Abstract
Quenching is a key approach to obtain high acetylene yield in the process of coal tar pyrolysis to produce acetylene in a thermal plasma reactor due to the thermodynamic characteristics of acetylene. Experiments of coal tar pyrolysis were carried out in a lab-scale H2/Ar plasma reactor under various quenching conditions. Meanwhile, thermodynamic analysis was performed to assist the optimization of quenching temperature and the maximization of acetylene yield. As quenching media in the experiments, hydrogen, argon, methane, and water were used separately to study the influence of quenching process on acetylene yield and specific energy requirement. The experimental results indicate that the acetylene concentration in quenched product gas was significantly affected by quenching operation, and the acetylene yield was significantly affected by quenching medium flow rate. The acetylene yields of 24.6, 17.8, 44.9 and 23.6 wt% can be reached by using hydrogen, argon, methane, and water as quenching media, respectively. The specific energy requirement analysis indicates that process energy efficiency can be improved by a suitable quench operation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Thermal plasma pyrolysis of coal, coal derived liquids and hydrocarbons, provides a direct route to produce acetylene with no direct CO2 emission and no needs for a large amount of water [1–4]. This process can be implemented according to the free energy data of acetylene [5]: the Gibbs function of acetylene becomes lower than other low-molecular-weight hydrocarbons when the system temperature is higher than 1500 K. In other words, a gas mixture with acetylene as primary component as well as H2, C2H4, and CH4 as by-products will be produced once the feedstock materials are rapidly heated up to an extremely high temperature. Thermal plasma has the advantages of ultra-high temperature (over 10,000 K in core area [6]) and high heating rate, being capable to rapidly heat the feedstock up to the temperature required by acetylene generation [7].
The possibility of this process was first reported in the early 1940s [8, 9]. Huels Corp. in Germany produced acetylene successfully by using methane as feedstock in an 8 MW pilot plant plasma reactor with 70.5 % methane conversion and 51.4 % yield of acetylene. Since 1960s, a wide range of researches on the pyrolysis process of various feedstock materials (such as coal, coal tar, ethylene, ethane, toluene, benzene and so on) in thermal plasma have been carried out in lab- and pilot-scale plasma reactors. Thermal plasma has been proved to be powerful enough to convert various feedstock materials to valuable products. However, the pyrolysis process is strongly affected by the feedstock types and pyrolysis reaction temperatures [10–22]. Thermodynamic analyses indicate that the acetylene concentration in the pyrolysis product gas is greatly affected by the mass ratio of C/H in the gas phase at the thermodynamic equilibrium state, and the reacting flow should be quenched rapidly enough to prevent the decomposition of acetylene [23, 24]. Bond et al. [11] found that only 70 % of added acetylene was obtained in the gas products when acetylene was injected into a thermal plasma jet. Krukonis et al. [12] studied acetylene decomposition by injecting acetylene into plasma streams of argon, helium, nitrogen, and hydrogen, and confirmed that acetylene would be continuously decomposed to hydrogen and carbon black without the subsequent quenching step and hydrogen acts as a chemical preserver of acetylene during the quenching process. Chakravartty et al. [13] reported that the yield of acetylene was significantly increased by changing the position to inject quenching media in the reactor. Polak reported that the 2 ms retardation in quenching process resulted in a decrease of the yield of acetylene from 15.5 to 10.0 % in plasma cracking methane process [25]. Thermal plasma provides a unique route to produce acetylene, and quenching operation is one of the key approaches to maintain the acetylene concentration in the gas phase and to obtain high process energy efficiency. However, the mechanism of a quenching process is still not well known. Thorough researches on quenching operation for thermal plasma pyrolysis process are necessary to further understand the process and its development.
In this work, experiments were carried out to investigate the effects of quenching process on coal tar pyrolysis to produce acetylene in a lab-scale H2/Ar plasma reactor at the atmospheric pressure. The influences of various parameters (including the quenching medium type, initial quenching temperature, and quenching media flow rate) on the yields of the main gaseous products and specific energy requirement were investigated.
Experiments
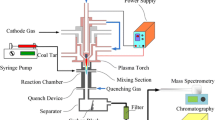
A reaction system including a plasma torch, a downer reactor, a coal tar inject system, a quenching device and a gas analyzing system, was set up to carry out the process of coal tar pyrolysis to acetylene. The experimental apparatus is shown in Fig. 1.
Experimental apparatus for thermal plasma pyrolysis of coal tar. 1 H2 Cylinder, 2 Ar Cylinder, 3 Raw material container, 4 Quenching material container, 5 Gas–solid separator, 6 Cathode, 7 Plasma nozzle, 8 Anode, 9 Power supply, 10 Thermo couple, 11 Quenching apparatus and Quenching media inlet, 12 Reactor, 13 Thermo couple, 14 Metering pump, 15 Products analysis system, 16 Vent-pipe
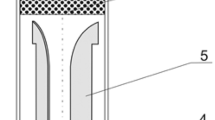
A plasma generator with maximum electric power input of 20 kW was used to generate a high temperature plasma jet. The plasma generator consists of three parts: (1) the plasma torch, (2) a nozzle-shaped anode made of copper with an inner diameter of 6 mm, and (3) a cathode made of tungsten. The electrodes were protected by cooling systems to reduce electrode wear and tare. A mixture of H2/Ar gas (40 vol% H2 and 60 vol% Ar) at a flow rate of 15 L/min was used as plasma working gas. The arc voltage, determined by the arc length, is also dependent on the arc current, nozzle diameter, gas composition, and gas flow rate. The direct current for the arc was supplied from an insulated gate bipolar transistor (IGBT) rectifier power with a maximum output of 160 A current and 140 V open-circuit voltages. A downer reactor with an inner diameter of 20 mm was used for coal tar pyrolysis. The reactor consists of four parts: (1) the coal tar inlet, (2) the reaction chamber, (3) the temperature measurement channel, and (4) the quenching medium inlet. The reactor length can be adjusted to achieve quenching at various vertical distances to the nozzle. The inner reactor temperature was measured by four C type high temperature thermal couples (NANMAC corp. A12D, accuracy of 0.75 %). The vertical distance from the four thermal couples to the plasma nozzle was 60, 70, 80 and 90 mm, respectively. At the bottom of the reactor, a cone-shaped quenching apparatus with a shrinking inner diameter from 20 to 10 mm was operated effectively to stop the decomposition of acetylene to hydrogen and soot, as shown in Fig. 2.
Coal tar samples produced by Sanjiang Coal Chemical Co., Ltd (at Shanxi province, China) were used as feedstock. The moisture content of the samples was 8.01 wt% and the density was 1.08 g/cm3. The C, H, O, S and N element content of the coal tar after dehydration were 82.74, 8.17, 7.91, 0.05 and 0.45 %, respectively. Piston type injector was used to pump coal tar into reactor perpendicular to the side wall below the plasma generator so as not to affect the stability of the arc. The coal tar feeding rate was within a range of 10–30 g/min.
Each feeding gas was controlled by a set of Mass Flow Controllers (MFCs, ALICAT 21-1-1-1000-24-AC-KM6008, accuracy of 0.1 %). The flow rate of the quenching water was controlled by a ball valve and measured with flow meter (NEXON, FTB200, accuracy of 0.5 %). An on-line mass spectrometer (Extrel CMS, MAX300-LG) was used to monitor the component concentration in pyrolysis gas. The fluctuations of the gas concentration became stabilized, which illustrates the reactor has reached a steady state. The gas species concentration at steady state was measured by two GCs (Agilent Technologies, Micro-GC 490). A filter (Swagelok, SS-4TF-7) was installed at the vent-pipe to collect the particulate carbon black and the unconverted coal tar to prevent the gas detector from being polluted.
The hydrogen elemental conversion (CH) of coal tar is used to evaluate the progress of coal tar pyrolysis reactions, defined as
where HTPG is the hydrogen elemental mass flow rate of the gas products in g/min; HWG is the hydrogen elemental mass flow rate of the plasma working gas in g/min; CT is coal tar feeding rate in g/min; and HC is mass fraction of hydrogen species in the coal tar.
The yield of acetylene and carbon monoxide is generally defined as
where YG is the yield of a particular product in wt%; and MFL is mass flow rate of a particular product in g/min.
The feedstock specific input power is defined to express the energy density in the reactor of the process, with the expression as following,
where SIP is specific input power in MJ/kg; and IP is input power in kW.
The specific energy requirement that express the economic value of the process is defined as
where SER is specific energy requirement in kwh/kg C2H2; and MFA is mass flow rate of acetylene in kg/h.
Results and Discussion
Different from traditional reactors, thermal plasma reactors can offer extremely high temperatures, massive amounts of active atoms and intense heat transfer. Coal tar undergoes a rapid heating process immediately as it is injected into the thermal plasma reactor. Evaporation and thermal cracking reactions follow to release a large amount of small molecular species (such as CO, H2, CH4, C2H4, etc.). Acetylene is produced in milliseconds at a gas phase average temperature about 1500–4000 K due to the thermodynamic characteristics of acetylene. In coal tar pyrolysis process, thermal energy is the driving force and the gas-phase reactions are mainly controlled by the thermodynamic equilibrium [21–24]. The maximum value of acetylene concentration is determined by the gas phase temperature before quenching, and rapid quenching is essential to prevent acetylene decomposition. In quenching step, the quenching medium selection and its flow rate play important roles. As mentioned above, the quenching temperature needs to be established first. After that, experimental studies on various quenching media are carried out to investigate the properties of the quenching operation.
Effect of Specific Energy Input on Reaction Process
Both mass/heat transfer and reaction temperatures in a typical plasma pyrolysis process are affected by the energy input. The influence of power input was investigated by using the index of specific input power see Eq. (3). The first series of experiments were carried out at different levels of specific input power without a subsequent quenching process. Hydrogen elemental conversion from coal tar to gas products and the reactor internal temperature are two of the most influential parameters used to demonstrate the effect of power input on the pyrolysis process.
Figure 3 is a graph of the hydrogen elemental conversions at different levels of coal tar specific input power without quenching for the pyrolysis gas flow. The hydrogen elemental conversion increases rapidly as the coal tar specific input power increases from 4.2 to 22.5 MJ/kg, while the change becomes flat afterwards. The hydrogen elemental conversions obtained in the experiments were only 8.5 and 15 wt% at the specific input powers of 4.2 and 6.8 MJ/kg, respectively. However, a high hydrogen elemental conversion up to 88.4 wt% can be obtained at a specific input power of 22.5 MJ/kg. Coal tar is a by-product in coal carbonization and coal pyrolysis process, and it has various structures such as alkane structures, aliphatic structures and aromatic structures, etc. According to the characteristics of liquid hydrocarbons pyrolysis [22], alkanes are easier to be broken up than other structures, which are mainly caused by the difference of molecular structures. The results above show that the hydrogen elemental conversion is no longer affected much when the specific input power is higher than 22.5 MJ/kg under current experimental conditions.
The errors for the hydrogen elemental conversions are in between 3 and 50 % (see error bars in Fig. 3) based on the three repeated experiments. The error generated at high specific input power (3 % at 43.6 MJ/kg) was much less than that at low specific input power (50 % at 4.2 MJ/kg). This phenomenon may indicate that the coal tar pyrolysis reactions are incomplete and relatively unstable at low specific input power because of insufficient thermal energy supplied.
Solid residue generated in the reaction process was collected and sampled for ultimate analysis. The ultimate analysis results of the solid residue can be used to calculate the gas phase elemental compositions which are the basis for a thermodynamics analysis. At the specific input power of 22.5 MJ/kg, solid residue yield was 35.1 wt% with the C, H, and O element contents of 86.64, 2.74 and 9.85 %, respectively. Figure 4 shows the mole fraction of each species by thermodynamics calculation from the elemental compositions of gas phase at 22.5 MJ/kg; and the calculation was explained in detail in the work by Wu et al. [24]. The line of acetylene mole fraction in Fig. 4 indicates that the maximum acetylene concentration in gas phase appears in the temperature range of 1800–3000 K, which agrees well with the previous researches on this process [23, 24]. The calculation results also indicate that the C2H2 concentration drops rapidly once the gas temperature is below 1800 K. The C2H2 formation is very sensitive to the temperature; the initial quenching temperature appears to be of primary importance to ensure C2H2 at maximum concentration in the C–H equilibrium system.
Figure 5 shows the variation of the internal reactor temperature with respect to the axis distance to the plasma nozzle at different coal tar specific input powers. Under the experimental conditions, the average temperature becomes lower as the specific input power becomes lower and vice versa. The internal reactor temperature drops gradually as the reactor length extends. But it increases as the specific input power increases at a given axis position as shown in Fig. 5. The specific input power of 10.0, 22.5 and 34.8 MJ/kg correspond to 685, 1368 and 1686 K of the internal reactor temperature at a reactor length of 90 mm. These temperatures increase to 973, 1792 and 2072 K, respectively when the reactor length reduces to 80 mm. The results demonstrate that high specific input powers can provide sufficient thermal energy for coal tar pyrolysis to acetylene, but the temperature inside a plasma reactor has a large temperature gradient in the vertical direction. Considering the hydrogen elemental conversions and the thermodynamics analysis results, the coal tar specific input power of 22.5 MJ/kg with a quenching at 80 mm (far from the plasma nozzle) can be used as the optimal operating conditions.
Effect of Quenching Gases on Acetylene Yield
The effects of quenching media and quenching media flow rates on the process were further investigated based on the optimal operation conditions (i.e. at coal tar specific input power of 22.5 MJ/kg and reactor length of 80 mm). The ratio of medium volume flow rate of a quenching medium to coal tar feeding rate, termed as the quenching media flow factor (QMFC), was used to describe the strength of quenching process, is defined as,
where the unit of QMFC is in L/g (coal tar); and GFL is the quenching medium volume flow rate in L/min;.
Experiments were carried out by using argon, hydrogen, and methane as quenching media under different quenching medium flow factors.
As shown in Fig. 6, the acetylene yield is much higher when a quenching medium is used compared to a case without quenching media (i.e. 10.2 %). The quenching results are heavily dependent on the selection of a particular quenching gas. The cause to the low acetylene yield in the case of no quenching is the fact that a large amount of acetylene undergoes strong decomposition reactions when the gas phase temperature decreases slowly as reported in the literature [13, 23–25]. When a quenching medium is injected into the reactor, the acetylene in the gas phase is kept intact by rapid cooling. The acetylene yield with argon as the quenching medium increases consistently with the argon gas flow rate increases. For example, they were 15.1 and 17.8 wt% at a quenching medium flow factor of 1 and 3 L/g (coal tar), respectively. The argon gas acts mainly as a cooling agent. The higher the gas flow rates are, the stronger the cooling effects become at a fixed mass ratio of C/H of the gas phase. Similar phenomena were observed on hydrogen as the quenching gas. However, hydrogen has better quenching effect than argon at the same quenching medium flow factor. For example, at a quenching medium flow factor of 3 L/g (coal tar), the acetylene yield was 24.6 wt% with hydrogen as compared to 17.8 % with argon. The main reason is that hydrogen has a higher molar specific heat capacity and thermal conductivity than argon. Furthermore, added hydrogen increases the mole ratio of H/C of the gaseous phase, which in turn increases the acetylene concentration in the gas phase [24].
Different from using argon and hydrogen as quenching media, the acetylene yield in the case of using methane was significantly increased and a maximum value was observed by varying methane flow rate. Methane is viewed as ideal feedstock to produce acetylene in thermal plasma reactors [8]. When methane is used as quenching medium, it both cools down the gas phase and simultaneously cracks into acetylene. This consumes thermal energy, which helps the gas phase temperature to decrease rapidly. Acetylene yield increases rapidly when the quenching medium flow factor changes from 0 to 2 L/g (coal tar). This is caused both by the additional acetylene generated from methane cracking and the increased mole ratio of H/C. However, when the quenching medium flow factor increases further, acetylene yield drops remarkably. This is mainly due to the fact that less methane can be cracked as the gas phase temperature drops much faster with overload methane injected.
The errors for acetylene yields were between 6 and 15 % (see error bars in Fig. 6) based on the three repeated experiments. The error generated with hydrogen as quenching medium (6 %) was much less than the case with argon and methane as quenching media (12 and 15 %, respectively). These results indicate that adding hydrocarbons into the quenching process makes the formation of acetylene more unstable due to additional side reactions.
A large amount of thermal energy is required to produce acetylene because the formation of acetylene from coal tar is strongly endothermic. Figure 7 shows the influence of quenching medium flow factor on the specific energy requirement in this lab-scale process. The SER was 61.2 kWh/kg C2H2 without quenching, which is much higher than the value of using gas quenching media. When using argon and hydrogen as quenching media, the lowest SER was 35.1 and 25.4 kWh/kg C2H2 at 2 L/g (coal tar) quenching medium flow factor, respectively. The results indicate that high energy efficiency can be obtained under effective quenching operations. When methane was used as the quenching medium, the lowest SER was reduced to 13.9 kWh/kg C2H2 under the same quenching medium flow factor, which demonstrates that using hydrocarbons as the quenching media can greatly improve the process economies. Also as shown in Fig. 7, the SER drops rapidly when the quenching medium flow factor increases from 0 to 2 L/g (coal tar), and then the trend goes down as the quenching medium flow factor increases further. This clearly means that over quenching is not helpful to fully utilize the thermal energy.
Effect of Water Quenching on Acetylene Yield
Water was also used as the quenching medium in the experiments at a coal tar specific input power of 22.5 MJ/kg and a reactor length of 80 mm. Figure 8 plots the trends of acetylene yield and carbon monoxide yield as a function of the quenching medium flow factor. The acetylene yield increases as the quenching medium flow factor changes from 0 to 0.13 L/g (coal tar), and a maximum value of 23.6 wt% can be found at 0.13 L/g (coal tar). This illustrates that reacting flow can be quenched rapidly enough by water. The acetylene yield drops slightly as the quenching medium flow factor increase further. This is due to the fact that excessive water leads to lower gas equilibrium temperature and hence less acetylene is generated in the gas phase. The errors of acetylene yield as shown by the error bars in Fig. 8 were below 4 % based on the three repeated experiments, which indicates that using water as the quenching medium is an effective and stable approach to cool down the gas phase.
As shown in Fig. 8, the carbon monoxide yield increases continuously as the quenching medium flow factor increases. A large amount of carbon monoxide was produced because of the reactions between water and carbon at high temperatures. The carbon monoxide yield increases rapidly at relatively low quenching medium flow factors and approached to a plateau at the relatively high flow factors. The hydrogen generated by water gas shift reaction has a positive effect on stopping acetylene decomposition due to the increased mole ratio of the H/C in the gaseous equilibrium. However, unnecessary oxygen is introduced in the reactions with water, bringing in a negative effect on the formation of acetylene [4]. This is the reason why the maximum acetylene yield generated by using water as quenching medium is lower than that with hydrogen. The errors of carbon monoxide yield were between 5 and 8 % (see error bars in Fig. 8).
Figure 9 shows the influence of quenching medium flow factor on the specific energy requirement in the lab-scale process using water. The SER drops rapidly as the quenching media flow factor increases from 0 to 0.13 L/g (coal tar), and a minimum value of 26.4 kWh/kg C2H2 can be found at 0.13 L/g (coal tar). The minimum SER with water as the quenching medium is much lower than the one with argon, but a little higher than the one with hydrogen. The results indicate that water is a relatively good quenching medium to achieve rapid quenching outcome with a low cost, even though the oxygen content has a negative impact on the energy efficiency. The SER increases at the relatively high quenching medium flow factor, exhibiting the same trend as in previous experiments.
Conclusions
This work presents a comprehensive study of the quenching effects on coal tar pyrolysis to acetylene process in H2/Ar plasma. The hydrogen elemental conversion increases gradually as coal tar specific input power increases. Appropriate quenching temperature and maximized acetylene yield are established by the thermodynamic equilibrium analyses on coal tar pyrolysis process. Measurements of the internal temperatures are used to determine an appropriate reactor length, which in turn ensures the flow is well quenched. Hydrogen, argon, methane and water were used as quenching media for the experiments. The experimental results indicate that the acetylene yields can be significantly increased by an effective quenching. The maximum acetylene yield was 24.6, 17.8, 44.9 and 23.6 wt% in the case of using hydrogen, argon, methane, and water, respectively. The quenching outcomes are heavily dependent on the selection of a quenching medium. The acetylene yield increases continuously as the quenching medium flow factor increases in the case of using hydrogen and argon, while a maximum value can be found in the case of using methane and water. Oxygen content in quenching medium has a negative effect on the formation of acetylene. The SER was greatly affected by the quenching medium flow factor. The lowest SER of 13.9 kWh/kg C2H2 was obtained by using methane as quenching medium at the quenching medium flow factor of 2 L/g (coal tar). The estimation of SER in the lab-scale apparatus can be used to estimate the economics of the quench process. Light hydrocarbons having good reactivity in thermal plasma are preferred quenching media to achieve integrated energy utilization for such an ultrahigh temperature process.
References
Chen JQ, Cheng Y, Xiong XY, Wu CN, Jin Y (2009) Research progress of coal pyrolysis to acetylene in thermal plasma reactor. Chem Ind Eng 28:361–367
Cheng Y, Chen JQ, Ding YL, Xiong XY, Jin Y (2008) Inlet effect on the coal pyrolysis to acetylene in a hydrogen plasma downer reactor. Can J Chem Eng 86:413–420
Chen J, Cheng Y (2009) Process development and reactor analysis of coal pyrolysis to acetylene in hydrogen plasma reactor. J Chem Eng Jpn 42:103–110
Yan BH, Xu PC, Guo Y, Jin Y, Cheng Y (2012) Experimental study on coal pyrolysis to acetylene in thermal plasma reactors. Chem Eng J 207:109–116
Plooster MN, Reed TB (1959) Carbon–hydrogen–acetylene equilibrium at high temperatures. J Chem Phys 31:66–72
Boulos MI (1991) Thermal plasma processing. IEEE Trans Plasma Sci 19:1078–1089
Nicholson R, Littlewood K (1972) Plasma pyrolysis of coal. Nature 236:397–400
Gladisch H (1962) How Huels makes acetylene by dc arc. Hydrocarb Process Petrol Refin 41:159–164
Holmen A (2009) Direct conversion of methane to fuels and chemicals. Catal Today 142:2–8
Bond RL, Ladner WR, Mcconnell GIT, Galbraith IF (1963) Production of acetylene from coal, using a plasma jet. Nature 200:1313–1314
Bond RL, Ladner WR, Mcconnel GIT (1966) Reactions of coal in a plasma jet. Fuel 45:381–395
Krukonis VJ, Gannon RE, Modell M (1974) Deuterium and carbon-13 tagging studies of the plasma pyrolysis of coal. Adv Chem Ser 131:29–41
Chakravartty SC, Dutta D, Lahiri A (1976) Reaction of coals under plasma conditions—direct production of acetylene from coal. Fuel 55:43–46
Holmen A, Oisvik O, Rokstad OA (1995) Pyrolysis of natural gas: chemistry and process concepts. Fuel Process Technol 42:249–267
Bittner D, Baumann H, Klein J (1985) Relation between coal properties and acetylene yield in plasma pyrolysis. Fuel 64:1370–1374
Plotczyk WW, Resztak A, Szymanski A (1995) Plasma processing of brown coal. Int J Mater Prod Technol 10:530–540
Leutner HW, Stokes CS (1961) Producing acetylene in a plasma arc. Ind Eng Chem 53:341–342
Ibberson VJ, Sen M (1976) Plasma-jet reactor design for hydrocarbon processing. Trans Inst Chem Eng 54:265–275
Laflamme CB, Jurewicz JW, Gravelle DV, Boulos MI (1990) Thermal plasma reactor for the processing of gaseous hydrocarbons. Chem Eng Sci 45:2483–2487
Fincke JR, Anderson RP, Hyde T, Detering BA, Wright R, Bewley RL, Haggard DC, Swank WD (2002) Plasma thermal conversion of methane to acetylene. Plasma Chem Plasma Process 22:105–136
Cheng Y, Yan BH, Li TY, Cheng Y, Li X, Guo CY (2015) Experimental study on coal tar pyrolysis in thermal plasma. Plasma Chem Plasma Process 35:401–413
Yan BH, Xu PC, Li X, Guo CY, Jin Y, Cheng Y (2012) Experimental study of liquid hydrocarbons pyrolysis to acetylene in H2/Ar plasma. Plasma Chem Plasma Process 32:1203–1214
Wang F, Guo WK, Yuan XQ, Zhao TZ (2006) Thermodynamic study on production of acetylene from coal pyrolysis in hydrogen plasma. Plasma Sci Technol 8:307–310
Wu CN, Chen JQ, Cheng Y (2010) Thermodynamic analysis of coal pyrolysis to acetylene in hydrogen plasma reactor. Fuel Process Technol 91:823–830
Polak LS (1966) Chemical processes in low-temperature plasmas. Pure Appl Chem 13:345–360
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, X., Wu, C. & Han, J. Quenching Experiment Study on Thermal Plasma Pyrolysis Process of Coal Tar. Plasma Chem Plasma Process 36, 869–880 (2016). https://doi.org/10.1007/s11090-016-9697-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-016-9697-2