Abstract
The recombination (synthesis and conversion) of nitric oxide was investigated using dielectric barrier discharge reactor at atmospheric pressure. In this work, products identification and its evolution of different gas components have been studied. In the NO/O2/N2 systems, nitric oxide (NO) can be removed via chemical oxidation and chemical reduction, and corresponding products are NO2 and N2, respectively. In the O2/N2 systems, N2O5 producing from the interaction of NO3 with NO2 was also observed. There is an optimum SED at which the highest NOx yield and best NO conversion efficiency will be achieved. In the H2O/O2/N2 systems, the formation of NO2, HNO2 and HNO3 were observed in both NF and NO conversion. The N2O molecule, as a byproduct of plasma chemical reaction, was observed in all the experiments when the H2O or O2 is presence in the simulated gas. The lowest energy cost of NO conversion is achieved at the SED of 1250 J/L.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen oxide (NOx), which represents both NO and NO2, is one of the main causes of some environmental problems such as acid rain, photochemical smog, and stratospheric ozone chemistry. On the other hand, the increasing demand from the global population will intensify the demand of nitrogen fixation, which will supply the energy for all life on the Earth. For the past few decades, a great deal of interest has been expressed to the analysis of the role of non-thermal plasma (NTP) in different reaction systems involving NO synthesis [1] and NO conversion [2]. The sequence of transformations of initial reactive species and electric energy into products and thermal energy is usually referred to as the mechanism of the plasma-chemical process. Collision between energetic electrons and substrate molecules (N2, O2 and H2O) create a mix of reactive species including reactive nitrogen species (RNS: N, NH and \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\)) and reactive oxygen species (ROS: O, HO, HO2 and O3) [3, 4]. Evolution of NO in NTP process involves: (1) reactions between NO and reactive species; (2) reactions between different reactive species. Dielectric barrier discharge (DBD) is a prominent technology to generate NTP under a moderate operating condition (atmospheric pressure and room temperature) [5]. Competition with the conventional chemical techniques for NF (Haber–Bosch) and DeNOx (Selective catalytic reduction, three-way catalyst) motivated a long history research and development effort to increase the product yield and energy efficiency of DBD process. All these efforts are focus on reaction kinetics [4, 6], operating parameters [3, 7] and plasma catalysis [1, 8,9,10]. Consequently, the evolution characteristics of NO involving production and loss is an interesting subject for DBD process.
NO synthesis using DBD process has been studied extensively [6, 8, 11]. The production of NO from molecular nitrogen is extremely energy intensive due to the large bond strength of nitrogen (945 kJ mol−1 or 9.79 eV). Breaking the bond can proceed efficiently via reactions of vibrationally-excited nitrogen molecules (N2*(vib)). NO synthesis in NTP are controlled by an endothermic step involving N2*(vib): O(3P) + N2*(1Σg) → NO + N, following by a secondary exothermic process: N + O2 → NO + N [11, 12], which is known as the Zeldovich mechanism. The electronically excited species, \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\), plays the most important role in NO production. Energy stored in \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\) may be consumed to overcome the high activation barriers of dinitrogen reactions. Teodoru et al. [6] presented a numerical model for NxOy remediation in humid air involving ~ 540 reactions. NH radicals combined with \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\) play a key role in NO synthesis. O, N, OH and HO2 play a role in both NO production and NO conversion.
DBD has been intensively investigated to eliminate NO in the exhaust gas stream since 1990s [2, 3]. Penetrante et al. [3, 7, 13] propose that the electron mean energy in the DBD reactor is weakly dependent on reactor parameters under identical gas composition and temperature. The performance of DBD reactor for NO conversion are mainly dependent on energy consumption and gas composition. For DBD in H2O/O2/N2 system, NO is mainly oxidized to NO2 and HNO2 by O and OH, respectively [3, 14, 15]. Jõgi et al. [16] proposed that the presence of back-reaction NO2 + O→NO + O2 leads to the saturation of NO oxidation in O2/N2 system. NTP generated in gas with oxygen shows high oxidative potential, which is beneficial for the conversion of NO to NO2.
Investigation of NF and NO removal in DBD reactor, even for a system as simple as H2O/O2/N2, requires a reaction mechanism involving many species such ions, atoms, radicals, and molecules. Among all of the plasma chemical reactions, which one is the dominant reaction depending on the quantity of active species and the reaction rate involving these species. The co-existence of reactive species for NO formation and loss make DBD chemistry such a complex system. Effects of energy input and gas composition on the performance of DBD chemical process are the most industrial interests. However, there are no systematic studies on NO formation and loss in one investigation.
Present study is devoted on investigation of the effect of SED and gas composition on NO evolution and byproducts formation in DBD process. The simulated gas has been introduced into the DBD reactor with a constant flow rate under the ambient temperature and pressure. In “Energy Consumption and of DBD System” section, the effect of gas composition on electrical characterization has been investigated. In the next two sections, the performance of DBD reactor was investigated in six different systems: (1) NO synthesis in O2/N2, H2O/N2 and H2O/O2/N2 systems (“NO Formation in DBD System” section); (2) NO removal in NO/O2/N2, NO/H2O/N2 and NO/H2O/O2/N2 systems (“NO Conversion in DBD System” section). Finally, a comparison of NOx and N2O concentration as a function of SED with and without initial NO was investigated in “NOx Evolution and N2O Production” section.
Experimental Setup
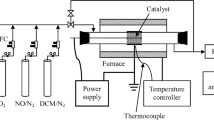
The schematic of the experimental setup is shown in Fig. 1. NTP was obtained in a coaxial cylinder-type DBD reactor energized by a high-voltage power supply. The discharge gap is fixed at 2 mm with a total discharge volume of 2.5 mL. Gas composition and flow rate were controlled by mass flow controllers (MFC). Water vapor was added to the feed by bubbling N2 through an H2O saturator kept at 303 K. A total flow rate of 0.3 ± 0.01 L/min, which was equal to a residence time of 0.5 s, was maintained in DBD stage. Six different systems were used to investigate the effect of oxygen and water vapor on DBD performance, as shown in Table 1.
Electrical Characterization
The power supply employed in the experiments delivered a sinusoidal AC output voltage of up to 20 kV, with frequency varying from 5 to 20 kHz. The output ac voltage was applied to the stainless steel rod in the reactor and measured by using a capacitive divider (1000:1), where the wave form of the sinusoidal ac voltage is displayed on the oscilloscope. The other electrode made of copper mesh (20 mesh) was grounded through either a charge-probe capacitor Cm = 0.47 μF or a resistor R = 50 Ω in series with a switch as shown in Fig. 1. The discharge power of the DBD reactor was determined from the enclosed area of Lissajous figure. The specific energy density in joules/liter (J/L) was calculated using the formula:
where the P is the discharge power calculated from V–Q Lissajous figure (J/s), Q is the flow rate of gas in L/s.
Chemical Analysis
The concentration of NO, NO2 and N2O was analyzed online by Nicolet iS50 Fourier transform infrared (FTIR) spectrometer (Nicolet iS50, Thermo Fisher scientific, America, pathlength is 2.4 m) operation in the adsorption mode, with a resolution of 4 cm−1. Characteristics features were obtained for NO2 in the range of 1660–1540 cm−1 [17], for NO in the range of 1980–1860 cm−1 [1, 17], for N2O in the range of 2270–2140 cm−1 [18] and for O3 the range was 1080–940 cm−1. The above chosen wavenumber ranges provided signature FT-IR plot characteristic of each molecular species, and obey the Beer–lambert law: I = I0 exp(− συNL), where συ is absorption cross-section, N the molecular density and L the absorption path length. The absorbance of each line was then given by A = ln(I0/I). The oxygen content were detected by flue gas analyzer (Kane, KM9106).
The energy cost in mega-joules per mole for the conversion of NO was calculated using the formula:
where NOinlet and NOoutlet are the NO concentration of inlet and outlet of the reactor in ppm; SED is the specific energy density (J/L); “A” is a constant equal to 24.5, 1 mol is equal to 24.5 L at 25 °C.
Results and Discussion
Energy Consumption and of DBD System
The DBD geometry designed for this research is a typical coaxial-tube with a double quartz wall and coupled to an ac high-voltage (HV) source, as shown in Fig. 1. The measured probe voltage across the capacitor Cm (0.47 μF) is proportional to the charge crossing the electrode [19, 20]. The measured operating voltage and charge values are plotted against each other in Q–V figures, which are better known as Lissajous figures. Using on period of voltage measured at the point of M and N (Fig. 1), the characteristic V–Q Lissajous curves at applied power of 24 W were plotted (Fig. 2). The energy dissipated for discharge per one cycle at applied power of 24 W is varied between different systems: 1.04 × 10−3 J for the O2/N2, H2O/N2 and H2O/O2/N2 system at 9.92 kHz; 0.98 × 10−3 J for the N2 system at 9.80 kHz. The ratio of discharge power to applied power for 9.92 and 9.80 kHz are 43 and 40%, respectively. In presence of H2O and O2, the effect of gas composition on power used to discharge is not obvious at a fixed applied power.
DBD reactor is generally considered as a capacitive load. The slope of the V–Q Lissajous figure before electrical discharge, A–B or C–D, represents capacitance of DBD reactor which is estimated to 7.2 pF. Dielectric capacitance of dielectric tubes (Cd), slope of B–C and A–D, are estimated to 16 pF for plasma on. The results in Fig. 2 show that gas composition has little effect on the capacitance of DBD reactor. Breakdown voltage (Ub), the lowest voltage value at which the gas turn to plasma, can be also obtained from Q–V Lissajous figures. The Ub shows a little decrease with O2 or H2O feeding into DBD reactor compared with pure nitrogen plasma. For a power supply with a given voltage and frequency, lower Ub indicate longer discharge duration.
The discharge power of different applied power calculated from Q–V Lissajous figures are shown in Fig. 3. The average discharge power (Pave) of different systems was calculated from the discharge power of DBD reactor at a fixed applied power. Figure 3 (dashed line) shows the calculated Pave of discharge power as a function of applied power. The increase of average discharge power is nearly linear with the applied power increasing from 10 to 30 W. The changes of discharge power among different gas components are negligible for a given set applied power lower than 24 W. The ratio of discharge power to applied power increases with frequency decreasing from 10.2 to 9.8 kHz.
NO Formation in DBD System
Identification of NxOy and HNOx by IR Spectroscopy
Figure 4 presents typical FTIR spectra of different systems (O2/N2, H2O/N2 and H2O/O2/N2) at SED of 2080 J/L. The main products contain NO, NO2, N2O, N2O5, HNO2 and HNO3, and the corresponding spectral performance are listed in Table 2.
In the O2/N2 system, NO and NO2 were observed in the outlet gas by using the FTIR around 1900 and 1600 cm−1, respectively. The limiting step in NO production is the breaking of the strong triple bond of the N2 molecule (9.77 eV). The NO produced from the chain reaction O(3P) + N2*(1Σg) → NO + N and N + O2 → NO + O will be quickly oxidized to NO2 by O3 and O [11, 27]. The spectra of N2O5 and N2O were also observed. The spectral behavior of N2O5 is very smiliar to that of N2O4: νas(N–O) at 1720–1750 cm−1, νs(N–O) at 1300–1340 cm−1, and δ(NO2) at 750 cm−1. In addition, N2O5 manifests a band near 860 cm−1, which is different with N2O4. As shown in Fig. 4b (E-1), the band near 860 cm−1 combined with band at 1700 and 1320 cm−1 may be the spectral behavior of N2O5. The known routes for N2O5 formation are via strong oxidation of N2O4 by ozone, interaction of NO3 with NO2, or dehydration of HNO3 [18]. In the O2/N2 system, N2O5 may be mainly produced from the interaction of NO3 with NO2.
In the H2O/N2 system, the main products are NO and NO2 with small fraction of HNO2. The IR behavior of HNO2 and HNO3 are similar: 1680–1700, 1260–1300 and 790–820 cm−1 for HNO2; 1700, 1300–1330, 880–900 cm−1 for HNO3. The spectral behavior of HNO2 and HNO3 overlap with each other near 1700 and 1300 cm−1. The spectral differences between the two are 790–820 cm−1 for HNO2 and 880–900 cm−1 for HNO3. As shown in Fig. 4b, the bands at 791 and 853 cm−1 are believed to represent the N–O single bond valence vibrations of trans- and cis-tautomery of HNO2 molecule, respectively (E-3 and E-5). In the case of H2O/N2 system, the NO can be formed from the reaction between NH radical and hydroxyl radical (OH) [6].
Lowest electronically excited state of N2, \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\), and first electronically excited states of N, N(2D), play a key role in the formation of NH and OH [6].
The HNO2 and NO2 in the H2O/N2 plasma are generated form the reaction [28, 29]:
In the H2O/O2/N2 system, the main products are NO and NO2 with small fraction of HNO2 and HNO3. NO can be also formed by the reaction [6]:
An additional shoulder at 1698 cm−1, and bands at 1360 and 1314 cm−1 were observed, which may be attributed to HNO3 [30]. Very little information, however, can be found in the literature concerning the quantitative analysis of nitric acid vapor in such gas mixtures. Owing to the reaction of nitrogen oxides with HO, nitric acid vapor (HNO3) and nitrous acid vapor (HNO2) can be formed, especially at higher concentrations of nitrogen dioxide [6].
Effect of SED on NO and NO2 Concentration
NO and NO2 concentration as a function of SED for different gas compositions (O2/N2, H2O/N2 and H2O/O2/N2) are shown in Fig. 5. The O3 was only observed in the O2/N2 plasma with the SED below 1600 J/L. The formation of ozone is strongly affected by temperature because of thermal decomposition of ozone, and the formation of NO will further aggravate the depletion of O3. For a fixed SED, NO concentration of different systems are ordered as follows: H2O/N2 > O2/N2 > H2O/O2/N2. NO2 concentration of different systems follows the opposite order.
In the O2/N2 system, the dissociation of O2 and N2 is the main chemical process. The energy cost of O2 dissociation in atmospheric pressure NTP is 6.0 eV for production of two O atoms in the ground state and 8.4 eV for production of one O atom in the ground state and second in the O(1D) state [31]. Most of O(1D) is quenched by O2 to the ground state O(3P). Since electron mean energy in DBD process is between 3 and 5 eV [7], most of the O atoms in dry air come from the impact with excited electronic states of N2. It is well known that dissociation energy of oxygen (5.2 eV/O2) is lower than that of nitrogen (9.79 eV/N2) [32]. O2 dissociation is energetically favorable reaction pathways compared with N2 dissociation. At low SED, the oxidation of NO to NO2 is accomplished via R9.
The reactor temperature increases with the increase of SED. It is not favor for NO oxidation, which is an exothermic reaction. A significant amount of the O radicals can reduce NO2 back to NO:
Therefore, the NO2 concentration increase firstly then decrease with the increase of SED.
In the H2O/N2 system, NO concentration is higher than NO2 concentration. It is clear that the rate of NO2 formation from the reaction R4 and R5 involving NO, HO and HNO2 is slower than that of NO formation. NO concentration increase with SED increasing from 400 to 2000 J/L then decrease with SED further increasing. The reason for this is that the increase of NO concentration and SED will cause product’s reaction involving N.
In H2O/O2/N2 system, the increase of NO2 concentration was nearly linear with the increase of SED from 400 to 3000 J/L in plasma. In the presence of H2O and O2, the importance of O(1D) lies in their high reactive activity with contaminant molecules and their reactions with H2O to produce HO [6]. NO generated form NTP process will be quickly oxidized to NO2 and HNO2.
NO Conversion in DBD System
In NO/O2/N2 system, the outlet products are NO and NO2 with small fraction of byproduct N2O (Fig. 6, E-2). N2O5 formation was not observed in this system. The initial NO will suppress the formation of ozone. NO3 forms from NO2 oxidation is also suppressed in the presence of NO. Since N2O5 are mainly formed from oxidation of N2O4 by ozone and interaction of NO3 with NO2, the formation of N2O5 would be suppressed with addition of NO. Atomic N and O are two major reactive species which contribute to reduction and oxidation, respectively. NO was mainly oxidized to NO2 by atomic O at SED of less than 1000 J/L. NO and NO2 interconvert into each other via reaction NO + O→NO2 and NO2 + O→NO + O2. The increase of O concentration with the increase of SED can improve the back reaction NO2 + O→NO + O2, which will reduce the conversion of NO-to-NO2. As shown in Fig. 7b, NO2 concentration increase firstly then decrease with the increase of SED in the NO/O2/N2 system. NO conversion efficiency increases with SED increasing from 400 to 2000 J/L (Fig. 7). Further increase of SED will reduce the conversion efficiency of NO-to-N2 due to product’s reaction of N2 and O2 to form NO. The energy cost of NO conversion increases nearly linear with the increase of SED in NO/O2/N2 system (Fig. 9). Limitless increase of SED is not favor for NO conversion due to the interconversion of NO and N2, NO and NO2.
The characteristic FTIR spectrum of NO, NO2, N2O, HNO2 and HNO3 were observed in the NO/H2O/N2 system, as shown in Fig. 6 (E-4). NO2 concentration is smaller than other two systems, which indicates the conversion efficiency of NO-to-NO2 is lower in NO/H2O/N2 system. The HO and N are main species contribute to NO conversion. The NO-to-NO2 conversion occur in chain reactions involving HO. Quantitative analysis of HNO3 vapor in such gas mixtures was not carried out in this work. The relative content of HNO2 was calculated from the intensity of FTIR spectra from 720 to 910 cm−1. Figure 8 presents the integral area of HNO2 FTIR spectrum of different systems. The evolution of HNO2 content with the increase of SED follows the similar trend as the evolution of NO2 concentration. The NO is oxidized to HNO2 by OH, further the NO2 is formed by the reaction HNO2 with HO. The most important chain breaking reactions are the formation of nitric acid (HNO3) from the collision reaction between HO and NO2.
In the NO/H2O/O2/N2 system, the co-existence of ROS and RNS have been proved in, thus the NO can be removed through oxidation and reduction. Oxidizing agent (O, HO and HO2) is generated from collision reaction. In terms of their oxidizing potential, HO (2.8 V) is second only to fluorine (2.87 V) and exceeds other oxidizing agents such as O3 (2.07 V), O2 (1.23 V), and nitric acid (0.94 V). The chain reaction involving HO radicals play an important role in the NO conversion during NTP chemical process. As shown in Figs. 5b and 7b, NO2 concentration increases with SED increasing from 500 to 3500 J/L in H2O/O2/N2 system with and without initial NO, which is different compared with that in O2/N2 and H2O/N2 system. The reason for this may be that NO2 molecules are produced from the reaction between bulk gas H2O, O2 and N2.
Figure 9 shows the energy cost as a function of SED for different gas compositions. In the NO/O2/N2 system, the energy cost for NO conversion increase linearly with the increase of SED. In the presence of H2O, the energy cost decreases with SED increase from 500 to 1250 J/L, then increases with further increase of SED. There are two reasons for this: the first one is that NO oxidation efficiency increase firstly then decrease with further increase of SED, the other reason is that NO is formed from bulk gas at higher SED. It seems that there is an optimal SED to achieve lowest energy cost for NO conversion (1250 J/L in this work).
NOx Evolution and N2O Production
Nitrogen oxides (NOx: NO and NO2) as a function of SED for different gas composition are shown in Fig. 10a. The quantitative gap of NOx concentration between DBD processes with and without initial NO decreases with SED increasing from 400 to 2500 J/L. For the SED more than 2500 J/L, the difference of NOx concentration between DBD systems with and without initial NO are maintained at a constant. For DBD process, the NTP is initiated by the collision between energetic electrons and bulk gas (H2O, O2 and N2). The evolution (production or loss) of NOx in DBD reactor is determined by the concentration of radicals and excited species such as \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\), N, O, HO and NH. Since these reactive species play an important role on both formation and loss of NOx, it is infeasible to increase products yield by unlimited increase of SED due to the product’s reaction.
N2O is a greenhouse gas and also contributes to stratospheric ozone depletion. In this section, special attention is given to N2O. As a byproduct of NTP process, N2O was observed in all NTP processes. N2O concentration as a function of SED is shown in Fig. 10b. N2O is usually considered as a by-product of NO conversion by NTP process. Zhu et al. [15] and McLarnon et al. [3] claimed that NTP alone convert NO to NO2, then part of NO2 will be reduced by N to form N2O via reaction NO2 + N→N2O. However, the N2O concentration decrease with NO initial concentration increasing from 0 to 500 ppm. The reason for this is that NO molecule is an excellent source for physical quenching of \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\), which would suppress the N2O formation in DBD reactor. For the NTP generated by DBD, N2O is mainly a product from NTP process itself. The N2O molecules are mainly formed upon collision of \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\) with O2 [33]. Partial N2O may be formed from the reaction involving O(1D) and NH radicals [6, 33]. The reaction rate coefficient of R14 is 3.5 × 10−37 cm6/s [34]. The competing reaction route N2 + O(1D) → O(3P) + N2 resulting in the quenching of O(1D) has reaction rate coefficient of 2.5 × 10−11 cm3/s. The NO concentration of R15 is always at ppm level. Therefore, the R13 is the dominant source of N2O formation.
On the other hand, the reactive species (O(1D) and \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\)) also play an important role on N2O conversion [33, 35].
N2O concentration reaches a saturation with SED increasing from 500 to 1500 J/L, as shown in Fig. 10b. The N2O concentration shows ignorable changes with SED increasing from 1500 to 3000 J/L. The reason for this may be that to \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\) plays a dual-function role on N2O formation and loss. The increase of \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\) and N2O concentration improves the product’s reaction contributing to N2O loss. O(1D) also plays an important role on the N2O conversion.
Conclusion
In the DBD applications, the plasma chemistry is initiated from the collision reaction between energetic electrons and substrate molecules. The atomic oxygen (O) and hydroxyl radical (OH) generated from dissociation of O2 and H2O play an important role on the formation and conversion of NO, NO2, HNO2, HNO3 and N2O. The co-existence of different reaction pathways, such as N2-to-NO, NO-to-N2, NO-to-NO2, NO2-to-NO, N2-to-N2O, NO-to-HNO2, NO-to-HNO3 and HNO2-to-NO2 make the DBD such a complex system. Due to interconversion of reactant and product, limitless increase of SED is not favor for energy efficiency of DBD reactor. N, O and OH can react with either reactants or products, thus leading the interconversion of N2 and NO, NO and NO2, NO and HNO2, respectively. The byproducts N2O was observed in all DBD process. N2O was mainly formed by the collision reaction involving reactive species such as \({\text{N}}_{ 2} ( {\text{A}}^{ 3}\Sigma _{\text{u}}^{ + } )\), N, O and NH.
In the DBD systems without initial NO, the amounts of NOx production increase with the increase of SED from 400 to 2000 J/L in the system O2/N2 and H2O/N2. Further increase of SED will take more atomic nitrogen N into the plasma chemical process, which is favor for the NO reduction. The production of N2O, NO2 and HNO2 increase with SED increasing from 500 to 3500 J/L in the system H2O/O2/N2.
In DBD system for NO conversion, there is an optimum SED at which the best NO conversion efficiency will be obtained. NO oxidation is the main reaction pathway in the DBD process in the presence of O2 and H2O. NO oxidation following with absorption, adsorption or fast selective catalytic reduction is an ideal technology for NOx removal.
References
Hessel V, Anastasopoulou A, Wang Q, Kolb G, Lang J (2013) Catal Today 211:9–28
Penetrante BM, Hsiao MC, Merritt BT, Vogtlh GE, Wallman PH (1995) IEEE Trans Plasma Sci 23:679–689
McLarnon CR, Penetrante BM (1998) Society of automotive engineers fall fuels and lubricants meeting 1998, San Francisco, CA, pp 19–22
Kossyi AYKIA, Matveyev AA, Silakov VP (1992) Plasma Sources Sci Technol 1:207–220
Fridman A, Chirokov A, Gutsol A (2005) J Phys D Appl Phys 38:R1–R24
Teodoru S, Kusano Y, Bogaerts A (2012) Plasma Process Polym 9:652–689
McLarnon CR, Penetrante BM (1998) Society of automotive engineers fall fuels and lubricants meeting, p 982434
Patil BS, Cherkasov N, Lang J, Ibhadon AO, Hessel V, Wang Q (2016) Appl Catal B 194:123–133
Miessner H, Francke KP, Rudolph R (2002) Appl Catal B Environ 36:53–62
Tonkyn RG, Barlow SE, Hoard JW (2003) Appl Catal B 40:207–217
Atkinson R, Baulch DL, Cox RA, Hampson RF, Kerr JA, Rossi MJ, Troe J (1997) J Phys Chem Ref Data 26:1329
Fridman A (2012) Plasma chemistry. Cambridge University Press, New York
Penetrante BM, Brusasco RM, Merritt BT, Pitz WJ, Vogtlin GE, Kung MC, Kung HH, Wan CZ, Voss KE (1998) Society of automotive engineers fall fuels and lubricants meeting, p 982508
Fujii YAT, Yoshioka N, Rea M (2001) J Electrostat 51–52:8–14
Zhu A-M, Sun Q, Niu J-H, Xu Y, Song Z-M (2005) Plasma Chem Plasma Process 25:371–386
Jõgi I, Levoll E, Raud J (2016) Chem Eng J 301:149–157
Nakamoto K (2009) Infrared and Raman spectra of inorganic and coordination compounds. John Wiley & Sons, Inc., Hoboken, New Jersey
Hadjiivanov KI (2000) Catal Rev 42:71–144
Grundmann CTS (2009) Int J Heat Fluid Flow 30:394–402
Borcia G, Anderson CA, Brown NM (2003) Plasma Sources Sci Technol 12:335–344
Kantcheva M, Cayirtepe I (2006) J Mol Catal A Chem 247:88–98
Bibart CH, Ewing GE (1974) J Chem Phys 61:1284–1292
Cantrell CA, Davidson JA, McDaniel AH, Shetter RE, Calvert JG (1988) Chem Phys Lett 148:358–363
Guillory WA, Hunter CE (1971) J Chem Phys 54:598–603
McGraw GE, Bernitt DL, Hisatsune IC (1965) J Chem Phys 42:237
Al-Abduly A, Christensen P (2015) Plasma Sources Sci Technol 24:065006
Golde M (1988) Int J Chem Kinet 20:75–92
Wang T, Sun B-M, Xiao H-P, Wang D, Zhu X-Y, Zhong Y-F (2013) Plasma Chem Plasma Process 33:681–690
Yin S-E, Sun B-M, Gao X-D, Xiao H-P (2009) Plasma Chem Plasma Process 29:421–431
Jones LH, Badger RM, Moore GE (1951) J Chem Phys 19:1599
Eliasson B, Hirth M, Kogelschatz U (1987) J Phys D Appl Phys 20:1421–1437
Zhao G-B, Hu X, Argyle MD, Radosz M (2004) Ind Eng Chem Res 43:5077–5088
Higashi SUM, Suzuki N, Fujii K, Trans IEEE (1992) Plasma Sci 20:1–12
NIST chemical kinetics database. http://kinetics.nist.gov/kinetics/index.jsp
Green JHD (2001) Plasma Chem Plasma Process 21:459–481
Acknowledgements
This study was primarily supported by National Key R&D Program of China (2017YFC0210303-01). This work was also partly supported by National Natural Science Foundation of China (21677010, U1660109).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, X., Wang, J., Yi, H. et al. Nitrogen Fixation and NO Conversion using Dielectric Barrier Discharge Reactor: Identification and Evolution of Products. Plasma Chem Plasma Process 38, 485–501 (2018). https://doi.org/10.1007/s11090-018-9876-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-018-9876-4