Abstract
In this experiment, a gas–liquid two-phase discharge water treatment inverse device was designed independently to treat the actual workshop intermediate dye wastewater from a chemical plant. Firstly, the effects of initial concentration of wastewater, initial pH, circulation flow rate of solution, content of Fe2+, content of H2O2, and addition of tert-butanol on the organic removal rate and decolorization rate of dye wastewater treatment were investigated. The results showed that Fe2+ and tert-butanol would react with the active particles (H2O2, ·OH) and inhibit the degradation of the dye wastewater, resulting in the decrease of both organic matter degradation rate and decolorization rate. The experimentally degraded dye wastewater mainly contained benzoic acid and its derivatives in addition to dye molecules, thus the degradation mechanism of benzoic acid was mainly analyzed. Then, the actual dye wastewater treated by low-temperature plasma was combined with the traditional biological treatment technology. The biochemical properties of the wastewater treated by low-temperature plasma technology were greatly improved, and the B/C was increased from the initial 0.17 to 0.33. The effluent after the combined biological method could meet the effluent discharge standard, and the final CODcr reached 198 mg/L, BOD5 reached 65 mg/L, and pH and chromaticity reached 6.39 and 50, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dye wastewater is an industrial wastewater that is difficult to treat because of its highly variable water quality, high chromaticity, high biological toxicity and continuous bioaccumulation, difficulty in biodegradation, and high organic matter content (Chethana et al. 2016; Habiba et al. 2017; Saravanan et al. 2013). The composition of dye wastewater is complex, the actual dyeing wastewater is a mixture of various wastewaters discharged during the production process of dyeing enterprises, resulting in large variations in the quality of dyeing wastewater, with COD content as high as 2000–3000 mg/L and ammonia nitrogen content ranging from a few mg/L to 1000 mg/L (Chethana et al. 2016); due to the presence of color groups and chromophores in the dyestuff, it shows excellent pollution, and the dyeing material cannot be fully transferred to the textile during the dyeing process, and part of it remains in the water, thus making the wastewater coloration large (Liu 2020); dye wastewater contains metal pollutants such as chromium (Cr), copper (Cu), and iron (Fe), all of which are cumulative and may enter the food chain, thus causing bioaccumulation (Chethana et al. 2016; Habiba et al. 2017); dye wastewater contains metal pollutants and has a complex dye molecular structure (e.g., methyl orange and Congo red), and dye wastewater also contains many azo groups, heterocycles, benzene rings, amine groups, and other groups, making the dye wastewater poorly biochemical and difficult to biodegrade (Chatterjee et al. 2009; Chen et al. 2010; Qin et al. 2021). Some dyestuffs are carcinogenic, teratogenic, and mutagenic (Haque et al. 2021), and if dyestuff wastewater is discharged directly or indirectly without reasonable treatment, it will cause irreversible consequences to the environment and human health when it enters the ecosystem. At present, the main traditional methods of dye wastewater treatment process are physicochemical method, biochemical method, advanced oxidation method, and integrated treatment technology with multiple technologies coupled with each other. Advanced oxidation technologies (AOPs) can generate a large number of reactive groups such as ·OH, which decompose organic matter in dye wastewater through electron transfer, electrophilic addition, and dehydrogenation reactions, converting organic matter in dye molecules into easily biodegradable intermediates or directly mineralized into simple inorganic substances such as CO2 and H2O (Huang et al. 2021; Cui and Yin 2021). Therefore, in this paper, an experimental setup of gas–liquid two-phase discharge coupled with conventional biotechnology was designed to treat actual fuel wastewater.
Low-temperature plasma technology is one of the advanced oxidation technologies, and the main forms of discharge in the treatment of wastewater are classified as gas-phase discharge, liquid-phase discharge, and gas–liquid-phase discharge (Bruggeman et al. 2016). Gas–liquid two-phase discharge was first proposed by Yee et al. (1998). The principle of the advanced oxidation process of gas–liquid plasma is mainly the oxidative degradation of organic matter in wastewater using chemically active substances such as ·OH, O3, and H2O2 generated by the discharge, and a variety of physical effects such as high-energy electron radiation, shock waves, and ultraviolet light are generated during this discharge, which may also have disinfection and sterilization effects on wastewater (Jiang et al. 2014; Hyoung-Sup et al. 2015; Hwang et al. 2018). Compared with other advanced oxidation processes, it has the characteristics of fast degradation rate, short process, no secondary pollution, and wide applicability (Tijani et al. 2014). Malik et al. (2002) used pulsed corona discharge to treat methylene blue wastewater and investigated the effects of discharge voltage, solution conductivity, and other parameters on the removal rate of methylene blue, respectively. The experimental results showed that the energy efficiency of the reaction system as well as the degradation rate of methylene blue could be significantly increased when oxygen was introduced into the system. Aziz et al. (2019; 2017; 2018) proposed a novel planar descending film dielectric barrier discharge reactor and treated wastewater containing methylene blue, and the experimental results showed mineralization rates as high as 88%. Zhang et al. (2018) used dielectric barrier discharge plasma. Simultaneous removal of Cr(VI) and acid orange 7 (AO7) from aqueous solutions showed that there was a synergistic effect between Cr(VI) reduction and AO7 degradation, and the presence of Cr(VI) improved the degradation efficiency of AO7, which reached 89% at a voltage of 120 V. It was also demonstrated that the degradation efficiency of Cu(II), Co(II), Ni(II), Mn(II), and Fe(III) addition had no significant effect on the degradation of AO7. Chen et al. (2019) treated methyl orange (MO) synthesis wastewater by gas-phase discharge plasma method, and the experimental results found that the decomposition of MO could be promoted when Fe2+ was properly added, and the lower pH could favor the Fenton reaction and promote the degradation of MO. Tecer et al. (2020) used a cold plasma reactor to treat 30 L of textile wastewater and the experimental results showed that the efficiency of chemical oxygen demand (COD) and total suspended solids (TSS) reached 99%. Sanchez et al. (2019) used Fe2+ as a catalyst to treat 0.5 mM of acid black 210 (AB210) dye and achieved 99.9% removal at 180 min of treatment. Iervolino et al. (2020) used DBD and H2O2/DBD reactors to degrade acid orange 7. The experimental results showed that the H2O2/DBD reactor was more effective, obtaining about 80% color change and simultaneous mineralization at 2.5 min. Zhang et al. (2022) independently designed a gas–liquid two-phase discharge reactor to degrade alizarin red at alizarin red concentration of 50 mg/L, pH 7.21, at a discharge power of 43.5 W, a circulation flow rate of 52 L/h, and a carrier gas velocity of 20 mm/s. The removal of alizarin red reached 94.6%.
However, dual-media barrier gas–liquid two-phase discharge is currently facing problems of high energy consumption, low economic feasibility, incomplete decomposition of organic components, and easy generation of by-products, but it has some economic advantages if used as a front-end process to increase the biochemical properties of high concentrations of hard-to-degrade dye wastewater, in combination with traditional biological methods for treating actual dye industry wastewater. Biological methods are simple, inexpensive, environmentally friendly, and have the advantages of large number of microorganisms that are easy to obtain and maintain and low preparation conditions (Varjani et al. 2020). Biological methods are often used to degrade dye wastewater and it is a natural method with negligible energy consumption; however, not all dye wastewater is biodegradable and the efficiency of this method is low when the wastewater is not biodegradable (Njiki et al. 2020), for example, indoles containing double-ring gelling structure are difficult to be treated with ring breaking by traditional biological processing technology (Luo et al. 2021). Pre-chemical oxidation can improve the biochemical properties of industrial wastewater. Njiki et al. (2020) studied and optimized the kinetics of biodegradation of azo and triphenylmethane dyes using an integrated process combining biological treatment and sliding arc plasma. The experimental results showed that low-temperature plasma technology coupled with biodegradation significantly reduced the energy cost of treating textile wastewater by accelerating the kinetic rate of the wastewater.
Although many scholars have applied low-temperature plasma technology to dye wastewater treatment in a wide range of research areas in recent years, they have mainly focused on single or common simulated dye wastewaters and have not seen any research on the application of the technology to the treatment of actual dye wastewater (Tange et al. 2020). The main objective of this paper is to study the actual dye industrial wastewater as the object and adopt a self-designed gas–liquid two-phase discharge water treatment reactor in order to increase the gas–liquid mass transfer efficiency and increase the concentration of ozone and active radicals such as hydroxyl radicals in the solution in order to enhance the oxidation process of the actual dye wastewater, improve the treatment effect of the hard-to-degrade organic matter in the wastewater, and improve its biochemical properties.
Experiment
This project adopts self-designed dual-media barrier gas–liquid two-phase media barrier discharge water treatment reactor to degrade dye wastewater. Through the gas phase discharge generated active substances driven by the carrier gas through the venturi and wastewater miscible, the jet action makes a large number of micro and nano bubbles generated in the water, with the micro and nano bubbles in the water expansion, fragmentation, cavitation effect, local high temperature, and high pressure coupled with strong shock waves lead to the splitting of O2 and H2O in the water, the generation of ·OH, ·H, and O·, while in the liquid phase discharge generated ·OH, O3, and H2O2 reactive groups, high-energy electron bombardment, UV photolysis, and high-temperature pyrolysis destroy complex macromolecular compounds such as benzene, naphthalene, and anthracene, which constitute the color-rendering groups, reduce the color of wastewater, improve the B/C of wastewater, and reduce the difficulty for subsequent biochemical treatment. The treatment system effectively uses liquid circulation to lower the reactor temperature, isolate the wastewater from the electrode, improve the electrode life, and can be discharged stably. The method is also extremely scalable, and can significantly reduce treatment energy consumption and greatly improve degradation rates by combining with one or more other technologies or by adding suitable catalysts to the reaction system.
Source and nature of the dye wastewater
The dyestuff wastewater used in the experiment was taken from the actual workshop dyestuff intermediate wastewater of a chemical plant in Liaoning, which mainly contained benzoic acid and its derivatives in addition to dyestuff molecules. The actual water quality indexes of dye wastewater are shown in Table 1.
Experimental system
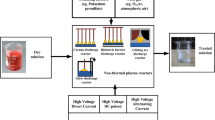
The experimental system mainly consists of a plasma module and an activated sludge biological treatment module, as shown in Fig. 1. The dye wastewater was first treated by the experimental self-designed dual-media barrier gas–liquid two-phase media barrier discharge water treatment reactor. The activated sludge was then taken from a sewage plant in Shenyang, Liaoning Province, China, and used for biological experiments after 1 month of domestication and cultivation, and the mixed liquid suspended solids (MLSS) of the domesticated sludge was 5000 mg/L. The domesticated sludge was added to the actual dye wastewater treated by low-temperature plasma technology under optimal operating conditions in a volume ratio of 1:9 in a beaker, sealed with a permeable sealing film, and placed in a constant temperature shaker (THZ-100, Shanghai YETOP Instruments Co., Ltd., China) at a shaking speed of 120 r/min for 8 h.
The plasma module mainly consists of a wastewater treatment unit, a gas supply unit, and a liquid circulation unit, as shown in Fig. 2.
The gas enters the high-pressure discharge chamber of the gas–liquid two-phase discharge water treatment reactor through the pressure reducing valve and rotor flow meter, and the working gas generates O3, ·OH, H2O2, and other high-energy active groups under the excitation of high-frequency high-voltage power supply. The wastewater water sample is pumped into the main reaction chamber of the wastewater treatment device by a submersible pump through the inlet pipe, and then fully mixed with the active substances generated by the discharge in the gas–liquid two-phase discharge reactor through the venturi jet through the circulation pump, and the organic matter in the wastewater is gradually degraded.
Wastewater treatment device
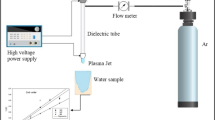
The wastewater treatment device mainly includes a gas–liquid two-phase discharge reactor, a high-frequency high-voltage power supply (CTP-2000 K, Nanjing Suman Electronics Co., Ltd., China), a voltage regulator, and a digital display oscilloscope. The structure of the discharge reactor is schematically shown in Fig. 3. The reactor adopts the gas–liquid two-phase mixed discharge, separating the liquid to be treated and the electrode into two chambers, avoiding the corrosion and loss of the electrode, while the outer liquid flowing phase can be used as the electrode liquid cooling system. The reactor has an external body made of organic glass, an internal medium made of quartz glass, a high voltage electrode made of nickel–chromium alloy, and a grounding electrode made of copper mesh. The discharge spacing is 9.0 mm, and the effective discharge area length is 12 cm.
Gas supply device
The gas supply device mainly includes electromagnetic air pump, gas pipeline, and rotameter. The gas flow rate is controlled at 20.0 mm/s in the experiment.
Liquid circulation device
Liquid circulation device mainly includes liquid circulation pool, venturi injector, submersible circulation pump, etc. Among them, the volume of the liquid circulation pool is 500 mL; the venturi injector is used for gas–liquid mixing; and the submersible circulation pump (flow rate 450 L/H, head 7 m) is placed at the bottom of the liquid circulation pool.
Analysis method
COD
COD of the water samples was determined by COD dissipation instrument (RD-125, Rovibon, Germany) and COD rapid tester (MD-200, Rovibon, Germany).
BOD5
BOD5 was measured by BOD tester (LH-BOD601, Beijing Lianhua Yongxing Technology Development Co., Ltd., China).
pH
The pH of the solution was measured by digital pH meter (FE28, Mettler Toledo Instruments (Shanghai) Co., Ltd., China).
Colorimetricity
Colorimetric determination using the dilution multiplier method (GB11903-89).
Conductivity
The conductivity of the solution was measured by digital display conductivity meter (DDB-303 A, Shanghai Precision Scientific Instruments Co., Ltd., China).
Ozone concentration
The gas-phase ozone concentration was measured by UV-2100 (IDEAL MACHINE TECH INC) meter; and the liquid-phase ozone concentration was measured by sodium indigo disulfate spectrophotometry (Chavez et al. 2016).
Organic matter removal rate
The removal efficiency of organic matter was calculated as formula (1):
where η is the removal efficiency, %; CA is COD in wastewater before treatment, mg/L; and CB is COD of wastewater after treatment, mg/L.
Results and discussion
Effect of circulation flow rate on the removal rate of organic matter
Initial conditions: the initial COD of wastewater was 14,000 mg/L, pH value was 2.23, discharge voltage was 25 kV, and carrier gas flow rate was 20.0 mm/s.
Take 1000 mL of dye wastewater in a beaker, add coagulant for coagulation and sedimentation for about 30 min, take 250 mL of supernatant in a beaker and dilute it to 500 mL, set the circulation variable to 16.0 L/h, 28.0 L/h, 40.0 L/h, 52.0 L/h, and 64.0 L/h respectively for the experiment, and the degradation results are shown in Fig. 4 and Table 2.
As shown in Fig. 4 and Table 2, the removal rate of organic matter in the actual dye wastewater increased, then decreased and then increased with time. At 10 min of reaction, the highest removal efficiency of 64.6% was achieved at a flow rate of 64.0 L/h. At 20 min of reaction, the removal rate was less than 50%. With further reaction, the removal rate at a circulation flow rate of 52.0 L/h was higher than that at other circulation flow rates, and the decolorization rate reached 92%. The reason is that in the early stage of the discharge reaction, the easily degradable organic matter in the wastewater is rapidly oxidized and decomposed, but as the discharge reaction proceeds, the unsaturated large-molecule organic pollutants are oxidized and decomposed into small-molecule organic matter, resulting in a second increase in COD value, i.e., a decrease in removal rate. When the concentration of dissolved ozone and active groups such as hydroxyl groups in water increases, small molecule organic matter is further oxidized and decomposed, causing the removal rate to increase again. When the flow rate was at 64.0 L/h for a long time, the water flow rate was too large and too much air was introduced. Under the action of the jet of the jet, a large number of ultra-micro nano bubbles broke up and increased the collision between the solution and the reactor wall, which reduced the concentration of dissolved ozone in the water, so the flow rate of 52.0 L/h was chosen for the subsequent experiment.
Effect of initial COD concentration on removal efficiency
Initial conditions: pH of wastewater was 2.14, liquid circulation flow rate was 52.0 L/h, discharge voltage was 25 kV, and carrier gas flow rate was 20.0 mm/s.
Take 1000 mL of dye wastewater in a beaker, add coagulant for coagulation and sedimentation for about 30 min, take 500 mL, 250 mL, and 125 mL of supernatant in a beaker, and dilute it to 500 mL, and the degradation results are shown in Fig. 5 and Table 3.
From Fig. 5, it can be seen that the organic removal rate increased, then decreased and then increased with time. When the reaction was carried out for 10 min, the removal rate of organic matter in the solution with initial COD concentration of 14,100 mg/L reached 45.8%, and the removal rate of COD initial concentration of 8100 mg/L was 46.9%, while the removal rate of organic matter in the undiluted raw water was only 7.7%. As can be seen from Table 3, the decolorization rate of the solution with the initial COD concentration of 14,100 mg/L reached 50% after 10 min of reaction, which was basically the same as the other conditions. When the reaction was carried out for 60 min, the decolorization rate reached 82%. From the overall degradation trend, the removal rate of organic matter in the solution with initial COD concentration of 8100 mg/L and 14,100 mg/L was not much different, but the decolorization rate of organic matter in the solution with initial COD concentration of 14,100 mg/L was better than that when the initial COD concentration was 8100 mg/L. The reason is that the reactor is able to generate a large number of active radicals in a short period of time, and enough active particles have an increased number of effective collisions at an initial COD concentration of 14,100 mg/L without causing waste of active particles, so that the removal rate of COD is greatly increased in a short period of time, providing advanced mineralization of organic matter. However, the number of active radicals produced is limited, which leads to a decrease in degradation efficiency when the concentration is too high.
Effect of initial pH on removal rate
Initial conditions: the initial COD of wastewater was 14,000 mg/L, the liquid circulation flow rate was 52.0 L/h, the discharge voltage was 25 kV, and the carrier gas flow rate was 20.0 mm/s.
Take 1000 mL of dye wastewater in a beaker, add coagulant for coagulation and sedimentation for about 30 min, take 250 mL of supernatant in a beaker and dilute it to 500 mL, adjust the initial pH of the solution to 2.40, 4.75, 7.18, 9.68, and 11.21, respectively, and the degradation results are shown in Fig. 6 and Table 4.
As shown in Fig. 6 and Table 4, the removal rates of wastewater with different initial pH values were 52.2%, 14.1%, 38.6%, 59.2%, and 31.5%, and the decolorization rates were 82%, 34%, 52%, 64%, and 72%, respectively, after 60 min of discharge reaction. Under the alkaline condition, the removal rate of organic matter from the actual dye wastewater was significantly accelerated compared with that without pH adjustment. When the initial pH was adjusted to 7.58, the removal rate was 58.36% after 20 min discharge treatment, while the removal rate was only 23.23% at pH > 9, and the degradation of organic matter was even worse at pH < 6.
The reason for this is that when the initial pH of the wastewater is adjusted to 9.68, the production rate of hydroxyl groups is high and the yield of benzoic acid into 2-hydroxybenzoic acid is higher, increasing the removal rate of benzoic acid and thus the degradation rate of organic matter (Khlyustova et al. 2020). However, when the pH is 9.68, the organic matter in the wastewater that does not contain functional groups presenting color is easily degraded by ozone and reactive groups such as hydroxyl groups, so the organic matter removal rate is higher under this condition, but the decolorization rate is lower. When the initial pH of the solution is adjusted to 4.75, a large amount of H+ in the solution converts monomeric oxygen into HO2 and O2– which are less reactive, so the removal rate under this condition is lower. When the initial pH of the solution was adjusted to 11.21, it could be seen that the degradation trend was different from other conditions, which was due to the fact that OH− would remove the ·OH from the wastewater in a short time, so that the active particles in the wastewater were reduced and most of the organic substances in the wastewater, such as benzoic acid, were reacted to form oxygenated acid salts, which could not be completely oxidized and decomposed in a short time, so the concentration of COD changed at a slower rate. However, the COD degradation rate still showed a continuous increasing trend at pH 11.21, indicating that other active particles (such as O3 and H2O2) played a dominant role in the process.
Effect of Fe2+ on removal rate
Initial conditions: the initial COD of wastewater was 14,000 mg/L, pH value was 2.56, liquid circulation flow rate was 52.0 L/h, discharge voltage was 25 kV, and carrier gas flow rate was 20.0 mm/s.
Take 1000 mL of dye wastewater in a beaker, add coagulant for coagulation and sedimentation for about 30 min, take 250 mL of supernatant in a beaker and dilute it to 500 mL, add FeSO4 solution with concentration of 10.0 mg/L, 50.0 mg/L, and 100.0 mg/L, respectively, and the degradation results are shown in Fig. 7 and Table 5.
As shown in Fig. 7 and Table 5, the removal of organic matter from the dye wastewater was 52.2%, 48.4%, 38.1%, and 27.5%, and the decolorization rates were 80%, 32%, 44%, and 34%, respectively, after 60 min of discharge reaction. As the reaction proceeded, precipitates appeared in the solution. The reason is that the wastewater itself contains a large amount of acidic substances, and the Fe2+ added in the solution can have Fenton reaction with H2O2 in the solution, and Fe2+ is oxidized to produce Fe3+, and Fe3+ produces colored complexes with organic acids, and the complexes are more stable and difficult to be further degraded, which makes the decolorization rate of the wastewater greatly reduced. Therefore, the addition of Fe2+ alone in this actual wastewater treatment does not promote the removal of organic matter, but decreases the decolorization rate. The addition of Fe2+ caused the first increase and then decrease in the degradation of benzoic acid due to the scavenging reaction of Fe2+ with ·OH radicals, which slowed down the reaction rate and corroborated the decrease in the removal of organic matter from the dye wastewater (Deshpande et al. 2019).
Effect of H2O2 on removal rate
Initial conditions: the initial COD of wastewater was 14,000 mg/L, pH was 2.26, liquid circulation flow rate was 52.0 L/h, discharge voltage was 25 kV, and carrier gas flow rate was 20.0 mm/s.
Take 1000 mL of dye wastewater in a beaker, add coagulant for coagulation and sedimentation for about 30 min, take 250 mL of supernatant in a beaker and dilute it to 500 mL, add H2O2 solution with concentration of 10.0 mg/L, 50.0 mg/L, and 100.0 mg/L, respectively, and the degradation results are shown in Fig. 8 and Table 6.
From Fig. 8 and Table 6, it can be seen that the organic removal rate increased with the concentration of H2O2 in the wastewater when the concentration of H2O2 was certain, and when the concentration of the over H2O2 solution was too high, it would inhibit the degradation of organic matter in it instead. When the reaction time reached 30 min, the removal rates of organic matter in the wastewater were 17.3%, 33.9%, 28.6%, and 34.8%, respectively. The highest decolorization rate of up to 90% was achieved when the addition amount of H2O2 was 10 mg/L. When the reaction was carried out for 60 min and the addition amounts of H2O2 were 0 mg/L, 10 mg/L, and 50 mg/L, the removal rates were higher than 50%. The reason is that the actual dye wastewater composition is complex, and H2O2 has a strong oxidation ability under acidic conditions, and it can further react and decompose into oxygen and water with the organic matter in the wastewater that has not been oxidized by active groups such as ozone and hydroxyl radicals under the UV light and high temperature conditions generated by the discharge, which promotes the production of ozone and improves the removal rate of organic matter. And benzoic acid is mainly degraded by H2O2 or with the destruction of ·OH, and thus the high concentration of H2O2 increases the degradation rate of organic matter in a short period of time (Khlyustova et al. 2020), but then the reason for the decrease is the automatic decomposition of H2O2 into oxygen and water and the reorganization of ·OH radicals (Deshpande et al. 2019). Although H2O2 can promote the production of ·OH, too high H2O2 concentration will act as a radical scavenger and make the degradation of organic matter less efficient.
Effect of the content of tert-butanol on the removal rate
Initial conditions: the initial COD of wastewater was 14,000 mg/L, pH value was 7.54, liquid circulation flow rate was 52.0 L/h, discharge voltage was 25 kV, and carrier gas flow rate was 20.0 mm/s.
Take 1000 mL of dye wastewater in a beaker, add coagulant for coagulation and sedimentation for about 30 min, take 250 mL of supernatant in a beaker and dilute it to 500 mL, add tert-butanol solution with concentrations of 50.0 mg/L and 100.0 mg/L, respectively, and the degradation results are shown in Fig. 9 and Table 7.
It can be seen from Fig. 9 and Table 7 that the removal efficiency of wastewater organics gradually became lower when the concentration of tert-butanol in the solution increased, indicating that tert-butanol had a certain inhibitory effect on the degradation reaction of highly concentrated organic wastewater organics. When the reaction was carried out for 60 min, the removal rates were 52.2%, 46.7%, and 40.4%, and the decolorization rates were 82%, 48%, and 60%, respectively. The reason is that tert-butanol is a typical hydroxyl scavenger, it will preferentially react with hydroxyl radicals, and the conductivity of the solution will also increase due to the addition of tert-butanol, which will inhibit the degradation of organic matter and lead to the decrease of removal rate. Therefore, the role of hydroxyl radicals is important for the whole reaction system when treating actual dye wastewater.
Role of benzoic acid and degradation process
This actual dye wastewater contains a large amount of benzoic acid and its derivatives, and the degradation of benzoic acid has a large impact on the organic degradation rate and decolorization rate of the wastewater. Wei et al. (2007) excited benzoic acid with 266 nm laser and found three channels for benzoic acid to produce OH. Wang et al. (2022) experimentally found that benzoic acid is promoted by O3 to produce O3·−, HO2·, O2·−, and ·OH, ·OH can also activate the reaction of benzoic acid with ozone, promote the degradation of benzoic acid, and destroy its benzene ring or open ring. The previous experiments all showed an increasing–decreasing-increasing trend in the degradation rate of organic matter in dye wastewater, probably due to the rapid degradation of dye molecules, benzoic acid, and their derivatives in dye wastewater by the plasma generating a large number of reactive radicals in a short period of time at the beginning of the reaction. However, the degradation rate decreased due to the limited generation of reactive radicals by low-temperature plasma technology. As the reaction proceeded, the organic matter in the dye wastewater started to decrease and benzoic acid continued to produce O3·−, HO2·, O2·−, and ·OH to increase the oxidizability of the wastewater with the promotion of O3 generated by the plasma, which in turn led to a slow increase in the organic matter degradation rate of the dye wastewater around 30 min. Zhang et al. (2019) studied the reaction between benzoic acid and ·OH, and it was found that a larger amount of 6-hydroxybenzoic acid (6-HAB), 3-hydroxybenzoic acid (3-HAB), and 4-hydroxybenzoic acid (4-HAB) would be produced first during the reaction process, which was eventually mineralized to H2O and CO2. The degradation mechanism of benzoic acid is shown in Fig. 10.
Research on combined biochemical method for treating actual dye wastewater
The dual-media barrier gas–liquid two-phase discharge reactor can effectively remove the target pollutants from simulated dye wastewater. Although this technology is still facing the problems of high energy consumption and low economic feasibility, it has some economic advantages if used as a front-end process to increase the biochemical properties of highly concentrated hard-to-degrade dye wastewater in combination with traditional biological methods for treating actual dye industry wastewater.
Since the determination of BOD5 and CODcr is more convenient, economical, and fast compared to other water quality indicators in wastewater, the ratio of BOD5/CODcr (B/C) is often used as a criterion to determine the biochemical strength of wastewater. Usually, the larger the B/C ratio is, the higher the biochemical of wastewater and the easier it is to be degraded by microorganisms; the smaller the ratio is, the more difficult it is to be degraded by microorganisms. When B/C > 0.4, the wastewater is easy to be biodegraded; when 0.3 < B/C < 0.4, the wastewater is biodegradable; when 0.1 < B/C < 0.3, the wastewater is poorly biodegradable; when B/C < 0.1, the wastewater is difficult to be biodegraded or non-degradable. The water quality indexes of the actual dye wastewater after the combined treatment are shown in Table 8.
Conclusion
In this paper, we designed our own gas–liquid two-phase discharge water treatment device with actual dye industry wastewater as the research object, and through experimental research, the following conclusions were drawn.
-
(1)
The initial COD concentration of wastewater, the initial pH, and the circulating flow rate of the solution have a strong influence and correlation on the removal rate of organic matter, and the highest decolorization rate and organic matter removal rate of wastewater from actual dyestuff.
-
(2)
The addition of Fe2+ alone in the wastewater does not promote the removal of organic matter, but decreases the decolorization rate; the addition of H2O2 alone in the wastewater increases the removal rate of organic matter with the increase of H2O2 concentration, but inhibits the degradation of organic matter when the H2O2 concentration is too high; the addition of tert-butanol in the wastewater causes hydroxyl radicals to react with it preferentially, thus inhibiting the degradation.
-
(3)
Under plasma degradation conditions, the reaction of benzoic acid and its derivatives with O3 and ·OH in dye wastewater affects the degradation efficiency of the wastewater to some extent.
-
(4)
The biochemical properties of the actual dye wastewater treated by the combined gas–liquid two-phase discharge biochemical method were substantially improved, with B/C raised from 0.17 to 0.33, and the effluent met the effluent discharge standards, with CODcr reaching 198 mg/L, BOD5 reaching 65 mg/L, and pH and chromaticity reaching 6.39 and 50, respectively.
Data availability
The data and materials for this experiment are available.
References
Aziz KHH, Miessner H, Mahyar A et al (2019) Removal of dichloroacetic acid from aqueous solution using non-thermal plasma generated by dielectric barrier discharge and nano-pulse corona discharge [J]. Sep Purif Technol 216:51–57
Aziz KHH, Miessner H, Mueller S et al (2017) Degradation of pharmaceutical diclofenac and ibuprofen in aqueous solution, a direct comparison of ozonation, photocatalysis, and non-thermal plasma [J]. Chem Eng J 313:1033–1041
Aziz KHH, Mahyar A, Miessner H et al (2018) Application of a planar falling film reactor for decomposition and mineralization of methylene blue in the aqueous media via ozonation, Fenton, photocatalysis and non-thermal plasma: a comparative study [J]. Process Saf Environ Prot 113:319–329
Bruggeman PJ, Kushner MJ, Locke BR et al (2016) Plasma-liquid interactions: a review and roadmap [J]. Plasma Sources Sci Technol 25(5):053002
Chethana M, Sorokhaibam LG, Bhandari VM et al (2016) Green approach to dye wastewater treatment using biocoagulants [J]. ACS Sustain Chem Eng 4(5):2495–2507
Chatterjee S, Lee DS, Lee MW et al (2009) Enhanced adsorption of Congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide [J]. Biores Technol 11(100):2803–2809
Chen SH, Zhang J, Zhang CL et al (2010) Equilibrium and kinetic studies of methyl orange and methyl violet adsorption on activated carbon derived from Phragmites australis [J]. Desalination 1–3(252):149–156
Cui YM, Yin RC (2021) The progress of treatment methods of dye wastewater [J]. Sci Technol Rev 18(39):79–87
Chen Q, He BB, Ma YPX et al (2019) Influence of the pH value on the degradation of an azo dye of methyl orange by air discharge plasma. Plasma Process Polym 5(16)
Chavez AM, Rey A, Beltran FJ et al (2016) Solar photo-ozonation: a novel treatment method for the degradation of water pollutants [J]. J Hazard Mater 317:36–43
Deshpande BD, Agrawal PS, Yenkie MKN (2019) Advanced oxidative degradation of benzoic acid and 4-nitro benzoic acid—a comparative study. Adv Basic Sci 2019:2142
Habiba U Siddique TA, Joo TC et al (2017) Synthesis of chitosan/polyvinyl alcohol/zeolite composite for removal of methyl orange, Congo red and chromium(VI) by flocculation/adsorption. Carbohydr Polym 157:1568–1576
Haque MM, Haque HM, Md Khaled M et al (2021) Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J Biol Sci 28(1):793–804
Huang QL, Chen CJ, Zhao XL et al (2021) Malachite green degradation by persulfate activation with CuFe2O4@biochar composite: efficiency, stability and mechanism [J]. J Environ Chem Eng 9(4):105800
Hyoung-Sup K, Wright K, Joshua PA et al (2015) Inactivation of bacteria by the application of spark plasma in produced water [J]. Sep Purif Technol 156(2):544–552
Hwang I, Jenog J, You T et al (2018) Water electrode plasma discharge to enhance the bacterial inactivation in water [J]. Biotechnol Biotechnol Equip 32(2):530–534
Iervolino G, Vaiano V, Palma V, et al (2020) Enhanced azo dye removal in aqueous solution by H2O2 assisted non-thermal plasma technology. Environ Technol Innov 19
Jiang B, Zheng JT, Qiu S et al (2014) Review on electrical discharge plasma technology for wastewater remediation [J]. Chem Eng J 236:348–368
Liu Q (2020) Pollution and treatment of dye waste-water. 4th International Symposium on Resource Exploration and Environmental Science, (514):052001, pp 7
Luo YH, Yue XP, Jiang YR et al (2021) Recent progress of advanced oxidation processes in indole degradation [J]. Chem Indust Eng Prog 2(40):1025–1034
Khlyustova A, Sirotkin N (2020) Plasma-assisted oxidation of benzoic acid [J]. Front Chem Sci Eng 4(14):513–521
Malik MA, Ubaid-ur-Rehman, Ghaffar A et al (2002) Synergistic effect of pulsed corona discharges and ozonation on decolourization of methylene blue in water. Plasma Sources Sci Technol 11(3):236–240
Njiki A, Kamgang-Youbi G, Nola M, et al (2020) Biodegradation kinetic studies and optimization for the elimination of azoic and triphenylmethane dyes using an integrated process combining biological treatment and gliding arc plasma. J Chem Technol Biotechnol 1(96):273–282
Qin B, Gu JC, Yin P et al (2021) Research progresses on dye wastewater treatment technology [J]. Environ Prot Chem Indus 1(41):9–18
Saravanan R, Karthikeyan S, Gupta VK et al (2013) Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination [J]. Mater Sci Eng C Mater Biol Appl 33(1):91–98
Sanchez JV, Segundo CT, Palacios EM et al (2019) Elimination of AB210 dye in residual textile water by glow-discharge plasma application [J]. Desalin Water Treat 150:361–366
Tijani JO, Fatoba OO, Madzivire G et al (2014) A review of combined advanced oxidation technologies for the removal of organic pollutants from water [J]. Water Air Soil Pollut 225(9):2102
Tecer LH, Gunduz A, Atav R et al (2020) Investigation of the treatment of textile wastewater with cold atmospheric plasma reactor (Profoks) and reuse of recycled water in reactive dyeing process of cotton [J]. J Natl Fibers 6(19):2382–2389
Tange K, Nomura S, Nakajima J et al (2020) Methylene blue decomposition via various in-liquid plasma methods [J]. J Japan Inst Energy 8(99):99–103
Varjani S, Rakholiya P, Ng HY et al (2020) Microbial degradation of dyes: An overview. Bioresource Technol
Wei Q, Sun JL, Yue XF et al (2007) Photodissociation dynamics of benzoic acid monomer at 266 nm: the OH product channel [J]. Chem Phys Lett 1–3(448):11–15
Wang Z, Lin XZ, Huang YX, et al (2022) The role of hydroxylation on⋅OH generation for enhanced ozonation of benzoic acids: reactivity, ozonation efficiency and radical formation mechanism. J Hazard Mater :431
Yee DC, Chauhan S, Yankclevich E et al (1998) Degradation of perchloroethylene and dichlorophenol by pulsed-electric discharge and bioremediation [J]. Biotechnol Bioeng 59(4):438–444
Zhang CX, Sun YB, Yu ZQ et al (2018) Simultaneous removal of Cr(VI) and acid orange 7 from water solution by dielectric barrier discharge plasma [J]. Chemosphere 191:527–536
Zhang SY, Shen XJ, Li JR et al (2022) Study on degradation of alizarine reds in simulated dye wastewater by gas-liquid two-phase discharge plasma. Chem Eng Process 181
Zhang XH, Zhang CX, Sun XM et al (2019) Mechanism and kinetic study of the reaction of benzoic acid with OH, NO3 and SO4- radicals in the atmosphere [J]. RSC Adv 33(9):18971–18977
Author information
Authors and Affiliations
Contributions
JZ (first author): investigation, writing — original draft.
JL: methodology, validation.
SZ: investigation, formal analysis.
XS* (corresponding author): conceptualization, resources, writing — review and editing, project administration, funding acquisition.
Corresponding author
Ethics declarations
Ethical approval
We guaranteed that the works we submitted were original content, and there was no bad behavior such as plagiarism and fabrication of data. At the same time, we guaranteed that this manuscript has not been submitted to other journals for submission, and there was no phenomenon of submitting to different journals at the same time.
Consent to participate and publish
All authors have agreed to participate in this experiment and consent to the publication of this work.
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme L. Dotto
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Shen, X., Li, J. et al. Experimental study on the treatment of dye wastewater by plasma coupled biotechnology. Environ Sci Pollut Res 30, 57989–58001 (2023). https://doi.org/10.1007/s11356-023-26590-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26590-5