Abstract
A multi-needle-to-plate pulsed discharge plasma reactor was designed to investigate its potential for polyvinyl alcohol-containing wastewater (PVA) treatment. The effects of some operational parameters such as PVA initial concentration, pulse peak discharge voltage, air flow rate, solution pH value, and iron additives on PVA degradation were examined. The results indicated that PVA could be effectively degraded from aqueous solutions. PVA degradation efficiency was 76.0 % within 60 min’s discharge plasma treatment with 1.5 mmol L−1 Fe2+ addition. Decreasing PVA initial concentration and increasing pulse peak discharge voltage were both beneficial for PVA degradation. There existed appropriate air flow rate for obtaining great PVA degradation efficiency in the present study. A little acid environment was conducive to PVA degradation. The presence of Fe2+ and Cu2+ could both benefit PVA degradation, and the increment of Fe2+ and Cu2+ concentrations to a certain extent could enhance its degradation efficiency, as well as energy yield. PVA possible degradation mechanisms were discussed, and the degradation processes were mainly triggered by the reactions of PVA with \(^{ \cdot } {\text{OH}}\) radicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyvinyl alcohol (PVA), a well known recalcitrant compound, has been widely used as a sizing agent in the textile industry [1], and it is also used in polymerization process, adhesives, paper-coating, surfactants, and detergent-based industries [2–4]. The global annual consumption of PVA has been over 1 mt, and it is predicted that its consumption is increasing at a rate of about 3.5 % annually during 2009–2014 [5]. PVA is a stable polymer with water activity. In aquatic environment, PVA usually exists as colloids, and it takes about 900 days to be decomposed completely [6], which is detrimental to the ecosystem and can accumulate in the human body through food chain [3, 4]. Once PVA-containing wastewater is discharged into natural water bodies without efficient treatment, the water surface will be covered by large area of foam due to the strong surface activity of PVA, and then the activity of aerobic microorganisms will be inhibited by oxygen absence [7]. Even worse, PVA can promote the migration of deposited and bound heavy metals from sediments into water streams [8]. Therefore, it is of great importance and necessity to effectively treat PVA-containing wastewater.
Lots of methods have been used for PVA wastewater treatment such as adsorption [9], biological treatment [10–12], and membrane filtration [13, 14]. Adsorption method is just the phase transfer of pollutant and can not remove pollutant completely. Conventional biological systems do not efficiently degrade PVA because PVA has a low BOD5/COD ratio (BOD5/COD = 0.01), indicating the poor biodegradability and the capacity of most microbial species for PVA degradation is very specific and limited [10]. Studies have shown that only 60 % of PVA was removed under aerobic conditions within 4 months’ treatment and 0–12 % of PVA was removed in anaerobic sludge within 77 days’ treatment [11]. In addition, the generation of foam in biological reactors during PVA wastewater treatment results in difficult operation and low treatment efficiency [12]. PVA can clog the membranes used in membrane-based wastewater treatment techniques [14]. Therefore, it is imperative to develop more effective methods to treat PVA-containing wastewater.
Recently, advanced oxidation processes (AOPs) are receiving great emphasis on PVA-containing wastewater treatment, such as Fenton and sonochemistry method [15–17], and it has been proved that the biodegradability of PVA wastewater can be enhanced after treatment. Non-thermal discharge plasma, one of newly developed AOPs, has aroused considerable interest because of its environmental compatibility [18, 19]. During discharge plasma processes, the ensuring electron-molecule interactions generate highly reactive non-thermal plasma, which are strongly oxidizing environments due to the presence of large number of chemically active species, such as ozone, H2O2, \(^{ \cdot } {\text{OH}}\) and \(^{ \cdot } {\text{OOH}}\) radicals, O atoms, and ions (O2 −, O2 +, H3O+, O3 −); simultaneously, physical effects concomitantly generated during discharge plasma process, such as strong electric field, ultraviolet light, shock waves and pyrolysis, can also improve pollutants degradation. Previous studies have demonstrated that organic compounds in wastewater can be effectively removed by non-thermal discharge plasma [18–20]. However, there is still no systematic research on PVA degradation in wastewater using non-thermal discharge plasma. The objective of this study was to explore the feasibility of PVA-containing wastewater treatment using pulsed discharge plasma. PVA degradation behavior and roles of radical species were investigated, and PVA degradation processes in the presence of Fe2+ and Cu2+ were examined. The possible mechanisms of PVA induced degradation by pulse discharge plasma were also discussed.
Experiments and Methods
Materials
PVA (analytical reagent) was purchased from the Chemical Plant of Yangling, China. All other organic and inorganic reagents were analytical grade and were used as purchased without further purification. PVA was dried at 105 °C for 24 h before use.
Pulse Discharge Plasma System
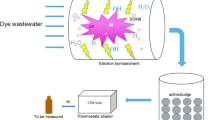
The schematic diagram of the experimental apparatus for PVA-containing wastewater treatment was illustrated in Fig. 1. The reaction system consisted of a pulsed high-voltage power supply and a multi-needle-to plate reactor. High-voltage pulses were generated using the combination of a 0–50 kV adjustable DC power supply, a storage capacitor (C e), an adjustable trim capacitance (C p) and rotation spark gap switches (RSG1, RSG2). In the power supply system, C e and C p were charged respectively by the changes of the rotation spark gap switches position, and then C p was discharged towards to the reactor, forming pulse discharge. The pulse frequency and C p were 35 Hz and 3 nF in the present research, respectively. The pulse rise time was less than 100 ns, and the pulse width was less than 500 ns.
For the multi-needle-to-plate reactor, the reactor vessel was made of a Plexigas™ cylinder (100 mm inner diameter and 250 mm length). The high voltage electrode (7 stainless-steel hypodermic pinheads, Φ = 1.5 mm), which produced positive streamer corona discharge at their needle tips, was located in the cylinder. The seven needles, comprised of one needle in the center and the other six distributed uniformly around a circle of 50 mm radius, were secured within a resin disc. Silicone insulation encased the hypodermic pinheads that protruded from the resin disc, with only 1 mm exposed beyond the silicone insulator. The ground electrode was a stainless-steel disc (Φ = 60 mm). The distance of adjacent needle was 15 mm. The distance between the high voltage electrode and the ground electrode was adjustable, and in the present research it was 15 mm.
The total volume of the treated PVA-containing wastewater was 300 mL, which was circulated by peristaltic pump and cooled by water bath during circulation. Air at atmosphere pressure flowed continuously into the reactor through the stainless steel needles.
Analysis
PVA concentration was determined using a 722 sp-type VIS-Spectrophotometer (UV-2802, Shanghai, China). PVA reacted with I2-KI solutions in boric acid to form a complex which exhibited selective absorption at 640 nm. A series of solutions with known PVA concentrations were firstly reacted and appropriate amounts of the I2-KI and boric acid solutions were added to blue-green solutions. The absorbance values at 640 nm were recorded and the relationship between absorbance and PVA concentrations was fitted [21].
Solutions of 0.1 mol L−1 HCl and NaOH were used to adjust solution pH value, respectively. An Orion 828 pH meter was used to measure solution pH. H2O2 concentration was measured using titanium potassium oxalate as reported by Sellers [22].
The peak pulse voltage and current were measured with a Tektronix TDS1012B-SC digital oscilloscope equipped with a Tektronix P6015A high voltage probe and a Tektronix P6021 current probe. The typical pulsed voltage and current waveforms obtained in the experiment were shown in Fig. 2.
Results and Discussion
Effect of PVA Concentration on Its Degradation
Figure 3 showed the effect of PVA initial concentration on its degradation by pulse discharge plasma. Herein the pulse peak discharge voltage was 22 kV, air flow rate was 5 L min−1, and initial solution pH was 4.95. As shown in Fig. 3, PVA degradation efficiency decreased with its initial concentration increased from 408–720 mg L−1 at the same discharge treatment time. It was indicated that the initial concentration affected PVA degradation behavior. When PVA initial concentration was 720 mg L−1, PVA degradation efficiency was 21.2 % within 60 min of discharge treatment time. However, when the initial concentration was 408 mg L−1, PVA degradation efficiency enhanced to 37.9 %. It might be the reason that the degradation amount of PVA was affected by input power of pulse discharge plasma. Therefore, under invariable input power, the degradation efficiency decreased with the increase of initial concentration. In addition, PVA degradation efficiency was close with the initial concentration of 324 and 408 mg L−1, and about 38.1 % of PVA was degraded within 60 min of discharge treatment time at 324 mg L−1. This might be due to the fact that there were enough active species reacted with PVA molecules in these two cases.
The following experiments are conducted at initial PVA concentration 324 mg L−1.
Effect of Pulse Peak Discharge Voltage on PVA Degradation
Pulsed discharge plasma can generate a large number of active species (such as high energy electrons, ions, radicals, and O3), ultraviolet light, shock waves, and heat etc., which are directly related to the pulse discharge voltage. To a certain extent, increasing pulse peak discharge voltage can improve the energy of each pulse, which benefits pollutant degradation. Hence, pulse peak discharge voltage is an important parameter to evaluate the potential of discharge plasma system for pollution control.
The effect of pulse peak discharge voltage on PVA degradation was shown in Fig. 4. Herein the air flow rate was 5 L min−1, and initial solution pH was 4.95. It was found that PVA degradation efficiency increased with the pulse peak discharge voltage. At pulse peak discharge voltage 20 kV, about 21.1 % of PVA was degraded within 60 min’s discharge plasma treatment, while it was enhanced to 39.5 % at pulse discharge voltage 24 kV within the same treatment time. It is generally believed that more energetic electrons are produced at higher pulse peak discharge voltage, leading to the accelerated formation of active species such as ozone, H2O2, and \(^{ \cdot } {\text{OH}}\) radical [23].
The mechanisms for \(^{ \cdot } {\text{OH}}\), ozone, and H2O2 formation were similar to those by electron irradiation, radiolysis and photochemistry. They were formed by the excitation and dissociation of the water molecules in the certain systems [24, 25]:
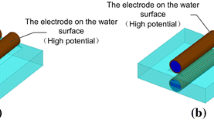
These chemically active species have strong oxidative potentials, which accelerate PVA degradation. Hence, the increase in pulse peak discharge voltage contributes to the increase in active species formation, which in turn enhances the PVA degradation efficiency. On the other hand, the pulse discharge generated in bubbles propagated by streamers directly into liquid phase. The discharge photos under different pulse peak discharge voltages were shown in Fig. 5. Obvious changes in plasma-liquid reaction surface were observed under different peak discharge voltages. The plasma-liquid reaction surface was disturbed more severely at higher pulse peak discharge voltage, which improved active species mass transfer and benefitted PVA degradation.
The following experiments are conducted at pulse peak discharge voltage 24 kV.
Effect of Air Flow Rate on PVA Degradation
The effect of air flow rate on PVA degradation was illustrated in Fig. 6. Herein the initial solution pH was 4.95. As shown in Fig. 6, there existed maximum PVA degradation efficiency with the change of air flow rate, which was obtained at the air flow rate 5.0 L min−1 in the experiments. When the air flow rate increased from 3.0 to 5.0 L min−1, there was an 8.4 % rise in PVA degradation efficiency within 60 min’s discharge plasma treatment. However, it exhibited downward trend with the further increase of air flow rate.
The existence of carrier gas could cause the change of discharge characteristics. Pulsed discharge plasma occurred more easily with a certain amount of carrier gas supplement, which was due to the fact that low pressure was generated near the pinpoint when the carrier gas passed through it, and thus the density of the carrier gas would decrease, and therefore the corona onset voltage decreased [26]. The results were similar with those of Zhang et al. [27], who pointed out that the increase of air flow rate to a certain extent was helpful for pollutant degradation; however, with the further increase of air flow rate, the residence time of active species such as ozone in aqueous was shortened and the utilization efficiency of the active species was decreased. A certain amount of air flow rate enhanced mass transfer of active species from gas phase to aqueous phase, and the stirring effect of PVA solutions by gas flow and the collision probability of PVA molecules with active species were both enhanced, which accelerated PVA degradation.
The discharge photos under different air flow rates were shown in Fig. 7. The plasma-liquid reaction surface was disturbed a little more severely at higher air flow rate, which improved active species mass transfer. However, lower PVA degradation efficiency was still observed at air flow rate 7.0 L min−1 than that at 5.0 L min−1; this might be attributed to the residence time of active species.
In addition, the dependencies about PVA degradation under different air flow rates seemed to be parallel at later treatment stage. This could be attributed to the fact that some degradation intermediates would compete with PVA molecules for active species.
Therefore, the optimized air flow rate is 5.0 L min−1 in this study.
Effect of Solution pH Values on PVA Degradation
During discharge plasma process in aqueous, the variety and property of chemically active species are quite different in variable solution pH. Therefore, it is of great significance to examine the effect of solution pH on PVA degradation during pulse discharge plasma in aqueous.
The effect of solution pH on PVA degradation was shown in Fig. 8, and the initial solution pH was 4.95 without pH adjustion. Solution pH imposed an apparent effect on PVA degradation. When solution pH values increased from 3.20 to 4.95, PVA degradation efficiency improved; however, there was an about 10 % decline in PVA degradation efficiency at solution pH value 8.10, as compared with that at solution pH 4.95. The greatest PVA degradation efficiency was achieved at pH 4.95 in the present research.
This may be presumed that more \(^{ \cdot } {\text{OH}}\) radical is produced from pulse discharge plasma in a little acid condition; while in an alkaline solution, \(^{ \cdot } {\text{HO}}_{2}\) ion will be decomposed by the discharge, which scavenges \(^{ \cdot } {\text{OH}}\) radical [28]. Similar phenomenon was also observed by Zhang [29], in whose research on dinitrophenol degradation in aqueous by discharge plasma, greater pollutant degradation occurred in a little acid condition than in an alkaline solution, and which was attributed to \(^{ \cdot } {\text{OH}}\) radical scavenged in alkaline conditions. On the other hand, the oxidative capacity of \(^{ \cdot } {\text{OH}}\) radical at higher solution pH was also low [30], and the useless decomposition of H2O2 was usually favored at relative higher solution pH [31]. Therefore, the observed PVA degradation efficiency decreased with solution pH increased from 4.95 to 8.10.
In addition, the observed PVA degradation efficiency was lower at solution pH 3.20 than that at pH 4.95, which might be due to the fact that \(^{ \cdot } {\text{OH}}\) radical could be consumed by excessive H+ at low pH value [30, 32].
During pulse discharge in aqueous with air as the carrier gas, NO −x could be generated, which affected the changes of solution pH. After pulse discharge occurred in PVA solutions (pH = 4.95, without pH adjustion) for 60 min, obvious change in solution pH was observed and it decreased to 3.11. On the one hand, the decrease of solution pH was not beneficial for PVA degradation, which could be confirmed by PVA degradation experiments in solution pH 4.95 and 3.20, where higher degradation efficiency occurred in pH 4.95; on the other hand, the decrease of solution pH could also enhance PVA degradation, for example, higher PVA degradation efficiency was observed in pH 7.00 than in 8.10. Therefore, the effects of changes of solution pH on PVA degradation were dependence on solution acid–base property, which directly affected active species activity and generation.
Effect of Cu2+ Addition on PVA Degradation
The effect of Cu2+ additive on PVA degradation was shown in Fig. 9. Herein, Cu(NO3)2 solutions were employed as the Cu2+ additive. Cu2+ additive imposed an apparent enhancement effect on PVA degradation. About 39.5 % of PVA was degraded within 60 min’s pulse discharge plasma treatment without Cu2+ addition; while there was an about 24.8 % rise in PVA degradation efficiency within the same treatment time with 1.5 mmol L−1 Cu2+ addition.
The enhancement effect of PVA degradation efficiency with Cu2+ addition could be attributed to more \(^{ \cdot } {\text{OH}}\) radical formation from H2O2 catalytic decomposition by Cu2+, as shown in Eqs. (8) and (9) [33]. Similar results were also reported by Zhang [29], where Cu2+ addition improved dinitrophenol degradation in aqueous during discharge plasma processes.
Effect of Fe2+ Addition on PVA Degradation
The effect of Fe2+ as the additive on PVA degradation by pulse discharge plasma was also tested and shown in Fig. 10. Herein, FeSO4 solutions were employed as the Fe2+ additive. It was found that Fe2+ additive enhanced PVA degradation greatly. About 39.5 % of PVA was degraded within 60 min’s pulse discharge plasma treatment without Fe2+ addition; while there was an about 36.5 % rise in PVA degradation efficiency within the same treatment time with 1.5 mmol L−1 Fe2+ addition. On the other hand, PVA degradation efficiency increased with the concentration of Fe2+ additive.
The corresponding energy yield with/without Fe2+ addition during pulse discharge plasma processes was presented in Fig. 11. Apparently, Fe2+ additive improved the energy utilization of this discharge plasma system. The energy yield was about 0.39 mg kJ−1 after 60 min of discharge plasma treatment without Fe2+ addition, which was enhanced to 0.7 mg kJ−1 with 1.5 mmol L−1 Fe2+ addition, that is, there was a 79.5 % increase in energy yield.
The addition of Fe2+ as catalyst enhanced the oxidizing power of the present discharge system because of the production of \(^{\cdot}{\text{OH}}\) radical in the solution by Fenton reactions, as shown in the following equations.
In addition, H2O2 concentration was measured within 30 min’s discharge treatment in four different medium, that is, direct pulse discharge in clean water without PVA, pulse discharge in PVA solutions, pulse discharge in PVA solutions with 1.0 mmol L−1 Cu2+ addition, and pulse discharge in PVA solutions with 0.8 mmol L−1 Fe2+ addition. The experimental results were shown in Table 1. Higher H2O2 concentration (6.4 mg L−1) was observed in clean water than that in PVA solutions (4.2 mg L−1), and this result indicated that H2O2 participated in PVA degradation processes. Moreover, obvious decrease in H2O2 concentration occurred when Fe2+ was added to PVA solutions, this could be attributed to Fenton reactions of Fe2+ and H2O2.
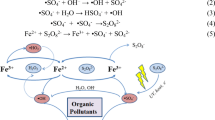
According to the above mentioned analysis, it was apparent that \(^{ \cdot } {\text{OH}}\) radical played an important role in PVA oxidation in the system. As a strong oxidant, \(^{ \cdot } {\text{OH}}\) radical could oxidize organics fairly quickly, by destroying chemical bonds such as C–H, C–C, C–O, and C=C [34]. For PVA, which contains lots of –OH functional group, like other organics, it can be broken down into smaller pieces under the attack of \(^{ \cdot } {\text{OH}}\) radicals. Further chain scission can occur to these smaller pieces to generate CO2 and H2O. Some ketones/enols may be formed during PVA oxidation by \(^{ \cdot } {\text{OH}}\) radical [35]. Furthermore, O3 can also react with organic pollutants through nucleophilic electrophilic and cyclo-addition reactions. The nucleophilic and electrophilic attacks of O3 yield some hydroxylated intermediates, and further attacks to these intermediates by O3 lead to forming small organic acids. Possible pathways of PVA degradation in the present system were described in Fig. 12.
Conclusions
The application of pulse discharge plasma-induced PVA degradation in wastewater was studied. Under the studied conditions, decreasing PVA initial concentration or increasing pulse peak discharge voltage resulted in higher PVA degradation efficiency. There existed a maximal degradation efficiency of PVA with the change of air flow rate, due to the retention of active species and their utilizations. A little acid environment benefited PVA degradation due to the formation of \(^{ \cdot } {\text{OH}}\) radicals and its greater utilization. The obvious enhancement effects of Fe2+ and Cu2+ additives on PVA degradation indicated that \(^{ \cdot } {\text{OH}}\) radicals played a decisive role in PVA degradation processes.
This study was a fundamental research effort, trying to offer an alternative solution for PVA-containing wastewater treatment.
References
Giroto JA, Guardani R, Teixeira ACSC, Nascimento CAO (2006) Chem Eng Process 45:523–532
Yang QX, Zhang WY, Zhang H, Li YH, Li CM (2011) Bioresour Technol 102:3790–3798
Tokiwa Y, Kawabata G, Jarerat A (2001) Biotechnol Lett 23:1937–1941
Tsujiyama S, Okada A (2013) Biotechnol Lett. doi:10.1007/s10529-013-1281-8
Inoguchi Y, Chinn H (2011) Polyvinyl alcohols, SRI consulting. http://www.sriconsulting.com/CEH/Public/Reports/580.1810/. Accessed 14 Nov 2011
Zhang B, Zhou Y (2003) Cotton Text Technol 31:17–20 (in Chinese)
Shan JC, Guan Y, Zheng QK, Han JS, Liu QS, Pu ZY (2009) J Appl Polymer Sci 113:860–867
Schonberger H, Baumann A, Keller W (1997) Am Dyestuff Rep 86:9–18
Zhang YH, Chen W, Lv GC, Lv FZ, Chu PK, Guo WM, Cui BL, Zhang R, Wang H (2012) Water Sci Technol 65:2055–2060
Phugare SS, Kalyani DC, Surwase SN, Jadhav JP (2011) Ecotoxicol Environ Safe 74:1288–1296
Li M, Zhu Z, Pan X (2011) Starch-Starke 63:638–691
Yu H, Gu G, Song L (1996) Environ Technol 17:1261–1267
Mo JH, Lee YH, Kim J, Jeong JY, Jegal J (2008) Dyes Pigments 76:429–434
Ji ZJ, He YS, Zhang GJ (2006) Desalination 201:255–266
Sun YY, Hua XY, Ge R, Guo AT, Guo ZY, Dong DM, Sun WT (2013) Environ Sci Pollut Res 20:5797–5805
Wu Y, Lian Y, Liu J, Zhu TH (2008) Water Treat Technol 34:32–34
Oh SY, Kim HW, Park JM, Park HS, Yoon C (2009) J Hazard Mater 168:346–351
Hu Y, Bai Y, Yu H, Zhang C, Chen J (2013) Bull Environ Contam Toxicol 91:314–319
Sun B, Aye NN, Gao Z, Lv D, Zhu X, Sato M (2012) J Environ Sci (China) 24:840–845
Wang HJ, Chen XY (2011) J Hazard Mater 186:1888–1892
Finley JH (1961) Anal Chem 33:1925–1927
Sellers RM (1980) Analyst 105:950–954
Wang HJ, Li J, Quan X (2008) Appl Catal B Environ 83:72–77
Sun B, Sato M, Clements JS (1999) J Phys D Appl Phys 32:1908–1915
Bian WJ, Zhou MH, Lei LC (2007) Plasma Chem Plasma Process 27:337–348
Barbara F, Romuald W (1993) IEEE Trans Elect Insul 28:932–940
Zhang YZ, Zheng JT, Qu XF, Chen HG (2008) Chemosphere 70:1518–1524
Gai K (2007) J Hazard Mater 146:249–254
Zhang JB, Zheng Z, Zhang YN, Feng JW, Li JH (2008) J Hazard Mater 154:506–512
Deng Y (2007) J Hazard Mater 146:334–340
Kang YW, Hwang KY (2000) Water Res 34:2786–2790
Herney-Ramirez J, Vicente MA, Madeira LM (2010) Appl Catal B Environ 98:10–26
Kaplan LA, Reasoner DJ, Rice EW (1994) J Am Water Works Assoc 86:121–132
Neyens E, Baeyens J (2003) J Hazard Mater 98:33–50
Zhang S, Yu H (2004) Water Res 38:309–316
Acknowledgments
The authors thank the National Natural Science Foundation, P.R. China (Project No. 21107085) and the Initiative Funding Programs for Doctoral Research of Northwest A&F University (2013BSJJ121) for the financial supports to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, T., Ma, T., Qu, G. et al. Performance Evaluation of Hybrid Gas–Liquid Pulse Discharge Plasma-Induced Degradation of Polyvinyl Alcohol-Containing Wastewater. Plasma Chem Plasma Process 34, 1115–1127 (2014). https://doi.org/10.1007/s11090-014-9565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-014-9565-x