Abstract
Recycling of organic waste is an increasingly hot topic in recent years. This issue becomes even more interesting if such processing leads to a source of hydrogen or syngas for fuel production. A process of high-temperature decomposition of lignite was studied on the plasma gasification reactor PLASGAS, where water-stabilized plasma torch was used as a source of high-enthalpy plasma. The plasma torch power was 120 kW and allowed heating of the reactor to more than 1000 °C. The material feeding rate in the gasification reactor was 30 or 60 kg per hour that is comparable to a small industrial production process. The efficiency evaluation of the thermal decomposition process was performed. Energy balance of the process was carried out as well as an influence of the lignite particle size and the addition of methane (CH4) on the synthesis gas composition. The ratio H2/CO was in the range of 1.5–2.5 depending on the experimental conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Since its discovery in the beginning of the last century, gasification of biomass has gained widespread popularity as one of the main competitor of the renewable energy sources. Thermal decomposition of many solid, liquid or gaseous organic materials allows producing a mixture of H2 and CO called synthesis gas (syngas). The obtained gas mixture can be used as a raw material for the synthesis of more complex organic fuels/lubricants or as a gas fuel directly. Also it can serve as a source of pure hydrogen. Many gasification systems and methods have been studied for the obtaining of syngas from different biomaterials [1,2,3,4,5,6,7,8,9,10,11].
The growing interest in this field led to search of new modern methods because of low syngas yield/quality using previous classical techniques.
At the present time, a gasification method list was replenished with new concepts as a unique gasifier, which was patented in 2008 [12]. This reactor has a compact design that combines gasification, gas cleaning and conditioning processes in one device and hence lower financial expenditure is needed. However, presence of tar in syngas also takes place.
Such processes as a multi-stage gasification combining pyrolysis and gasification [13], combination of pyrolysis and gasification at different locations [14] or combination of gasification with a partial oxidation stage [15] promise high process efficiency, sufficiently pure syngas with low tar concentration or even tar reduction by partial oxidation.
Combined gasification where produced syngas was immediately transformed into heat, power or pure hydrogen was also studied [16,17,18,19]. Advantage of this method is that the electrical energy and collected hydrogen can be transported somewhere in contrast to heat that must be used right away close to the gasification system.
Even gasification in a supercritical water was investigated [20]. The main disadvantage is a very high energy and financial expenditure. However, this technique offers a solution for processing wet biomass without pre-drying as well as the plasma gasification methods.
Gasification of various organic substances in plasma has also been actively studied in recent years [21,22,23,24,25,26,27,28,29]. A review of thermal plasma gasification is presented in [21,22,23], gasification of several organic materials is described in [24,25,26,27], coal gasification in arc plasma was studied in [28, 29] and in microwave plasma with power 75 kW [30]. Modeling of coal gasification in plasma reactor is presented in [31].
In this work, we studied gasification of lignite, low quality brown coal, with high humidity. Removal of water and drying of the material is difficult and thus direct usage of lignite as a fuel is limited, as well as gasification of this material by partial oxidation. Gasification of lignite was made in steam plasma, which was produced in a special plasma torch, where steam plasma was produced in a direct contact of water with electric arc discharge. Main characteristics of this torch are very high plasma enthalpy and temperature and thus low plasma flow rate needed for transport of sufficient energy into the gasification reactor. Comparing to the other published plasma gasification experiments [23,24,25,26,27,28,29,30], the results presented in this paper were obtained in the reactor with pretty high torch power and the material throughputs in conditions closer to small industrial manufacture.
Materials and Methods
Plasma Torch and Gasifier
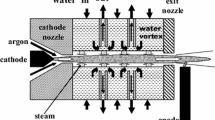
Plasma torch with stabilization of the arc by a mixture of an inert gas and water vapor generated thermal plasma. A detailed description of an operation principle can be found elsewhere [32, 33]. Gas (in our case—argon) is supplied along an inner thin tungsten cathode and then arc stabilized in water vortex. Thus the plasma has no impurities which could contaminate the synthesis gas. A rotating copper disc with water cooling serves as outer anode [34].
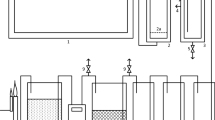
The scheme of plasma gasifier PLASGAS is presented in Fig. 1 [22, 35]. Plasma torch is placed at the top of the reactor. Temperature of inner surface is measured in several positions as showed in Fig. 1. Treated solid material is transported into the reactor by a feeder placed on the upper side of the reactor. Therefore, the material enters directly into the plasma jet.
The flow rates of input gases were measured using mass flow controller AALBORG GFC-57. The syngas flow rate was determined from measured Ar concentration. The argon was added at known input flow rate. The argon flow rate was measured with accuracy better than 10%, the argon concentration in syngas was measured with an accuracy better than 1%. A quadrupole mass spectrometer Pfeiffer Vacuum Omnistar GSD 301 was used as a main gas analyzer.
Materials and Conditions
Natural lignite is a fossilized biomass, which still keeps its natural structure. A series of the experiments were performed with crushed lignite (a humid non-hydrolysable complex organic powder).
The complete chemical composition of the tested material can be found in Table 1.
The chemical composition calculations were made according technological parameters given by a customer, because elemental analysis of the volatile matter was done with respect to the total lignite weight. Unfortunately, a chemical composition of the ash was not presented in the above mentioned data. This material usually contains high amount of water and hence it is not suitable for usual combustion process. The lignite humidity was measured in our laboratory before the experiment once again. Lignite was heated to 105 °C until its mass ceased to change. Resulted moisture differed from the initial by a few percent that was also took into account at chemical composition calculations. Material drying is quite difficult, because the water is bound in the inner structure of lignite. However, high humidity of the material is beneficial in thermal plasma treatment, where material is decomposed into its constituent atoms at high plasma temperatures, and dissociated water is a source of oxygen for complete lignite gasification and contributes to an increase of hydrogen content in produced syngas.
The experiments were performed in the plasma reactor PLASGAS [22] under the experimental conditions specified in the Table 2.
Two sets of experiments were made with lignite powder with two average particle sizes—less than 100 µm (50–100 µm) and more than 100 µm (100–500 µm). The powder was injected into plasma jet separately and also in a mixture with additional gas (methane) at various feed and flow rates. Methane was added into the reactor in order to study possibility of control of H2/CO ratio in produced syngas. Argon was used as a calibration gas for mass spectrometry analysis and for determination of syngas flow rate. Steam evaporated from a water vortex inside the plasma torch system was also taken into account in the chemical composition calculations (the details of the steam flow rate measurements can be found elsewhere [36]).
Nine experiments were performed with feeding rates of reactants specified in Fig. 2. Chemical composition of the input materials including lignite, water and methane is shown in Table 3. Only the components (H2, C, O,) are presented here, because of their presence were detected by mass spectrometer at the reactor outlet and nothing more. The ash and non-gasified part of lignite were collected at the bottom of the reactor in the form of lava. First four experiments were done using small lignite particles (less than 100 µm in diameter), large particles (above 100 µm in diameter) were used in the other five experiments.
The temperature of the reactor inner walls fluctuated in the range between 1300 and 1150 °C at the top of the reactor and between 1100 and 950 °C at the bottom.
Ratio of the full consumption of electrical net energy to the mass of treated lignite in the experimental series is shown in Fig. 3. The net energy available directly for decomposition process was evaluated taking into account the measured energy losses to the reactor walls and losses in plasma torch. The ratio of this energy to mass of treated material (process enthalpy) is also presented in Fig. 3.
Results and Discussion
Composition of gas reaction products was monitored by using mass spectroscopy. Examples of time evolution of gas composition are shown in Fig. 4. Flow rates of added gases, lignite feed rate and the electric parameters are indicated at the bottom of the picture. It is clearly seen gas composition had fast response to changing the input parameters (methane and argon flow rates in this case). Some time was needed after any changes in order to stabilize the system state.
Composition of syngas for nine experimental runs characterized in Figs. 2 and 3 is shown in Fig. 5. Besides H2 and CO also some amount of CO2 and CH4 was detected. Argon was added for measurement of syngas flow rate. Content of CO2 was between 5 and 13%, depending on feeding rates of input reactants. Content of methane was from 1 to 5% for experiments with methane input, and below 1% in the other experiments. Presence of CO2 can be explained by high content of oxygen in the chemical composition of the raw material.
After changes of conditions in the reactor, the stable situation was reached after long time. This was caused especially by a high thermal inertia of the reactor body, especially of the thick ceramic wall insulation. Therefore we used the data obtained in a time interval after the state with slow changes of measured parameters was reached. The relations between characteristics that were measured in the same time moment were used in evaluation of the results.
Methane addition into the reactor volume allowed partially compensating a stoichiometric surplus of oxygen and increasing of hydrogen yield. At the same time, the highest methane flow rate (150 slm) was already redundant. Thus, methane surplus leaved the reactor without reacting. It can be seen in results of the experiments 7 and 8 (see Fig. 5), where the H2/CO ratio was the same at increase of the methane volume percentage.
Resulted percentage of hydrogen in some cases reached more than 60% of the total gas volume. Hereby, this method of high temperature plasma treatment can be used for hydrogen production.
The hydrogen and carbon yield of the gasification process is illustrated in Fig. 6. The hydrogen losses could be due to formation of new water molecules, but water was not measured by mass spectroscopy. Some carbon molecules accumulated on the inner reactor walls in the form of soot. Both elements also remained part of an unreacted material, which was collected at the bottom of the reactor.
Methane addition played a considerable role in increasing the carbon yield. The particle size as well as material feed rate did not have a significant influence on hydrogen and carbon yields. Nevertheless we can suppose further possibility of the material feed rate increase without dramatic influence on the yield. Resource expenditure for deep material grinding presumably was not necessary as well.
Ratio of the Low Heating Value (LHV) of produced syngas (Esg) to full torch power (Etp—the electrical energy supplied to the plasma torch), to the net energy (Enet—the energy balance taking into account a coefficient of plasma torch efficiency (keff) and thermal losses during cooling of the reactor walls (Ecooling)), which was involved in the decomposition process and to the sum of full torch power and the chemical energy (Ech) contained into the feed material is showed in Fig. 7, where:
In our case the feed material chemical energy (Ech) was 12.57 MJ/kg.
Thus direct energy consumption in the form of network electricity helped to convert hard-to-reach lignite energy to come-at-able energy of the syngas during the plasma gasification process and in several experiments the amount of syngas energy was more than the electrical energy consumption and much more than the pure energy available directly for gasification process.
The difference between those ratios (see Fig. 7) allows to better estimate the amount of the total energy loss due to the thermal losses and the magnitude of the plasma torch efficiency coefficient. It means an analysis of different plasma gasification reactor constructions and plasma torch types can help to find an optimal way to reduce losses.
Figure 8 presents heating value of produced syngas. The low heating value of the syngas in all cases was in the range from 8 to 11 MJ/m3.
Conclusions
Plasma gasification leading to the production of syngas is an alternative to non-plasma methods of organic substances treatment. Plasma is a medium with the highest content of energy, and therefore substantially lower plasma flow rates are needed to supply sufficient amount of energy needed for gasification. The result is minimum contamination and dilution of the produced syngas by plasma gas and an easy control of syngas composition. In comparison with non-plasma methods the volume percentage of CO2 did not exceed 13% of the total amount [37]. The methane was under 5% threshold when it was observed. Nitrogen compounds were not detected due to lack of atmospheric air in the gasifier. The process also acts as energy storage—electrical energy is transferred into plasma enthalpy and then stored in the produced syngas.
Plasma treatment offers a better control over the temperature of the process, fast process response to changing experimental conditions, lower reaction volume and especially a suitable composition of produced syngas for Fisher–Tropsch process. The plasma gasification exploits the thermochemical properties of plasma. Decomposition of the material is achieved by the action of the kinetic energy of plasma particles, which is extremely high due to high temperatures of the plasma, and also the influence of radiation and chemically active species.
The specific features of plasma treatment were illustrated in this paper. Steam plasma was generated in a special plasma torch which is characterized by substantially higher enthalpy and temperature than commonly used thermal plasma generators. Thus, main advantages of plasma treatment were: the extremally high energy concentration that provided by the hybrid plasma torch and hence a possibility of direct treatment of moisture materials, a smaller amount of foreign components in a syngas composition.
Syngas with concentration of hydrogen and carbon monoxide more than 80% and the ratio of the respective components (H2/CO) between 1.7 and 2.7 was produced by gasification of high humidity lignite in steam plasma. Hydrogen yield was up to 80%, maximum carbon gas yield was close to 90%. Maximum ratio of the syngas calorific value to full plasma torch power reached 2.5, and the ratio of calorific value to energy spent for gasification process was up to 6.3. The total output/input energy ratio was in the range between 0.34 and 0.94.
In spite of minor differences in feed material chemical composition for all nine experiments (see Table 3) next conclusions can be done:
-
Gasification of the small lignite particles gives some advantage in H2/CO ratio at 30 kg/h feed rate and in total H2 and CO percentage content at 60 kg/h feed rate accordingly;
-
Small particles treatment with methane addition (75 slm) also showed better results in total H2 and CO percentage content at both feed rates.
Thus, the highest total H2 and CO percentage content with high H2/CO ratio in resulted syngas was reached using finely crushed lignite particles (50–100 µm) at both material feed rates (30 and 60 kg/h) with methane addition (75 slm). Methane addition at 75 slm of the gas flow rate allowed to rich the syngas by H2 and CO components. Moreover, the H2/CO ratio was also increased.
Usage of thermal plasma offers the possibility of treatment of materials with high content of water, which cannot be used for combustion or for non-plasma gasification without drying. In case of humid lignite, drying process is complicated and expensive. In plasma gasification, water contents in the material served as source of oxygen for more complete lignite gasification and for the increase of hydrogen contents.
Obtained synthesis gas can be a source of pure hydrogen. It also can be used directly as a gas fuel or as a raw material for a following liquid fuel production. No high molar mass compounds were detected by mass spectrometer in the produced gas composition at the reactor outlet. However, the solid products collected inside the reactor were not chemically analyzed.
References
Boerrigter H, van der Drift B (2005) “Biosyngas” key-intermediate for production of renewable transportation fuels, chemicals and electricity. In: 14th European biomass conference and exhibition, Paris. ECN report ECN-RX-05-181
Surisetty VR, Kozinski J, Dalai AK (2012) Biomass, availability in Canada, and gasification: an overview. Biomass Conv Bioref 2:73–85
Ye DP, Agnew JB, Zhang DK (1998) Gasification of a south Australian low rank coal with carbon dioxide and steam: kinetics and reactivity studies. Fuel 77:1209e19
Kajitani S, Suzuki N, Ashizawa M, Hara S (2006) CO2 gasification rate analysis of coal char in entrained flow coal gasifier. Fuel 85:163e9
Wu SY, Gu J, Li L, Wu YQ, Gao JS (2006) Gasification reactivity of rapid and slow pyrolyzed Shenfu chars with CO2 at high carbon conversions and elevated temperatures. J Fuel Chem Technol 34:339e43
Gomez-Barea A, Leckner B, Villanueva-Perales A, Nilsson S, Fuentes-Cano D (2013) Improving the performance of fluidized bed biomass/waste gasifiers for distributed electricity: a new three-stage gasification system. Appl Therm Eng 50:1453–1462
Meng X, de Jong W, Fu N, Verkooijen AHM (2011) Biomass gasification in a 100 kWth steam-oxygen blown circulating fluidized bed gasifier: effects of operational conditions on product gas distribution and tar formation. Biomass Bioenergy 35:2910–2924
Alauddin ZA, Lahijani P, Mohammadi M, Mohamed AR (2010) Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: a review. Renew Sustain Energy Rev 14:2852–2862
Sheth PN, Babu BV (2009) Experimental studies on producer gas generation from wood waste in a downdraft gasifier. Bioresour Technol 100:3127–3133
Seggiani M, Vitolo S, Puccini M, Bellini A (2012) Cogasification of sewage sludge in an updraft gasifier. Fuel 93:486–491
Plis P, Wilk RK (2011) Theoretical and experimental investigation of biomass gasification process in a fixed bed gasifier. Energy 36:3838–3845
Heidenreich S, Foscolo PU, Nacken M, Rapagna S (2008) Gasification apparatus and method for generating syngas from gasifiable feedstock material. PCT Patent Application PCT/EP2008/003523
Henriksen U, Ahrenfeldt J, Jensen TK, Gobel B, Bentzen JD, Hindsgaul C et al (2006) The design, construction and operation of a 75 kW two-stage gasifier. Energy 31:1542–1553
Koppatz S, Schmid JC, Pfeifer C, Hofbauer H (2012) The effect of bed particle inventories with different particle sizes in a dual fluidized bed pilot plant for biomass steam gasification. Ind Eng Chem Res 51:10492–10502
Houben MP, de Lange HC, van Steenhoven AA (2005) Tar reduction through partial combustion of fuel gas. Fuel 84:817–824
Negro SO, Suurs RAA, Hekkert MP (2008) The bumpy road of biomass gasification in the Netherlands: explaining the rise and fall of an emerging innovation system. Technol Forecast Soc Change 75(1):57–77
Gröbl T, Walter H, Haider M (2012) Biomass steam gasification for production of SNG—process design and sensitivity analysis. Appl Energy 97:451–461
Narvaez A, Chadwick D, Kershenbaum L (2014) Small-medium scale polygeneration systems: methanol and power production. Appl Energy 113:1109–1117
Shabani S, Delavar MA, Azmi M (2013) Investigation of biomass gasification hydrogen and electricity co-production with carbon dioxide capture and storage. Int J Hydrog Energy 38:3630–3639
Kıpcak E, Akgün M (2012) Oxidative gasification of olive mill wastewater as a biomass source in supercritical water: effects on gasification yield and biofuel composition. J Supercrit Fluids 69:57–63
Fabry F, Rehmet Ch, Rohani V, Fulcheri L (2013) Waste gasification by thermal plasma: a review. Waste Biomass Valoriz 4:421–439
Hrabovsky M, Hlina M, Kopecky V, Maslani A, Zivny O, Krenek P, Serov A, Hurba O (2017) Steam plasma treatment of organic substances for hydrogen and syngas production. Plasma Chem Plasma Process 37:739–762
Heberlein J, Murphy AB (2008) Thermal plasma waste treatment. J Phys D Appl Phys 41:053001
Moustakas M, Fatta D, Malamis S, Haralambous K, Loizidou M (2005) Demonstration plasma gasification/vitrification system for effective hazardous waste treatment. J Hazard Mater 123:120–126
Gomez E, Amutha Rani D, Cheeseman CR, Deegan D, Wise M, Boccaccini AR (2009) Thermal plasma technology for the treatment of wastes: a critical rewiev. J Hazard Mater 161:614–626
Rutberg PG, Kuznetsov VA, Serba EO, Popov SD, Surov AV, Nakonechny GV, Nikonov AV (2013) Novel three-phase steam-air plasma torch for gasification of high-caloric waste. Appl Energy 108:505–514
Ruj B, Ghosh S (2014) Technological aspects for thermal plasma treatment of municipal solid waste—a review. Fuel Process Technol 126:298–308
Qiu J, He X, Sun T, Zhao Z, Zhou Y, Guo S et al (2004) Coal gasification in steam and air medium under plasma conditions: a preliminary study. Fuel Process Technol 85:969e82
Messerle VE, Ustimenko AB, Lavrichev OA (2016) Comparative study of coal plasma gasification: simulation and experiment. Fuel 164:172–179
Uhm Han S, Na Young H, Hong Yong C, Shin Dong H, Cho Chang H (2014) Production of hydrogen-rich synthetic gas from low-grade coals by microwave steam-plasmas. Int J Hydrog Energy 39:4351–4355
Galvita V, Messerle VE, Ustimenko AB (2007) Hydrogen production by coal plasma gasification for fuel cell technology. Int J Hydrog Energ 32(16):3899–3906
Hrabovsky M, Kopecky V, Sember V, Kavka T, Chumak O (2006) Properties of hybrid water/gas DC arc plasma torch. IEEE Trans Plasma Sci 34:1566–1575
Hrabovsky M (2002) Generation of thermal plasmas in liquid and hybrid DC arc torches. Pure Appl Chem 74:429–433
Hrabovsky M, Konrad M, Kopecky V, Hlina J, Benes J, Vesely E (1997) Motion of anode attachment and fluctuations of plasma jet in dc arc plasma torch. High Temp Mat Process 1:167–178
Hrabovsky M (2011) Progress in biomass and bioenergy production. https://doi.org/10.5772/18234
Hrabovsky M, Konrad M, Kopecky V, Sember V (1997) Processes and properties of electric arc stabilized by water vortex. IEEE Trans Plasma Sci 25(5):833–839
Hatzilyberis KS, Androutsopoulos GP (2006) Lignite chemical conversion in an indirect heat rotary kiln gasifier. Therm Sci 10(3):181–197
Acknowledgements
The authors gratefully acknowledge support of the Grant Agency of CR under the Project Numbers GA15-19444S and GC17-10246J.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Serov, A.A., Hrabovsky, M., Kopecky, V. et al. Lignite Gasification in Thermal Steam Plasma. Plasma Chem Plasma Process 39, 395–406 (2019). https://doi.org/10.1007/s11090-019-09957-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-09957-w