln, wenn Samen mit LPDBD Luft/O2 und PAW O2 behandelt wurden. Dies deutet darauf hin, dass LPDBD-Plasma an einem erhöhten Level an reaktiven Sauerstoffspezies beteiligt gewesen sein könnte, was durch die Hochregulierung der APX- und CAT-Aktivitäten streng kontrolliert wurde und somit das Wachstum und die Entwicklung von Rapspflanzen auslöste. Diese Ergebnisse tragen zum Verständnis der Rolle und der Mechanismen der LPDBD-Technik bei. Durch den Einsatz dieser Technik können die Keimung und das Wachstum von Rapspflanzen verbessert werden.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As a result of reduction of agricultural lands and climate threats, crop production is at risk in many countries of the world. Although rapeseed (Brassica napus) is one of major oil-yielding crops in many countries, the production of rapeseed is decreasing due to several climatic (temperature, humidity, drought, etc.) and manmade factors (Ling et al. 2014; Zhao et al. 2016). Furthermore, application of chemical fertilizers and pesticides brings hazards to human health and environment. Therefore, strategies and technologies associated with no environmental risks should be used on a priority basis in agriculture.
Plasma technology showed agronomic importance in few crops. Improvement in seed germination and growth have been reported in few plant species (Šerá et al. 2009; Sera et al. 2010; Zhou et al. 2011) by atmospheric pressure cold plasma (APCP). Plasma treatment is drawing much attention due to its chemical-free capacity to tempt biological mechanisms in plants. In addition, APCPs are effective to inhibit seed contamination (Du et al. 2012; Mitra et al. 2014), water uptake mechanisms (Junior et al. 2016; Šerá et al. 2009), and drought tolerance (Ling et al. 2014). To date, most of the reports are concerned with atmospheric pressure dielectric barrier discharge (APDBD) plasmas. However, low pressure dielectric barrier discharge (LPDBD) technique is a vital source of non-thermal plasma due to its stability and large volume plasma as compared to APDBD plasma. Its potential effects are also applicable to the environment and energy conversion (Park et al. 2012; Sreethawong et al. 2007; Yu et al. 2009). Moreover, recent application of plasma activated water (PAW) has grown attraction to the plant physiologist due to its attractive features. Effectiveness of PAW on seed germination and growth rate of radish, tomato, and sweet pepper plants has been investigated (Sivachandiran and Khacef 2017). Long living reactive oxygen and nitrogen species as dissolved PAW are effective in the growth enhancement of plants (Sarinont et al. 2017).
Germination of seeds and subsequent plant growth and development depend on several physiological and biochemical indicators. Hydrogen peroxide (H2O2) is a reactive molecule that plays a dual role in physiological and developmental processes along with stress tolerance in plants. The positive and negative roles of H2O2 rely on its physiological conditions, concentrations and process specificities in plant systems (Wojtyla et al. 2016). Although the plasma treatment is used to enhance seed germination and plant growth, it is rather challenging to optimize the species production as it might elevate reactive oxygen species (ROS), such as superoxide anion (O2∙−) and hydroxyl radical (∙OH) and especially H2O2. While ROS and H2O2 function during imbibition and germination (Bailly et al. 2008), excess of these molecules may cause oxidative damage leading to cell death, membrane damage and protein degradation in plants. H2O2 is also considered as a signaling hub for the regulation of seed dormancy, germination, and antioxidant defense (Wojtyla et al. 2016). The α‑amylase is found in germinating seeds of many species. Starch reserves, which are transported as sugars and used by the growing embryo, are mobilized by α‑amylase (Kaneko et al. 2002). Recently, Roy et al. (2018) have shown that the low frequency glow discharge (LFGD) plasma was able to stimulate seed germination and growth in wheat. However, the role of LPDBD and PAW in rapeseed and its mechanisms leading to agronomic improvement have not yet studied. Therefore, we pooled a series of physiological, biochemical and molecular investigations to elucidate their role in rapeseed.
Materials and Methods
Plasma Production and Species Identifications
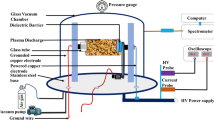
The plasma discharge reactor for seed treatment was made of a pyrex glass tube (inner diameter 12 mm, length 50 mm). One copper disk-type electrode (diameter 9 mm, thickness 0.5 mm) was placed at the lower end of reactor tube. Another copper disk-type electrode (diameter 8 mm, thickness 0.5 mm) was covered by a pyrex glass tube (outer diameter 10 mm, thickness 1 mm, dielectric constant 5.6) and was placed at the upper end of the reactor tube as shown in Fig. S1. The spacing between the two electrodes was maintained at 30 mm. A high voltage (5–10 kV, 3–8 kHz) bipolar sinusoidal power supply was fed across the electrodes for plasma generation. Wheat seeds to be treated were placed between the two electrodes of the reactor. The whole reactor setup was then placed into a vacuum chamber as shown in Fig. S1. The inside pressure of the chamber was reduced by a vacuum pump (FY-1C) and inside pressure was maintained at ~10 Torr. The flows of Ar, O2, and air to the chamber were controlled by three different gas flow controllers Yamato, KIT and 11P, respectively. The waveforms of the discharge voltage and current were recorded with voltage (HVP-08) and current (CP-07C) probes, respectively, along with a digital oscilloscope (GDS-1000B). The power absorbed by the plasma was determined from VI waveforms of the LPDBD plasma by using the formula \(P=\int _{0}^{T}v(t)i(t)dt\), where \(v(t)\) and \(i(t)\) are the discharge voltage and current, respectively, integrating over the period \(T\). The absorbed power in the plasma was found ~30 W for Ar/Air gas mixture measured at applied voltage 3 kV, frequency 4.5 kHz, electrode spacing 30 mm. The emitted spectra produced in the plasma were recorded with spectrometers (USB2000+XR1, slit size: 25 μm, grating: \(800\,\mathrm{lines/mm}\), optical resolution: \(1.7\,\mathrm{nm}\), wavelength range from 200–1100 nm and high resolution dual-channel spectrometer AvaSpec-2018, slit: 10 μm, gratting: 2400 lines/mm, optical resolution: 0.07 nm, wavelength range: 220–500 nm) for species identification and plasma diagnostics. In order to produce ROS and RNS in the LPDBD plasma; Ar/O2 (Ar: 60 \%, O2: 40 \%) and Ar/Air (Ar: 60 \%, Air: 40 \%) gas composition was used. The emitted spectrum from produced plasmas at voltage: 3 kV, electrode spacing: 30 mm, pressure (~10 torr) is depicted in Fig. S1(c). The major electronic transitions of nitrogen second positive system \(\mathrm{N}_{2}(\mathrm{C}^{3}\Pi _{\mathrm{u}}-\mathrm{B}^{3}\Pi _{\mathrm{g}})\) in the range \(294-380\,\mathrm{nm}\) and the first negative system \(\mathrm{N}_{2}^{+}(\mathrm{B}^{2}\Sigma _{\mathrm{u}}^{+}-X^{2}\Sigma _{\mathrm{g}}^{+})\) in the range 391–405 nm were found as RNS from Ar/Air plasma shown in inset the of Fig. S1. Atomic transitions of N+ were detected at wavelength 517.52 and 668.22 nm. From the figure it is seen that the transitions of \(\mathrm{O}\) radicals occured at 777.1 and 844.2 nm for Ar/O2 plasma. ROS was also observed as ionic transition of O+ and O2+ was found. The band transition of NO (\(A^{2}\Sigma ^{+}\rightarrow X^{2}\Uppi )\) was found in Ar/O2 plasma. Due to the presence of Ar gas, the transitions of Ar lines were detected in the spectrum in the range 685–914 nm and transitions of \(H_{\beta }\) and \(H_{\alpha }\) line were found for both Ar/Air and Ar/O2 plasmas. Rotational (\(T_{\mathrm{rot}})\) and vibrational (\(T_{\mathrm{vib}})\) temperatures were determined by simulating the first negative system \(\mathrm{N}_{2}^{+}(\mathrm{B}^{2}\Sigma _{\mathrm{u}}^{+}-X^{2}\Sigma _{\mathrm{g}}^{+})\) with the aid of LIFBASE spectroscopic software. The analyzed results revealed that \(T_{\mathrm{rot}}\approx 306\,\mathrm{K}\), \(T_{\mathrm{vib}}\approx 2.42\,\mathrm{kK}\) for \(Ar/Air\) and \(T_{\mathrm{rot}}\approx 323\,\mathrm{K}\), \(T_{\mathrm{vib}}\approx 2.58\,\mathrm{kK}\) for \(Ar/\mathrm{O}_{2}\) plasmas measured at 3 kV with dissipated power \(\sim 30\,\mathrm{W}\). The wheat seeds were selected randomly both for treatment and control. The experimental setup of plasma technique and seed placement with Ar/O2 and Ar/Air plasmas has been previously described (Rahman et al. 2018).
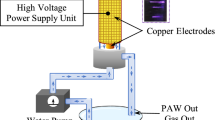
For the generation of PAW, a pyrex glass tube (inner diameter 9 mm and length 70 mm) was used as water discharge reactor for the preparation of PAW. The grounded copper electrode (diameter 0.5 mm) was inserted from the lower side of the tube and the powered disk-type copper electrode (diameter 8.5 mm) was placed at the upper side. Deionized water (50 ml) was put in the reactor tube and a small amount of Ar (0.25 lpm) and O2 (0.25 lpm) were flowed through the bottom side of the reactor tube and small bubbles were created in the water. A high voltage (3–6 kV, 3–10 kHz) power supply was connected to the electrodes for the generation of plasma. The distance between water surface and upper electrode was maintained at 15 mm. Arc discharge was generated at the water surface. Both ROS and RNS were produced in the water discharges as mentioned earlier. It is seen from the emitted spectra that both O and OH radicals were produced in O2 PAW, while only OH radicals were produced in Ar PAW. The emitted spectrum of Ar PAW indicates that the relative population density of OH radicals was enhanced (Roy et al. 2018) due to using Ar gas. The simulation of \(\mathrm{OH}(A^{2}\Sigma _{u}^{+}\rightarrow X^{2}\Pi _{g})\) band transition reveals that \(T_{\mathrm{rot}}\approx 1.78\,\mathrm{kK}\), \(T_{\mathrm{vib}}\approx 4.42\,\mathrm{kK}\) for \(H_{2}O/Ar\) and \(T_{\mathrm{rot}}\approx 1.93\,\mathrm{K}\), \(T_{\mathrm{vib}}\approx 4.58\,\mathrm{kK}\) for \(H_{2}O/O_{2}\) plasmas measured at 3 kV with dissipated power \(\sim 8\,\mathrm{W}.\) The dissolved long lived species (O3, H2O2, NO2) are also produced (Sarinont et al. 2017; Sivachandiran and Khacef 2017) in PAW.
Plasma Treatment and Plant Cultivation
The rapeseed seeds were selected randomly both for treatment and control. The seeds were treated by LPDBD plasmas with the following gas compositions: Ar/Air and Ar/O2. The seeds containers were placed inside the reactor. The treatment times followed for the seeds container were 90s. On the other hand, PAW was prepared by arc discharge O2 and Ar plasmas for a treatment time 10 min. Rapeseeds were immersed in PAW after 30 min from preparing time. Seedlings were then transferred to the hydroponic culture (Hoagland and Arnon 1950) containing the following nutrient elements (µM): KNO3 (16,000), Ca(NO3)2.4H2O (6000), NH4H2PO4 (1000), MgSO4.7H2O (2000), KCl (50), H3BO3 (25), Fe-EDTA (25), MnSO4.4H2O (2), ZnSO4 (2), Na2MoO4.2H2O (0.5) and CuSO4.5H2O (0.5). Under 10/14 h light and dark (550–560 µmol s−1 per µA) in a growth cabinet, the seedlings were cultivated in a 2 L container containing cultivation media of pH 6.0. Plants were cultivated for 4 days once transferred to solution culture and concurrently harvested.
Germination Percentage and Amylase Activity
The seeds (around 25) were placed in each 90 mm Petri dish containing two layers moistened with wet filter papers at the bottom for germination. The seed containing Petri dishes were incubated at 25 ℃ in an incubator for 3 d. Afterwards, additional distilled water was added daily to maintain sufficient moisture content for germination. The germination percentage was recorded after 3 d.
Amylolytic activity (α-amylase: EC 3.2.1.1) was measured as previously described (Valencia et al. 2000). Seeds were homogenized in citrate buffer 10 mM Na-NaCl-CaCl2 at pH 8. The mixture was added to 0.5% (w/v) starch solution and incubated at 37 ℃ for 15 min. Further, 2.5 ml of iodine reagent (I2; KI) was added and then the mixture was centrifuged at 4000 g for 10 min. Lastly the absorbance was taken at 580 nm.
Determination of Morphological Features and Chlorophyll (a and b)
For the analysis of chlorophyll, firstly weight of the young fresh leaves were taken. Then they were ground with mortar and pestle in 90% methanol. The sample mixtures were subsequently centrifuged at 12000 rpm for 5 min and the cell debris was discarded. For the analysis of chlorophyll a and chlorophyll b the absorbance of the clear supernatant was recorded at 662 and 646 nm respectively using spectrophotometer (UV-1650PC, Shimadzu.). The total concentration of chlorophyll (a and b) was obtained following the calculation (Lichtenthaler and Wellburn 1983).
Determination of Antioxidant Enzymes (SOD, APX, and CAT) in Seeds and Plants
SOD, APX and CAT enzymes were extracted according to the previously described protocol with slight modifications (Goud and Kachole 2012). Briefly, seeds/tissues were ground in phosphate buffer (100 mM, pH 7.0) using mortar and pestle. The homogenate was then centrifuged for 10 min (8000 rpm) and the supernatant was separated in new tubes. For the analysis of superoxide dismutase (SOD), 100 µl extract was added with 50 mM sodium carbonate/bicarbonate buffer (pH 9.8), 0.1 mM EDTA and 0.6 mM epinephrine (Sun and Zigman 1978). After 4 min, formation of adrenochrome was read at 475 nm in a UV-Vis spectrophotometer. APX activity was tested in a reaction mixture containing 0.1 mM EDTA, 50 mM potassium phosphate buffer (pH 7.0), 0.5 mM ascorbic acid, 0.1 mM H2O2, and 0.1 ml extract. The activity of APX was finally calculated using extinction coefficient of 2.8 mM−1 cm−1 based on the absorbance at 290 nm (Almeselmani et al. 2006). For CAT analysis, the reaction mixture contained 100 mM potassium phosphate buffer (pH 7.0), 6% H2O2 and 100 µl root extract. After adding the extract, the changes in OD were monitored at 240 nm absorbance (extinction coefficient of 0.036 mM−1 cm−1) using a UV spectrophotometer at 30 s intervals up to 1 min.
Determination of H2O2
Washed seed, root, and shoot were homogenized in 0.1% trichloroacetic acid (TCA) using mortar and pestle (Alexieva et al. 2001). At 10,000 rpm the homogenates were centrifuged for 15 min and the supernatant was collected and then mixed with potassium iodide (1 M) and phosphate buffer (10 mM, pH 7.0) and kept for 1 h at dark. Lastly, the optical density of the extract mixture was taken at 390 nm by a spectrophotometer (UV-1650PC, Shimadzu).
Analysis of Total Soluble Sugar
Soluble sugar in root and shoot was analyzed as previously described (Dubois et al. 1956). Briefly, fresh root and shoot were homogenized in hot (90 °C) aqueous ethanol (v/v 80%) and centrifuged at 12,000 rpm for 5 min. Subsequently, the clear supernatant was mixed with 0.2% of anthrone reagent. The sample mixtures were incubated in a boiling water bath for 8 min before placing on ice. The absorbance of the ice-cold samples was finally recorded at 620 nm.
Estimation of Total Soluble Protein
The total soluble protein was estimated in both roots and shoots by spectrophotometer (Guy et al. 1992). Firstly, washed root and shoot were weighed and homogenized with ice-cold mortar and pestle in buffer containing 2 mM EDTA (ethylenediaminetetraacetic acid), 50 mM Tris-HCl, pH 7.5 and 0.04% (v/v) 2‑mercaptoethanol. At 12,000 rpm and 25 °C temperature, the homogenates were then centrifuged. The supernatant was moved to cuvette filled with 1 ml CBB (Coomassie Brilliant Blue solution). Afterwards, the total soluble protein was determined following the absorbance of unknown samples taken at 595 nm in a spectrophotometer using the standard curve of BSA (bovine serum albumin).
Isolation of RNA and Gene Expression Analysis
For the analysis of expression of BnSOD, BnAPX, and BnCAT transcripts in roots, quantitative qRT-PCR (reverse transcription PCR) was performed. Briefly, roots (50–70 mg) were ground in liquid nitrogen using mortar and pestle to a fine powder. Then, the total RNA was extracted using the protocol instructed by SV Total RNA Isolation System (Cat. no. Z3100, Promega Corporation, USA). The superiority of RNA samples was subsequently verified by denaturing gel electrophoresis. After the quantification of RNA by UV-Vis Spectrophotometer (NanoDrop 2000), the first-strand cDNA was synthesized according to the protocol by GoScript™ Reverse Transcription System (Cat no. A5001, Promega Corporation, USA). RNase was used to treat the cDNA samples to eliminate possible RNA contamination. The real-time PCR analysis was performed in EcoTM real-time PCR system controlled by Eco Software v4.0.7.0 (Illumina, USA). Sequences of each gene-specific primer are presented in Table 1. Expression analysis was normalized with Actin as an internal control (Eco Software v4.0.7.0). The real-time PCR program was as follows: 3 min at 95 °C, 40 cycles of 30 s at 94 °C, 15 s at 58 °C and 30 s at 72 °C.
Statistical Analysis
Experiments were performed on the basis of randomized block design (CRBD) having three independent replications for each biological sample. The significance of each group data was analyzed statistically at P ≤ 0.05 by ANOVA one-way followed by Duncan’s Multiple Range Test (DMRT) in SPSS Statistics 20 software. Preparation of the graphical figures was done using GraphPad Prism 6.
Results
Germination Rate and Amylase Activity of Seeds
All plasma treatments (LPDBD Ar/Air, LPDBD Air/O2, PAW O2 and PAW Ar) showed significant improvement in germination rate and seedling vigor in rapeseeds compared to the seeds germinated without plasma treatment (Fig. 1). Among the plasma treatments, PAW O2 was found to be the most effective for increasing germination rate in rapeseed compared to the control (Fig. 1). Further, amylase activity was significantly increased in seeds of rapeseed due to any of the plasma treatments in comparison with control (Fig. 1).
H2O2 Concentration and Antioxidant Enzyme Activities in Seeds
No significant change was found in H2O2 concentration in seeds due to any of the plasma treatments (Fig. 1). Enzymatic analysis showed that SOD activity showed no significant changes in seeds of rapeseed compared to non-treated controls due to LPDBD Ar/Air and LPDBD Air/O2 plasmas. However, SOD activity significantly increased compared to controls when seeds were treated with PAW O2 and PAW Ar (Fig. 1). APX activity in seeds showed no significant changes due to any of the plasma treatments (Fig. 1). In addition, CAT showed no significant changes in seeds compared to controls when seeds were treated with LPDBD Ar/Air, LPDBD Air/O2 and PAW Ar (Fig. 1). However, a significant increase in CAT activity was found in PAW O2 treated seeds compared to the non-treated seeds (Fig. 1).
Morphological Characteristics of Rapeseed Seedlings
Root length and root dry weight did not show any significant changes compared to controls when seeds were treated with LPDBD Ar/Air and LPDBD Air/O2 plasmas (Fig. 2 and 3). However, a significant increase was found in root length and root dry weight in comparison with controls when seeds were treated by PAW O2 and PAW Ar during germination stage (Fig. 3). Further, shoot height of rapeseed significantly increased due to all type of plasma treatments compared to controls. In case of shoot dry weight, plants showed a significant increase only when seeds were treated with PAW Ar. However, LPDBD Ar/Air, LPDBD Air/O2 and PAW O2 showed no significant effect on shoot dry weight compared to controls (Fig. 3).
Total Chlorophyll (a and b) and Soluble Protein Concentration in Seedlings
Total chlorophyll concentrations (a and b) showed no significant changes in comparison with controls when treated with LPDBD Ar/Air (Fig. 4). A significant increase in total chlorophyll (a and b) concentration was found in leaves of rapeseed due to LPDBD Air/O2, PAW Ar, and PAW (Fig. 4). In addition, no significant change was found in total soluble protein in roots of rapeseed under different plasma treatments in comparison with the controls (Fig. 4). In addition, application of LPDBD Air/O2 caused a significant increase in total soluble protein in the shoot of rapeseed compared to controls. However, total soluble protein showed no significant changes in shoots when seeds were treated with LPDBD Ar/Air, PAW O2 and PAW Ar (Fig. 4).
H2O2 Concentration, Soluble Sugar, and Antioxidant Enzyme Activities in Seedlings
H2O2 concentration showed no significant variations in either root or shoot among the treatments (Fig. 5). LPDBD Ar/Air plasma showed no significant changes in total soluble sugar in roots of rapeseed compared to controls. However, total soluble sugar in roots showed significant increase when plants were grown from the seeds treated with LPDBD Air/O2, PAW O2 and PAW Ar plasmas (Fig. 5). In shoots, total soluble sugar showed no significant variations either treated with LPDBD Ar/Air or PAW O2. On the other hand, both LPDBD Air/O2 and PAW Ar caused a significant increase in total soluble sugar in shoot compared to controls (Fig. 5).
Analysis of antioxidant enzymes showed that none of the plasma treatment caused significant changes in SOD activity in either root or shoot of rapeseed compared to controls (Fig. 5). Further, APX activity showed a significant increase only under LPDBD Air/O2 plasma compared to controls in roots of rapeseed. However, the rest of the plasma caused no significant differences in root APX activity in rapeseed (Fig. 5). In the shoot, LPDBD Ar/Air and LPDBD Air/O2 showed no significant changes; however, both PAW O2 and PAW Ar caused a significant increase in APX activity compared with the plants grown from non-treated seeds (Fig. 5). Interestingly, PAW O2 and PAW Ar resulted in a significant increase in CAT activity in both root and shoot of rapeseed; while no changes was fond due to the other plasma treatments (LPDBD Ar/Air and LPDBD Air/O2) (Fig. 5).
Expression of Genes Related to Antioxidant Activities
Real-time PCR analysis showed no significant changes in BnSOD expression in roots of rapeseed plants originated from the seeds treated with any of the plasmas compared to controls (Fig. 6). However, expression of BnAPX and BnCAT significantly increased in roots when treated with LPDBD Air/O2 and PAW O2, respectively (Fig. 6). However, other plasma treatments showed no significant differences in BnAPX and BnCAT expression in roots of rapeseed compared with controls (Fig. 6).
Discussion
Germination and agronomic improvement in crops are highly advantageous with a view to mitigating the demand of the large population. Although plasma technology proved to be efficient in a very few plant species, the role of LPDBD plasma and its mechanistic basis for agronomic improvement in rapeseed was never investigated. This study reveals some new insights into the role of LPDBD plasma technology to induce the germination and growth of rapeseed plants.
In this study, LPDBD plasma treatments produced different functional reactive species. Exposure of rapeseed seeds to the LPDBD plasma treatments (Ar/Air and Air/O2) showed stimulating effect with respect to the germination rate and seedlings vigor in rapeseed. Previously, DBD (dielectric barrier discharge) plasma treatment was reported being useful for enhancing seed germination rate and vigor index in wheat plants (Guo et al. 2017; Meng et al. 2017). The O2 containing functional groups have enriched the surface of the seeds that result in essential improvement of wettability and finally influenced the germination (Roy et al. 2018). It was also reported that the incorporation of nitrogen on the seed surface enhances germination which is consistent with our findings (Volin et al. 2000).
In our study, LPDBD plasma treatment of seeds showed notable improvement in germination rate in rapeseed. PAW O2 proved to be the best to increase germination rate and thus, suggest that PAW O2 could be an efficient method to induce germination is rapeseed for large scale. Our biochemical analysis showed no distinct changes in H2O2 concentration in seeds due to plasma treatment. This suggests that the reactive species produced by our experiment did not deliver any drastic increase in superoxide level that may trigger higher H2O2 concentration in seeds. However, the accumulation of H2O2 is reported in seed physiology during imbibition, early germination stage and when seeds become hydrated (Bailly et al. 2008; Kranner et al. 2010). Further, H2O2 along with other ROS are often associated with seed deterioration and loss of seed vigor (Jeevan Kumar et al. 2015). During germination, the embryo generates starch degradation resulted in secretion of degradative enzymes, such as α‑amylase (Beck and Ziegler 1989). This α‑amylase in the aleurone layer plays critical roles in hydrolyzing the endosperm starch into metabolizable sugars, which provide the energy for the growth of roots and shoots (Beck and Ziegler 1989). The increase in amylase activity in seeds due to LPDBD plasmas reveals that this enzyme may be involved in inducing germination in rapeseeds.
In this study, the increased activities of SOD and CAT in seeds due to PAWs are correlated with the seed H2O2 concentration. This is probably done by the tight regulation of superoxide and H2O2 scavenging by means of elevated SOD and CAT, respectively. CAT, which is one of the main antioxidant enzymes, is associated with the H2O2 scavenging (Malar et al. 2016). The elevated CAT activity in seeds might be involved with the regulation of H2O2 induced by PAW. In most of the cases, PAW O2 and PAW Ar showed notable efficiency in the improvement of root and shoot characteristics in rapeseeds. Comparatively, plants grown hydroponically from the seeds treated with PAW Ar showed the improvement of root and shoot dry matter as well as length in rapeseeds. Similar agronomic improvements were previously demonstrated in poppy and tomato plants in response to atmospheric pressure cold plasmas (Meiqiang et al. 2005).
Interestingly, our studies showed the increase of total soluble protein in shoot and sugar in either root or shoot due to LPDBD Air/O2 and PAWs. It does reveal the effectiveness of LPDBD plasmas to enhance cell metabolism leading to enhanced growth and development in rapeseeds. These findings are consistent with previous results in wheat and Suaeda plants (Li et al. 2005; Sera et al. 2010). In addition, elevated soluble sugar due to LPDBD plasma treatment may also participate as a non-enzymatic antioxidant in rapeseed.
Induction of antioxidant enzymes is generally involved with the plasma treatment in plants. However, neither of the reports focused on the background scenario of ROS scavengers that plants induce in special certain conditions. In the present study, APX and CAT which are mainly active against excess H2O2 showed significant inductions subjected to LPDBD plasma treatments. The gene expression analysis of BnAPX and BnCAT showed a significant increase in roots of rapeseed due to LPDBD plasma treatments which is the confirmation of the biochemical analysis. It does suggest that the expected increase of H2O2 due to species produced through LPDBD plasmas is tightly regulated by increased APX and CAT activities in rapeseeds. Moreover, these antioxidant enzymes may also trigger cell metabolism. APX is mainly involved in the fine-tuning of H2O2 detoxification via its sulfhydryl group (Noctor et al. 2012). Interestingly, SOD activity and its corresponding BnSOD gene showed no significant changes in root and shoot tissues of rapeseed. It suggests that the rapeseed plants did get sensed by reactive superoxide (O2−) that might induce SOD activity under LPDBD plasma treatments.
Conclusion
This study reveals that LPDBD plasmas, preferably as activated water improved seed germination and agronomic characteristics in rapeseeds. This is achieved by the cellular regulation of ROS species and antioxidant activities. These findings will be critically useful to enhance germination and growth of rapeseeds through LPDBD plasmas.
References
Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat Plant. Plant Cell Environ 24:1337–1344
Almeselmani M, Deshmukh P, Sairam R, Kushwaha S, Singh T (2006) Protective role of antioxidant enzymes under high temperature stress Plant. Science 171:382–388
Bailly C, El-Maarouf-Bouteau H, Corbineau F (2008) From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. C R Biol 331:806–814
Beck E, Ziegler P (1989) Biosynthesis and degradation of starch in higher plants Annual review of plant. Biology (Basel) 40:95–117
Du CM, Wang J, Zhang L, Li HX, Liu H, Xiong Y (2012) The application of a non-thermal plasma generated by gas–liquid gliding arc discharge in sterilization. New J Phys 14:13010
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Goud PB, Kachole MS (2012) Antioxidant enzyme changes in neem, pigeonpea and mulberry leaves in two stages of maturity. Plant Signal Behav 7:1258–1262
Guo Q, Wang Y, Zhang H, Qu G, Wang T, Sun Q, Liang D (2017) Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci Rep 7:16680
Guy C, Haskell D, Neven L, Klein P, Smelser C (1992) Hydration-state-responsive proteins link cold and drought stress in spinach. Planta 188:265–270
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil Circular. California agricultural experiment station 347
Jeevan Kumar S, Rajendra Prasad S, Banerjee R, Thammineni C (2015) Seed birth to death: dual functions of reactive oxygen species in seed physiology. Ann Bot 116:663–668
Junior CA, de Oliveira Vitoriano J, Da Silva DLS, de Lima Farias M, de Lima Dantas NB (2016) Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Sci Rep 6:33722
Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M (2002) The α‑amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiol 128:1264–1270
Kranner I, Roach T, Beckett RP, Whitaker C, Minibayeva FV (2010) Extracellular production of reactive oxygen species during seed germination and early seedling growth in Pisum sativum. J Plant Physiol 167:805–811
Li W, Liu X, Khan MA, Yamaguchi S (2005) The effect of plant growth regulators, nitric oxide, nitrate, nitrite and light on the germination of dimorphic seeds of Suaeda salsa under saline conditions. J Plant Res 118:207–214
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions 11:591–592
Ling L, Jiafeng J, Jiangang L, Minchong S, Xin H, Hanliang S, Yuanhua D (2014) Effects of cold plasma treatment on seed germination and seedling growth of soybean. Sci Rep 4:5859
Malar S, Vikram SS, Favas PJ, Perumal V (2016) Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot Stud 55:54
Meiqiang Y, Mingjing H, Buzhou M, Tengcai M (2005) Stimulating effects of seed treatment by magnetized plasma on tomato growth and yield Plasma. Sci Technol 7:3143
Meng Y, Qu G, Wang T, Sun Q, Liang D, Hu S (2017) Enhancement of germination and seedling growth of wheat seed using dielectric barrier discharge plasma with various gas sources. Plasma Chem Plasma Process 37:1105–1119
Mitra A, Li Y‑F, Klämpfl TG, Shimizu T, Jeon J, Morfill GE, Zimmermann JL (2014) Inactivation of surface-borne microorganisms and increased germination of seed specimen by cold atmospheric plasma. Food Bioproc Tech 7:645–653
Noctor G et al (2012) Glutathione in plants: an integrated overview. Plant Cell Environ 35:454–484
Park G et al (2012) Atmospheric-pressure plasma sources for biomedical applications. Plasma Sources Sci Technol 21:43001
Rahman MM, Sajib SA, Rahi MS, Tahura S, Roy NC, Parvez S, Reza MA, Talukder MR, Kabir AH (2018) Mechanisms and signaling associated with LPDBD plasma mediated growth improvement in wheat. Scientific Reports 8(1)
Roy N, Hasan M, Talukder M, Hossain M, Chowdhury A (2018) Prospective applications of low frequency glow discharge plasmas on enhanced germination, growth and yield of wheat. Plasma Chem Plasma Process 38:13–28
Sarinont T, Katayama R, Wada Y, Koga K, Shiratani M (2017) Plant growth enhancement of seeds immersed in plasma activated water. MRS Adv 2:995–1000
Šerá B, Šerý M, Štrañák V, Špatenka P (2009) Does cold plasma affect breaking dormancy and seed germination? a study on seeds of Lamb’s quarters (Chenopodium album agg.). Plasma Sci Technol 11:750
Sera B, Spatenka P, Sery M, Vrchotová N, Hruskova I (2010) Influence of plasma treatment on wheat and oat germination and early growth. IEEE Trans Plasma Sci 38:2963–2968
Sivachandiran L, Khacef A (2017) Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: combined effect of seed and water treatment. RSC Adv 7:1822–1832
Sreethawong T, Thakonpatthanakun P, Chavadej S (2007) Partial oxidation of methane with air for synthesis gas production in a multistage gliding arc discharge system. Int J Hydrogen Energy 32:1067–1079
Sun M, Zigman S (1978) An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Anal Biochem 90:81–89
Valencia A, Bustillo AE, Ossa GE, Chrispeels MJ (2000) α‑Amylases of the coffee berry borer (Hypothenemus hampei) and their inhibition by two plant amylase inhibitors. Insect Biochem Mol Biol 30:207–213
Volin JC, Denes FS, Young RA, Park SM (2000) Modification of seed germination performance through cold plasma chemistry technology. Crop Sci 40:1706–1718
Wojtyla Ł, Lechowska K, Kubala S, Garnczarska M (2016) Different modes of hydrogen peroxide action during seed germination. Front Plant Sci 7:66
Yu L, Li X, Tu X, Wang Y, Lu S, Yan J (2009) Decomposition of naphthalene by dc gliding arc gas discharge. J Phys Chem A 114:360–368
Zhao C et al (2016) Field warming experiments shed light on the wheat yield response to temperature in China. Nat Commun 7:13530
Zhou Z, Huang Y, Yang S, Chen W (2011) Introduction of a new atmospheric pressure plasma device and application on tomato seeds. Agric Sci 2:23–27
Acknowledgements
We are grateful to Bioresearch Technologies, Denmark, for supplying primers.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Islam, F.B. Omar, S.A. Sajib, N.C. Roy, A. Reza, M. Hasan, M.R. Talukder and A.H. Kabir declare that they have no competing interests.
Caption Electronic Supplementary Material
Supplementary Fig. S1. a
Schematic diagram of LPDBD plasma for rapeseed treatment with Ar/O2 and Ar/Air gases, b V-I waveform of Ar/Air LPDBD plasma measured at applied voltage 3 kV and electrode spacing 30 mm and c emitted spectrum from Air/O2 and Ar/Air LPDBD plasmas at applied voltage \(3kV\) and electrode spacing 30 mm.
Supplementary Fig. S2. a
Schematic diagram for the generation of PAW with Ar and O2, b V-I waveform of H2O/Ar Arc measured at applied voltage 3 kV and electrode spacing 15 mm and c emitted spectrum from H2O/O2 and H2O/Ar Arc discharge plasmas at applied voltage 3 kV and electrode spacing 15 mm.
Rights and permissions
About this article
Cite this article
Islam, S., Omar, F.B., Sajib, S.A. et al. Effects of LPDBD Plasma and Plasma Activated Water on Germination and Growth in Rapeseed (Brassica napus). Gesunde Pflanzen 71, 175–185 (2019). https://doi.org/10.1007/s10343-019-00463-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10343-019-00463-9