Abstract
Gasification of several organic materials in steam plasma generated in a special plasma torch with a water-stabilized arc was investigated. Thermal plasma with very high enthalpy and low mass flow rate is produced in an arc discharge which is in direct contact with water. Biomass and several types of solid and liquid organic waste were gasified by plasma aided reactions of materials with water, carbon dioxide and oxygen. Composition of produced gas, energy balance of gasification process and gasification efficiency were determined from measured data. Synthesis gas with high content of hydrogen and carbon monoxide and very low content of carbon dioxide, light hydrocarbons and tar was obtained for all tested materials. Comparison of measured data with results of theoretical computations confirmed that steam plasma gasification produces syngas with composition which is close to the one obtained from thermodynamic equilibrium calculations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The recent focus on resource recovery in the waste industry, i.e. material and energy recuperation, has triggered the search for more advanced waste treatment technologies, from which thermal plasma treatment has been recognized as a promising method. Among various waste treatment processes, thermal plasma treatment offers specific performance characteristics. Heat supplied by plasma is used for melting and vitrification of inorganic materials and for gasification of organic substances. While decomposition of waste and dangerous materials in thermal plasmas has been intensively studied in the last decade [1–10], and industrial-scale systems for treatment of various types of waste have been installed [11–16], waste to energy process through plasma gasification of organics is a newly appearing application. The principal goal of the gasification is a production of fuel gases, principally the mixture of carbon monoxide and hydrogen, called syngas, characterized by maximum heating value and purity. Thermal plasma enables decomposition of organics by pure pyrolysis in the absence of oxygen, or with a sub-stoichiometric amount of added oxygen (gasification), leading to the production of high quality syngas, with high content of hydrogen and carbon monoxide and minimum presence of other components. In the gasification process, oxygen is supplied to balance carbon and oxygen molar concentrations for a maximum production of CO. As the main goal of this technology is the production of fuel gas, the energetic balance of the process as well as syngas quality and its high heating value are much more important than in the case of waste treatment, where the principal goal is material decomposition.
A number of non-plasma systems have been developed for the production of syngas from organic waste and biomass [17, 18]. In a widely used gasification process based on partial material oxidation, a solid or liquid carbonaceous material containing mostly chemically bound carbon, hydrogen and oxygen, reacts with air or oxygen. Oxidizing reactions provide sufficient exothermic energy to produce a primary gaseous product containing mostly CO, H2, CO2, H2O (steam), and a small amount of higher hydrocarbons. Heat from external sources is usually supplied into the reactor to control the process and the reaction temperature, but most of the heat required for the reaction usually comes from the calorific value of the material. The process is also known as autothermal. The principal limitations of autothermal gasification technologies are the low heating value of the syngas and the production of tar formed by complex molecules of hydrocarbons created during the process at lower temperatures. The gas from low-temperature gasification typically contains only 50% of energy in syngas components CO and H2, while the remainder is contained in CH4 and higher aromatic hydrocarbons [18]. The possibility of controlling syngas composition in autothermal process is limited. The need for production of clean syngas with controlled composition results in the use of technologies based on external energy supply. Energy can be carried into the gasification reactors by hot gases or solids (sand), a relatively new method is based on the use of thermal plasmas.
Plasma pyrolysis and gasification leading to the production of syngas [19] is an alternative to non-plasma methods of organic waste and biomass treatment. Plasma is a medium with the highest content of energy, and therefore substantially lower plasma flow rates are needed to supply the sufficient amount of energy, compared to other media used for this purpose. The result is minimum contamination and dilution of the produced syngas by plasma gas and an easy control of syngas composition. The process also acts as energy storage—electrical energy is transferred into plasma enthalpy and then stored in the produced syngas.
Plasma treatment offers a better control over the temperature of the process, higher process rates, lower reaction volume and especially an optimum composition of produced syngas. The process exploits the thermochemical properties of plasma. Decomposition of the material is achieved by the action of the kinetic energy of plasma particles, which is extremely high due to high temperatures of the plasma. In addition, the presence of charged and excited species makes the plasma environment highly reactive, which can catalyze homogeneous and heterogeneous chemical reactions. The main advantage of plasma consists in much higher enthalpy and temperature of plasmas compared to those of gases used in non-plasma methods. Thus, substantially lower plasma flow rates are able to carry sufficient energy for the process, and the composition of the produced syngas is not much influenced by plasma gas composition. Moreover, substantially less energy is required to heat the plasma to the reaction temperature.
Various plasma gases are used in plasma waste treatment systems, usually air, nitrogen, argon, steam, or carbon dioxide. Air plasma is the cheapest option, but produced gas is diluted by high amount of nitrogen, nitrogen presence can also contribute to formation of nitrogen oxides in output gases. Steam plasma offers optimal characteristics for waste treatment, namely high enthalpy (energy content in given amount of plasma), high heat transfer to treated material, and optimal composition (hydrogen and oxygen). The problem of plasma gases containing oxygen, and especially steam, follows from higher electrode erosion rates in arc torches. Electrode erosion is low for argon plasma, but argon plasma has low enthalpy and low thermal conductivity, and consequently low capacity of energy transport into the reactor and low rate of heat transfer from plasma to treated material.
The composition of produced syngas can be controlled by the selection of an additional oxidizing agent. Common oxidizing agents used for organics gasification are oxygen, air, CO2, steam or water. Oxygen and air gasification lead to the higher energy efficiency, as partial oxidation of material produce additional energy. A higher amount of oxygen results in an increase of CO2 content in syngas, in case of air the produced syngas is diluted by high amount of nitrogen. Steam and CO2 plasmas can be used for production of pure syngas containing high amount of hydrogen and CO and minimum other components, but energy efficiency is reduced as additional energy is needed for dissociation of CO2. Syngas with the highest calorific value is produced if molar concentrations of carbon and oxygen in all input reagents, i.e. in treated material, oxidizing agents and plasma, are balanced.
This paper presents the results of gasification of several organic materials in steam plasma generated in a special plasma torch with a water-stabilized arc, where plasma is produced in an arc discharge which is in direct contact with water. The specific feature of this type of plasma torch is very low flow rate of plasma and consequently very high plasma enthalpy and temperature. Although the plasma temperature is substantially higher than the temperature needed for gasification process, high plasma enthalpy and temperature offer specific advantages for gasification process, as will be shown in the paper. Gasification of organic materials by steam plasma stimulated reactions of materials with carbon dioxide, steam and oxygen was studied.
Experimental System
Water/Gas Plasma Torch
Plasma was generated in a dc arc plasma torch with stabilization of an arc by combination of gas flow and water vortex. This principle of arc stabilization is characterized by extremely high plasma enthalpy and temperature, and very low plasma flow rate [20, 21]. The typical arrangement of an arc chamber with water/gas stabilization is shown in Fig. 1. The cathode part of the torch is arranged similarly like in gas torches. Gas (argon) is supplied along tungsten cathode tip, vortex component of gas flow that is injected tangentially, assures proper stabilization of arc in the cathode nozzle. Argon plasma flows through the nozzle to the second part of the arc chamber, where an arc column is surrounded by a water vortex. The water-stabilized arc chamber is divided into several sections, where water is injected tangentially and water is kept by a centrifugal force on the walls of the chamber. The inner diameter of the vortex is determined by the diameter of the holes in the segments between the sections. The sections with tangential water injection are separated by two exhaust gaps, where water is exhausted out of the arc chamber. Interaction of the arc column with the water vortex causes evaporation from the inner surface of the vortex. The steam mixes with the argon plasma flowing from the cathode section. Steam evaporation is the main mechanism of plasma gas production, and the arc plasma is created by a mixture of argon with steam with prevailing contents of hydrogen and oxygen. An anode is created by a rotating copper disc with internal water cooling [22].
The values of basic parameters of plasma torch and generated plasma jet are given in Table 1. Plasma is composed of hydrogen and oxygen produced by water evaporation and dissociation, and small amount of argon. Molar concentrations of hydrogen, oxygen and argon species (atoms plus ions) are given in the table. Compared to gas/stabilized plasma torches which are commonly used for plasma waste treatment and organics gasification, the water/gas plasma torch is characterized by very low plasma flow rates and high values of plasma enthalpy and temperature. The utilization of high enthalpy, high temperature steam plasma offers several advantages for plasma gasification process. The temperature in the reactor could be easily controlled by a choice of combination of arc power and material feed rate. Very high temperatures can be achieved if necessary, moreover high level of UV radiation exists in positions in locations close to the plasma input to the reactor, which contributes to the dissociation of molecules of higher hydrocarbons. As only small amount of plasma gas enters the reactor, the composition of produced syngas can be controlled by a choice of added reactants in a wide range of ratio H2/CO. Moreover, plasma is composed mostly of hydrogen and oxygen and thus syngas is not diluted by the other gases.
Plasma Gasification Reactor
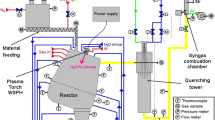
The scheme of plasma reactor PLASGAS is shown in Fig. 2. Plasma torch with hybrid water/gas stabilization of arc is attached at the top of the reactor. The water cooled walls of the reactor are separated from the inner reactor volume by 400 mm thick sandwich ceramic walls for a reduction of power loss. Temperature of inner surface of ceramic insulation was measured in several positions indicated in Fig. 2. The power loss to the walls at different parts of the reactor was determined from calorimetric measurements on cooling circuits. Treated solid material was supplied into the reactor by screw feeder with controlled feed rate. Material particles or liquid material stream enter plasma jet at a location about 500 mm downstream of plasma input. As most of treated organic materials had higher content of carbon than oxygen, a certain amount of oxygen was supplied into the reactor to balance total molar fractions of carbon and oxygen in input reagents. The oxygen content was increased by adding carbon dioxide, water or oxygen to achieve the value of equivalence ratio (ratio of molar concentrations of oxygen and carbon) close to 1.
Gaseous reaction products were fed into the quenching chamber where their temperature was reduced to 300 °C in water spray with automatically controlled flow rate. The gas was then exhausted by the water jet pump into the filter and cyclone where solid particles were separated. A water ejector installed between the filter and the cyclone maintained the reactor at the slight under-pressure of several hundreds of Pa. Gas was then fed into the afterburning chamber where it was combusted.
The measuring system included monitoring of plasma torch operation parameters, measurement of inner wall temperatures in several positions inside the reactor, calorimetric measurements on cooling water loops, and monitoring of flow rates of added reaction gases and water. The flow rate of produced syngas was measured using a Pitot tube flow meter. As this measurement was complicated by a high level of signal fluctuations, the other method of syngas flow rate evaluation was used which was based on a measurement of concentration of argon in produced gas for a case with known amount of argon added into the reactor. The gas temperature was measured at the input and output of the quenching chamber by thermocouples. A sampling probe for composition measurements was located at the reactor output before the quenching chamber, the second sampling probe was positioned after the filter. A quadrupole mass spectrometer Pfeiffer Vacuum Omnistar GSD 301 with direct inlet was used as a main gas analyser. A freezing unit is placed between the mass spectrometer and the sampling probe to avoid water condensation and the damaging of the mass spectrometer. From this reason, the efficiency of the gasification process is preferably calculated as a carbon yield.
The samples for tar analysis were withdrawn from the duct between the gasification chamber and the quenching chamber. The samples were captured onto DSC–NH2 adsorbent or silica gel positioned at the tube cooled by water spray. Liquid chromatography with UV and fluorimetric detection of the condensates was used for the analysis.
For a reduction of solid carbon production and an increase of CO output, various combinations of oxidizing media were added. Gas oxidizers (oxygen and carbon dioxide) were supplied through gas input points in the upper part of the reactor close to the plasma input. Water was mixed with supplied material in the input tube of the reactor.
Gasification of Biomass
Gasification Processes
Gasification of wood saw dust and wooden pellets were studied. Wood as most of organics contains more carbon atoms than oxygen atoms. To ensure maximum yield of carbon monoxide and to avoid formation of solid carbon, a certain amount of oxygen was supplied into the reactor to balance total molar fractions of carbon and oxygen in input reagents. Oxygen molar fraction was increased by an addition of oxygen, carbon dioxide, and water. Most of energy for endothermic process of material gasification is supplied by plasma, in case of usage of oxygen an additional energy comes from oxidation of volatilized material. If carbon dioxide or water are added, additional energy is needed for their dissociation. In an ideal gasification process all carbon atoms are bound in CO molecules and produced syngas is composed only from carbon monoxide and hydrogen. Ratio of molar fractions of hydrogen to carbon monoxide, which is an important parameter for syngas utilization, can be controlled by combination of added oxidation agents. Three basic chemical reactions for gasification of organic material represented by the formula CnCHnHOnO can be written as follows.
Gasification by reaction with oxygen:
Gasification by reaction with carbon dioxide:
Gasification by reaction with water:
where nC = c/MC, nH = h/MH and nO = o/MO are molar concentrations of carbon, hydrogen and oxygen in treated material M with mass fractions of carbon, hydrogen and oxygen equal to c, h and o, respectively.
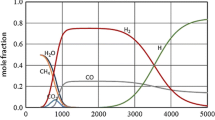
Various combinations of the three reactions (1–3) were used in the experiments. Besides these three basic reactions a number of other chemical reactions takes place in the reactor, for sufficiently long residence time an equilibrium state is achieved, and the composition of reactions product can be then determined by thermodynamic calculations. Figure 3 presents the temperature dependence of composition in a system containing mass fractions of carbon, hydrogen and oxygen for wood with added carbon dioxide and steam plasma corresponding to amounts of reagents specified in the figure caption. The equilibrium composition of this heterogeneous system was calculated using the method described in [23], the input data for calculations of standard reaction enthalpy and standard thermodynamic functions of system components were taken from database [24]. Molar fractions of components in the gas phase are shown for gas components H2, H2O, CO, CO2, CH4, and C2H2. For solid carbon (C(C)) the ratio of a number of solid carbon moles to a number of all moles in the gas phase is given. Formation of solid carbon can be suppressed if more oxidizing medium is added.
It can be seen that at temperatures above 1200 K the wood is decomposed into hydrogen, carbon monoxide and solid carbon with small amount of other components. For higher input of oxidizing media the production of solid carbon can be suppressed. It can be concluded that for production of syngas with high content of hydrogen and carbon dioxide it is necessary to supply not only energy needed for material volatilization and chemical reactions leading to syngas production, but also energy for heating of all reactants to the temperature 1200 K. Figure 4 shows components of energy balance of wood gasification by reaction with oxygen, carbon dioxide and water with amounts of oxidizing media corresponding to the Eqs. (1–3).
The first columns represent energy needed for transformation of wood into gas composed of CO and H2, calculated from the difference of heating value of CO/H2 mixture and heat of combustion of wood [25], the second column is energy spent for heating of all reagents to the temperature 1200 K. The total reaction enthalpy is a sum of all energies needed for gasification. Low heating values (LHV) of produced syngas, i.e. heating values without the heat produced by steam condensation, are shown in the last columns.
It can be seen that when oxygen is used as oxidizing agent, the ratio of syngas heating value to the total enthalpy needed for realization of the process is 7.7. When water or carbon dioxide are used, this ratio is almost the same for the both cases, and it is approximately 3. The ratios would be 7.9 and 3.4 if the energy obtained by cooling syngas from reaction temperature 1200–300 K was also utilized.
Detailed energy balance of gasification of 1 kg dry wood, with oxygen and CO2 at a ratio of oxygen/carbon moles = 1 and at reaction temperature of 1200 K is shown in Table 2. If CO2 is used as oxidizing medium the ratio of heating value of syngas to energy needed for syngas production is lower than in the oxygen process, as additional energy is needed for CO2 dissociation. The last two columns show the ratio of syngas LHV to energy which must be supplied by external source (energy consumption), and the ratio of syngas LHV to total energy input (energy supplied by external source plus heating value of wood).
The ratio of syngas LHV to energy consumed can be increased in the oxygen process if a higher amount of oxygen would be added. Then a part of the carbon is oxidized to CO2 and additional energy is produced in the reactor by this oxidation. LHV of produced syngas is then reduced for the same energy due to an increase CO2 concentration. This is used in large gasification units for higher material throughputs to reduce necessary power of plasma generator. The total energy efficiency, i.e. the ratio of heating value of syngas to total energy input, which is equal to the sum of energy consumed for the process and the heating value of wood, is almost the same for the both processes, as an increase of reaction energy due to CO2 dissociation is compensated by an increase of the heating value of syngas for the same value.
External energy needed for the process is delivered to treated material by plasma with some power losses, which are given by the power loss in the plasma torch and power loss in the reactor, energy can be lost also if heat transfer from plasma to material is not complete. Efficiency of plasma torches is usually between 60 and 80%, power loss in the reactor depends on its geometry and thermal insulation. The efficiency of utilization of plasma generator electrical energy for the process may be thus close to 50%, the total energy efficiency of the system would be in this case 82% for the oxygen process and 72% for the CO2 process.
Gasification of Wood Saw Dust and Wooden Pellets
Before material feeding, the reactor was heated to the wall temperatures about 1000 °C by electrical heating unit, after this temperature was reached the plasma torch was ignited and the reactor was heated to the wall temperatures 1200–1300 °C. After material feeding start the temperature of reactor walls went down and reached an equilibrium value depending on the torch power and material feeding rate. An example of a time development of inner wall temperatures at several positions within the reactor is shown in Fig. 5. The temperature Tg of produced gas was measured by the thermocouple positioned at the center of the output tube at position between the reactor and quenching tower is also shown in Fig. 5. The thermocouple was inserted into the central position in the time t = 10 min. Short period of heating of the reactor by plasma torch with the temperature increase followed by a decrease at the moment of start of material feeding (t1) is shown in the Fig. 5. It can be seen that the differences of temperatures at different positions in the reactor are not higher than 100 °C. Thus, good mixing of plasma with material and homogeneous heating of the reactor volume is achieved despite of the fact that plasma flow rate and dimensions of the plasma jet at the plasma torch output are small—the plasma jet core is 6 mm in diameter and about 10 cm long. The temperature of gas Tg at the output is lower as gas is cooled down as it flows in connecting output tube.
Temperatures Tw (1–6) of the reactor inner walls and temperature of produced gas Tg. Positions of Tw are indicated by numbers in Fig. 2. Wood 20 kg/h, CO2 125 slm, O2 43 slm. P = 100 kW, t1—time of start of material injection
Fir wood saw dust with humidity 7.7% (weight) and wooden pellets 13 mm long and 6 mm in diameter with humidity 7.0% were supplied into the reactor together with oxidizing media. The amounts of treated material and added oxidants are shown in Table 3. Runs 1–7 were made with fine saw dust, runs 8–10 with wooden pellets.
Figure 6 shows for all runs a ratio of arc power to material feed rate and the process enthalpy defined as the ratio of energy available for material treatment to amount of material. Energy available for material treatment was determined as the difference of the arc power and the sum of power losses in the plasma torch and to the reactor walls.
Figure 7 shows for all test runs the ratio of a number of oxygen atoms in added oxidizers to the number needed for balancing carbon and oxygen amount in all input reagents for production of CO from all carbon in treated wood. Oxygen input from steam plasma is icluded in the balance (O from H2O). The values of mass fractions of carbon, hydrogen and oxygen in the wood, used in computation, are c = 0.511, h = 0.064, o = 0425 respectively.
Syngas composition was measured on-line by the mass spectrometer. An example of the recorded syngas composition obtained for reaction of wood with carbon dioxide in steam plasma is shown in Fig. 8. Sudden changes of composition correspond to the changes of flow rate of argon, which was supplied into the reactor for determination of a flow rate of produced syngas from measured argon concentration at defined input flow rate of argon. This method of measurement of syngas flow rate was more exact than methods based on a usage of Pitot flow meter, which gave highly fluctuating signal due to fluctuations of pressure in the output tubes.
In Fig. 9 the measured syngas composition is shown compared with the composition corresponding to complete gasification of material to syngas. The theoretical composition was calculated under the assumption, that all carbon and oxygen in input reagents forms CO, in case of surplus of oxygen also CO2, and hydrogen in input reagents went to H2 in syngas. Content of water molecules in syngas was not measured due to problems with condensation in mass spectrometer, water was captured in a freezing unit at the input to the spectrometer. The formation of water molecules as well as production of solid carbon are main cause of differences between theoretical and measured composition.
It can be seen that in all cases the composition of produced syngas was close to the composition calculated for the case of complete gasification of material. In all cases a syngas with high concentration of hydrogen and carbon monoxide was obtained. Produced syngas contained also smaller amount of carbon dioxide, in most cases higher than theoretical value. Measured concentrations of methane and oxygen were less than 3%. Carbon dioxide was present in syngas also in runs with smaller amount of added oxygen. For higher amount of added oxygen the composition was close to theoretical values. Even in the cases with the highest feed rates of material (runs 7 and 10) the results were similar to runs with smaller feed rates. It may be concluded that in all cases the energy available for gasification (reaction enthalpy in Fig. 4) was higher than energy needed for the process. Also larger size of pellets has no effect on the resulting composition which was close to theoretical one similarly like for fine saw dust.
In all cases the content of tar and higher hydrocarbons in the produced gas was very low and substantially less than 10 mg/Nm3. This is lower than the tar content in most of non-plasma gasifiers, where the tar content for various types of reactors varies in the range from 10 mg/Nm3 to 100 g/Nm3.
Figure 10 presents the ratio of carbon and hydrogen in produced gas to the carbon and hydrogen in treated material, added gases and plasma. The carbon gas yield was in all cases higher than hydrogen yield, probably due to formation of water molecules which were not detected in measurement of syngas composition. The gasification efficiency was higher for wood saw dust, in case of pellets more solid carbon was produced.
In Fig. 11 the low heating values of syngas are shown which were calculated from measured gas composition, Fig. 12 presents the ratio of heating values of syngas to the torch power. Maximum achieved energy gain was 3.5 in the run 7 with the highest material feeding rate. It must be said that the experimental conditions were not optimised for maximum energy efficiency. The efficiency could be increased especially by increasing of material feeding rate.
Components of power balance of gasification in the run 7 are shown in Fig. 13. Power loss in the torch is 41% of the torch power, power loss into the reactor walls is relatively small due to good thermal insulation. Power needed for gasification, which was calculated using procedure described in [25], corresponds to endothermic reaction of production of syngas from solid wood and includes power produced by oxidation by reaction with added oxygen and oxygen produced by CO2 dissociation, syngas enthalpy corresponds to power needed to heat reaction products to the high temperature in the reactor which must be at least 1200 K as it can be seen in Fig. 3. Syngas heating value was calculated from measured composition of produced gas. Besides LHV of syngas, also energy corresponding to syngas enthalpy and energy of hot water in cooling systems of the torch and the reactor could be utilized, which would increase the total energy gain.
Gasification of Pyrolytic Oil
Sustainable development in transport includes proper handling of used tires. One of the treatment methods is pyrolysis of rubber from waste tires. The pyrolytic products (solid char, liquid pyrolytic oil and synthetic gas) are of poor quality and therefore investigations on upgrading of these products is important. We have studied the possibilities of pyrolytic oil upgrading in steam thermal plasma. In the experiments water, carbon dioxide and oxygen have been used as oxidizing media for partial oxidation of surplus of carbon in the oil to carbon monoxide.
Pyrolysis oil used in the decomposition process was produced from waste tires. Compositional analysis of the oil showed the high content of aromatic and polyaromatic hydrocarbons as well as 21 wt% of water. The elemental analysis of the water-free fraction of the oil was as follows: C—87.6, H—10.7, S—1.01 and N—0.66 (wt%). The low heating value and the density measurements of the water-free fraction were carried out as well—42.1 MJ/kg and 926 kg/m−3 (15 °C), respectively. An approximate molecular formula C5H8O follows from the analysis of elemental composition of pyrolytic oil. This formula was used in determination of amount of added oxidizers.
The main point of the experiment was to produce syngas with the maximal content of H2 and CO, so oxidizing media (H2O, CO2, O2 and the mixture of CO2 and O2) were added. The flow-rates of the oil and oxidizing media are summarized in Table 4. The pyrolytic oil was fed into water cooled feeding nozzle 0.5 mm in diameter. Oxygen and carbon dioxide were supplied directly into the reactor through gas inputs, liquid water was injected into the same nozzle as oil.
Produced syngas flows out of the reactor at temperature of approximately 1200 °C. Syngas composition measured at the output of the reactor for different types of oxidation is shown in Fig. 14. Argon from the plasma torch was neglected (the concentration of Ar varied under 1 vol%). Syngas analysis revealed high concentrations of H2 and CO for all types of oxidation.
The heating value of produced syngas was calculated from measured gas composition. The highest value of LHV showed the experiment with H2O (12.2 MJ/Nm3) as an oxidizing medium. The surplus of oxygen for this experiment was higher by 30% in comparison with the case of the stoichiometric amount of oxygen, which follows from the Eq. (3) for oil represented by the formula C5H8O. Carbon gas yield is for this experiment quite low so it can be deduced that a part of H2O (added as an oxidizing medium) is evaporated and it does not participate the gasification process (water in syngas was not analyzed). Efficiency of carbon gasification varied between 0.58 and 0.67 for gasification with water, for gasification with CO2 it was slightly lower, around 0.58. The highest efficiency of gasification 0.87 was obtained for oxygen, for the mixture of CO2 and O2 the efficiency was 0.80. The heating value of syngas is quite high due to high content of hydrogen and carbon monoxide. The highest content of H2 in syngas was in the case of oxidation with water, but part of the plasma enthalpy was lost for water dissociation. On the other hand, the use of oxygen brings some extra energy for gasification. The surplus of oxygen in the case when the mixture of CO2 and O2 was used reached 32.4%. The highest carbon yield was achieved but LHV is low due to the partial oxidation of the oil and therefore high CO2 content in syngas.
The reactor was opened after experiments and carbon black samples were withdrawn from two several positions within the reactor. Particles had regular spherical shape and their size ranged between 100 and 1000 nm, prevailing particles size was 100–200 nm. Results of elemental analysis of produced powder are in Table 5.
Small amount of Sulphur comes from the oil while Si comes probably from ceramic reactor lining and Cu from the plasma torch anode.
Measured syngas composition was compared with the one obtained by theoretical calculations. Figure 15a, b present results of calculation of equilibrium composition of gas, solid and liquid components of reaction products in dependence on temperature. Figure 15a shows molar fractions of components in the gas phase, Fig. 15b shows ratio of solid and liquid phase to the gas phase. It can be seen that similarly like in the case of wood gasification, syngas with high content of hydrogen and carbon monoxide and very low content of other components is obtained for temperatures above 1200 K.
Comparison of experimental and theoretical gas phase compositions is shown in Fig. 16. Measured concentrations are in the front columns, theoretical thermodynamic equilibrium values are in the columns in the back on the right hand side of experimental columns. The table also contains ratio of carbon to oxygen moles in the input reactants, ratio of input volume of water to oil, and ratio of input oxygen and carbon dioxide (in normal liters) to volume of oil (in ml).
Syngas with high content of hydrogen and carbon monoxide was obtained in all experiments. Measured composition was close to the one obtained by theoretical calculations in reactions of oil with oxygen and water. When carbon dioxide was used as one of oxidizing media, produced syngas contained higher amount of CO2 than theoretical values. Described decomposition process could be utilized for syngas production as well as for the production of spherical black carbon particles with the above-mentioned size.
Refuse-Derived Fuel
The next material used in gasification experiments was RDF—refuse-derived fuel processed from waste excavated from land sites and composed of municipal solid waste (59%) and industrial waste (41%). The material was composed of plastics 47%, wood + paper 24%, textiles 10%, and fines 18%. The results of ultimate material analysis are as follows: C 59.0%, O 33.2%, H 7,8%. The material was gasified at the rates of 20, 40 and 60 kg/h. Combinations of oxygen, carbon dioxide and water were added into the reactor together with treated material as oxidizing media. The torch power was 100 kW. Typical output of online mass spectrometer measurement is shown in Fig. 17.
Table 6 summarizes basic input and output characteristics for several experimental runs. Syngas output flow rate was calculated from measured argon concentrations for given input flow rate of argon. Reactions of syngas composition on changes of argon flow rate can be seen in Fig. 17. The composition presented in the Table 6 does not include argon, which was added for flow rate calibration, and argon from the torch (7 slm). Syngas with high content of hydrogen and carbon monoxide was produced, and smaller concentrations of carbon dioxide and methane. Table 6 also shows values of carbon gas yields calculated as a ratio of carbon amount in the produced gas to the sum of carbon in all input reagents.
Similarly like in experiments with other materials, plasma gasification of municipal waste produced syngas with high content of hydrogen and carbon monoxide. The conditions leading to very low content of other gas components (CO2, CH4, O2) were found. The carbon yield was dependent on the mass flow rate of treated material. This dependence is shown in Fig. 18.
Gasification of Other Materials
The results of gasification of materials described in the preceding chapters can be compared with the other tests of gasification of coal (lignite), plastics, and sunflower seed skins. The overview of basic input and output characteristics for these materials is presented in the Table 7. Table 7 shows the measured data for following materials:
-
Sunflower seeds skins.
-
Lignite—fine powder of soft brownish coal (humidity 45% weight).
-
Polyethylene pellets with diameter 3 mm.
-
Waste plastics from bottles crashed to pieces with dimensions 2–10 mm.
It can be seen in the table that syngas with high concentrations of hydrogen and carbon monoxide was produced for all materials. Content of carbon dioxide varied from very low values to 22% depending on the treated material and test conditions. Concentrations of CH4 and O2 was low in all cases. For all materials, the content of tar and higher hydrocarbons in the produced gas was substantially below 10 mg/Nm3. This content is lower than in most non-plasma gasifiers, where the tar content for various types of reactors varies from 10 mg/Nm3 to 100 g/Nm3. Similarly like in case of wood and pyrolytic oil the syngas composition was close to the one determined by thermodynamic equilibrium computations.
Discussion and Conclusions
Steam plasma gasification of various types of biomass and waste organics was investigated. Tested materials included fine saw dust and pulverized lignite, wooden pellets and sunflower seed skins, crashed waste polyethylene and pure polyethylene balls, crashed RDF, and liquid pyrolytic oil from treatment of car tires. The size of treated materials varied from submillimeter size powder (lignite) to particles of several centimeters (polyethylene waste, wooden pellets, RDF). Water content in materials varied from zero to 45%. Production of synthesis gas through the plasma assisted reactions of materials with oxygen, carbon dioxide and water was studied.
The experiments were made in the medium size plasma reactor with the plasma torch power up to 140 kW and material flow rates of order of tens kg per hour. Thus, test parameters were close to parameters in small industrial systems but on-line diagnostics and monitoring of process parameters corresponded to conditions in exact laboratory research. Plasma torch based on a principle of arc stabilization by a combination of water vortex and gas flow was used. The torch produces steam plasma jet with extremely low plasma mass flow rate and thus very high plasma temperature and enthalpy. The plasma flow rate was up to hundred times lower than in gas plasma torches which have been commonly used for waste treatment. Low plasma flow rate and very high enthalpy and temperature offer advantage of high heat transfer rate, ability of treatment of any material and reduction of energy needed for heating of plasma gas to the temperature needed for gasification process. The mass flow rate of plasma (18 g/min) was very low, compared with the flow rate of treated material (up to 1 kg/min). It was proved by the experiments that small size plasma jet with very low mass flow rate can produce homogeneous heating of the whole reactor volume, despite of very low dimensions of the jet core which were 6 mm in diameter and about six cm in length. This is caused by high level of turbulence produced in the reactor volume due to an interaction of high velocity, low-density plasma jet with reactor atmosphere and with injected treated material. The other source of an intensive turbulent mixing is rapid gasification of material particles, which produces gas with the volume more than 103 times larger than the volume of original solid particle.
Syngas with high content of hydrogen and carbon monoxide, high heating value, and very low content of other gases was produced by gasification of all tested materials. The content of tars was very low and it was more than one order lower than values found in non-plasma gasification technologies. Composition of syngas was close to the one determined by thermodynamic equilibrium calculations of composition of all input reagents. The composition could easily be controlled by added gasification media, which were added for balancing carbon and oxygen content in input reagents and for complete gasification of carbon contained in treated material.
Energy balance of gasification process is influenced by a choice of added gasification media. Analysis of energy balance was made for wood gasification. The highest energy production efficiency can be achieved if oxygen is used as gasification gas. For this case the heating value of produced syngas is more than 7 times higher than energy needed for the endothermic gasification process. This energy includes energy for material phase change, enthalpy of chemical reactions leading to syngas production, and energy needed for heating of all reagents to the temperature needed for optimum syngas composition. This temperature, determined by thermodynamic equilibrium calculations, is close to 1200 K. The energy for heating to this reaction temperature is a main component of total reaction enthalpy. If this energy of hot syngas was recuperated, the total energy efficiency of plasma gasification would be substantially increased. In the experiments the material throughputs was limited by limited capacity of the feeding mechanism and of the unit for syngas final combustion. Therefore maximum theoretical energy efficiency was not achieved in some experiments.
The ratio of calorific value of syngas to the input energy is lower for plasma treatment compared with non-plasma gasification technologies based on partial oxidation, which may be realized without any external energy source. However, heating value of syngas obtained by plasma treatment is much higher than in case of non-plasma technologies, as hydrogen and carbon gas yields are higher and syngas contains high concentrations of hydrogen and CO, which cannot be obtained in non-plasma technologies. The total energy efficiency of plasma gasification, i.e. the ratio of LHV of syngas to the sum of external energy input and the heating value of material, can be higher than for other technologies. Also the quality of syngas, especially very low content of tar, is important advantage of plasma treatment compared with other technologies. This should be taken into account in comparison of various gasification technologies, together with other plasma advantages like small process volume and very short start up and shut down times.
The main advantages of plasma gasification are a better control over the composition of the gas produced, its higher calorific value and reduction of undesired contaminants like tar, CO2, CH4 and higher hydrocarbons. Another advantage of plasma is a wide choice of materials to be treated. As the energy for the process is supplied by plasma, and chemical reactions in the reaction products are not the primary source of energy, the process can be applied to a wide range of organic materials and biomass. These advantages of plasma technology together with its higher energy consumption should be taken into account when evaluating technical and economic feasibility of plasma treatment.
References
Heberlein J, Murphy AB (2008) Thermal plasma waste treatment. J Phys D Appl Phys 41:053001
Gomez E, Amutha Rani D, Cheeseman CR, Deegan D, Wise M, Boccaccini AR (2009) Thermal plasma technology for the treatment of wastes: a critical review. J Hazard Mat 161:614–626
Ruj B, Ghosh S (2014) Technological aspects for thermal plasma treatment of municipal solid waste—a review. Fuel Process Technol 126:298–308
Moustakas M, Fatta D, Malamis S, Haralambous K, Loizidou M (2005) Demonstration plasma gasification/vitrification system for effective hazardous waste treatment. J Hazard Mater 123:120–126
Katou K, Asou T, Kurauchi Y, Sameshima R (2001) Melting municipal solid waste incineration residue by plasma melting furnace with a graphite electrode. Thin Solid Films 386:183–188
Sakai S, Hiaraoka M (2000) Municipal solid waste incinerator residue recycling by thermal processes. Waste Manag 20:249–258
Cheng TW, Chu JP, Tzeng CC, Chen YS (2002) Treatment and recycling of incinerated ash using thermal plasma technology. Waste Manag 22:485–490
Rutberg PG, Kuznetsov VA, Serba EO, Popov SD, Surov AV, Nakonechny GV, Nikonov AV (2013) Novel three-phase steam–air plasma torch for gasification of high-caloric waste. Appl Energy 108:505–514
Chen X, Badie JM, Flamant G (1997) Dynamics of complex chemical system vaporization at high temperature. Application to the vitrification of fly ashes by thermal plasma. Chem Eng Sci 52:4381–4391
Poiroux R, Rollin M (1996) High temperature treatment of waste: from laboratories to the industrial stage. Pure Appl Chem 68:1035–1040
Plasco Energy Group (2016). http://www.plascoenergygroup.com/. Accessed 17 Oct 2016
Pyrogenesis (2016). http://www.pyrogenesis.com/. Accessed 17 Oct 2016
CO Tetronics (2016). http://www.tetronics.com/. Accessed 17 Oct 2016
Solena (2016). http://www.solenagroup.com/. Accessed 17 Oct 2016
AlterNrg (2016). http://www.alternrg.com/. Accessed 17 Oct 2016
Retech (2016). http://www.retechsystemsllc.com/products/pact/. Accessed 17 Oct 2016
Boerrigter H, van der Drift B (2005) “Biosyngas” key-intermediate for production of renewable transportation fuels, chemicals and electricity. In: 14th European biomass conference and exhibition, Paris. ECN report ECN-RX-05-181
Surisetty VR, Kozinski J, Dalai AK (2012) Biomass, availability in Canada, and gasification: an overview. Biomass Conv Bioref 2:73–85
Fabry F, Rehmet Ch, Rohani V, Fulcheri L (2013) Waste gasification by thermal plasma: a review. Waste Biomass Valoriz 4:421–439
Hrabovsky M, Kopecky V, Sember V, Kavka T, Chumak O (2006) Properties of hybrid water/gas DC arc plasma torch. IEEE Trans Plasma Sci 34:1566–1575
Hrabovsky M (2002) Generation of thermal plasmas in liquid and hybrid DC arc torches. Pure Appl Chem 74:429–433
Hrabovsky M, Konrad M, Kopecky V, Hlina J, Benes J, Vesely E (1997) Motion of anode attachment and fluctuations of plasma jet in dc arc plasma torch. High Temp Mat Process 1:167–178
Coufal O (1994) Composition of the reacting mixture SF6 and Cu in the range from 298.15 to 3000 K and 0.1 to 2 Mpa. High Temp Mat Process 3:117–139
Coufal O, Sezemsky P, Zivny O (2005) Database system of thermodynamic properties of individual substances at high temperatures. J Phys D Appl Phys 38:1265–1274
Hrabovsky M, Konrad M, Kopecky V, Hlina M (2006) Pyrolysis of wood in arc plasma for syngas production. High Temp Mat Process 10:557–570
Acknowledgements
The authors gratefully acknowledge support of the Grant Agency of CR under the Project Number GA15-19444S.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hrabovsky, M., Hlina, M., Kopecky, V. et al. Steam Plasma Treatment of Organic Substances for Hydrogen and Syngas Production. Plasma Chem Plasma Process 37, 739–762 (2017). https://doi.org/10.1007/s11090-016-9783-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9783-5