Abstract
In this paper, we compare the characteristics of methane activation by diverse plasma sources. The test conditions of reactant flow rate and composition are fixed for each plasma source to eliminate any possible misleading effects from varying test conditions. Among the diverse characteristics of each plasma source, we focus on the electron energy and degree of thermal activation in evaluating the cost-effectiveness of methane decomposition. The reaction is evaluated based on the selectivity of specific products, including H2, C2H6, and C2H2. Among the tested plasma sources, those that provide a somewhat thermal environment have a rather high degree of warmness, resulting in higher methane conversion and lower operational costs. As the non-thermal characteristics of the plasma sources become stronger, the selectivity of C2H6 increases. This reflects C2H6 formation from the direct collision of CH4 with high-energy electrons. On the other hand, as the degree of warmness increases, the selectivity of H2 and C2H2 increase. The results give an insight into possible tools for process control or selectivity control by varying the degree of warmness in the plasma source. The process optimization and cost reduction of methane activation should be based on this concept of selectivity control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methane pyrolysis with an arc plasma source has been employed for some decades. The aim of this technique is to produce carbon black and acetylene when the arc plasma produces a high-temperature reaction field of up to 10,000 K. The technique has been applied at the plant scale for mass production [1, 2]. Apart from thermal arc plasma, diverse plasma sources such as corona, spark, microwave, and gliding arc have been studied with respect to the methane pyrolysis process. Specific processes where the plasma sources are applied include: (1) conversion of gas-phase fuel into liquid-phase fuel, (2) production of carbon nanotubes, (3) hydrogen production through partial oxidation and steam reforming, (4) CO2 recycling by dry reforming [3–10]. Among the technical aspects of plasma application, the cost issue is very important. Plasma inevitably consumes electricity to produce its discharge, and this is an inherent demerit of its industrial application. Actually, plasma can lessen the installation cost, compared to typical thermal pyrolysis, as long as the process is efficiently configured. However, the operating cost of plasma pyrolysis is higher than that of existing processes, which presents a hurdle to its application.
Reports on the plasma pyrolysis process generally introduce a specific plasma source and indirectly compare the process to other references. However, it is difficult to compare different processes, because power consumption is largely dependent on the process conditions, i.e., the concentration of gases, temperature and pressure of the reaction, and the efficiency of the power source. Thus, there is a need to study the direct comparison of plasma sources for methane pyrolysis. Direct comparison can provide objective validity for the discussion of plasma source optimization in terms of cost effectiveness and the efficient design of methane pyrolysis processes.
In this study, different plasma sources, which produce different densities and temperatures of electrons, as well as different gas temperatures, are compared in the methane pyrolysis process. The tested plasma sources are pulsed dielectric barrier discharge (Pulsed DBD), AC DBD, AC spark, pulsed spark, rotating arc, and hollow cathode plasma. The power consumption for each process is compared based on the process efficiency of methane decomposition to determine which plasma source is optimal. All of the plasma sources are generated under atmospheric pressure. The sources can be classified into two different groups according to the relative electron and gas temperatures: non-thermal plasma and warm plasma [11–13].
Many experimental and numerical studies on the rotational, vibrational, electron temperature, and electron and ion density properties of each plasma source have been reported [14–19]. However, how these different characteristics affect the specific process of methane decomposition is not yet fully known. Using this research as background, the present study investigates the process efficiency in terms of power consumption in methane pyrolysis using the aforementioned plasma sources, whose characteristics are well known. The results can be applied to optimize the pyrolysis process for the efficient production and conversion of methane-derived materials.
Experiment
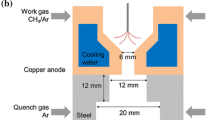
Figure 1 describes the experimental apparatus. The same measurement system and gas supply system is used throughout, and only the plasma sources are varied. The reactant is N2-balanced 5 % methane gas. The N2 and methane are quantitatively controlled by mass flow controllers, (Brooks, 5850E series) and are mixed before entering the reactor. Products are sampled after passing through a chiller, and a gas chromatograph (GC) is used to analyze the product.
In the series of experiments, the concentration of CH4 was designed to be 5 %, rather low concentration partly because higher concentration of CH4 can cause unnecessary instability of discharge and may cause generation of too much soot particles that is not favorable for the analysis of the process. Higher amount of N2 inevitably results in the generation of excited N2 species. However, the aspects of the excited N2 species itself also reflects the characteristics of each plasma source and hereafter, detail kinetics by excited species are not dealt separately.
Table 1 shows the test condition matrix for each plasma source.
The characteristics of the plasma sources can be found in the literature [9, 12, 13]. The method of generation and representative features of the sources are briefly given below.
DBD is generally generated by an AC power source. The configuration includes electrodes and a dielectric barrier (quartz, alumina, and so on) between the electrodes, as shown in Fig. 2a. The dielectric barrier is repeatedly charged and discharged by the AC power source, and protects against excessive current flow by preventing the transition of streamer to arc [20]. In this study, along with the AC power source that is typical for DBD generation, a DC pulse is also used to generate plasma with a higher electron temperature. The pulse power can accelerate the electron more than in the case of AC DBD with a higher electric field. The change in electron temperature may induce a different chemical reaction. Because the reactor is the same for the two different power sources, parameters such as the reaction time scale are fixed, though the characteristics of the generated plasma are different.
In general, plasma generated in AC DBD has the appearance shown in Fig. 2b, where micro-discharges are evenly distributed within the reactor volume, resulting in a lower current density in individual discharges. Electron number density is also low, ranging from 1012 to 1014/cm3, some orders of magnitude lower than that of arc. Typically, AC DBD produces electrons with a temperature of 5,000–10,000 K, in contrast to the gas temperature of the order of hundreds of K.
Figure 3 is a direct photograph of a spark discharge within a spark reactor. Tungsten pin electrodes are used for the discharge. The same pulse and AC power supplied for the DBD reactor are used. However, the discharge is focused in a much smaller volume than in the case of DBD reactor configuration. Thus, the plasma density is higher. The gas temperature is also reported to be higher, up to 1,000 K [21] in this spark configuration. Again, the pulse and AC power method is expected to generate different plasma characteristics. Pulse can generate a reaction environment with lower gas temperature and higher electron temperature, similar to that in the DBD reactor configuration.
Figure 4 shows the rotating arc setup. The reactor configuration consists of a cylindrical electrode with a conical electrode located inside. The reactant flow drives an arc string to proceed with a swirling motion within the reactor, resulting in less damage to the electrode compared to a typical thermal arc [7]. This configuration can be understood as a kind of three-dimensional gliding arc, of the type that has been applied for decades in diverse chemical processes. Gliding arcs generated by AC power are initiated at the point of minimum gap distance between electrodes, and slide down to a position where the distance between electrodes becomes larger. This increase in distance induces longer and thicker arc strings through convective heat and mass transfer. The change in arc string results in a gas temperature of about 2,000–3,000 K and an electron density of 1014–1015/cm3, signifying a temperature of about 1 eV [16]. The characteristics about the electron temperature and number density are not different in a rotating arc. The arc changes from thermal to non-thermal plasma as the arc string advances.
Figure 5 shows hollow cathode plasma being ejected through a hole of diameter 1 mm. Generation of the arc itself is similar to the case of rotating arc. However, hollow cathode plasma has the merit of a higher effective reacting volume. In the case of rotating arc, arc strings cannot cover all of the exit area. However, in the hollow cathode plasma, almost all of the reactant gas passes through the discharge region. Plasma passing through the small hole experiences more active heat transfer, and the high flow velocity results in a larger volumetric expansion of arc. These characteristics of hollow cathode plasma are expected to reflect those of non-thermal plasma.
The characteristics of the aforementioned plasma sources are summarized in Table 2. Rotating arc and hollow cathode plasma are expected to have a higher gas temperature and electron density compared to those of AC and pulsed DBD. Meanwhile, in the case of spark, the discharge is focused spatially and is expected to have a high electron density. As the distance between the electrodes is limited (usually less than 5 mm), the spark process has a rather lower current density. The gas temperature is higher than in AC and pulsed DBD, but lower than with rotating arc. This reasoning makes it possible to arrange these plasma sources according to the order of the so-called degree of non-thermal characteristics: DBD > Spark > Hollow cathode plasma > rotating arc.
Results and Discussion
Methane was decomposed in each plasma reactor. Reaction products, supplied power, and methane conversion rate were compared for all the reactor configurations. The results are shown in Table 3. In the case of methane activation by plasma, the primary radicals are CHx (x = 0–3), depending on the electron energy [13]. For this reason, C2 species can provide the largest portion of hydrocarbon products [13]. In addition, according to the reaction environment such as gas temperature, additive reactants and so on The kinetic path for C3H6 and C3H8 can be formed [13, 22]. Of course, H2 could be produced in the course of the dehydrogenation step. Considering this, C1–C3 hydrocarbons in the product are analyzed with H2. Methane activation accompanies soot generation and the relative generation of the amount of soot particle depends on what plasma source is used. Main path of soot generation in cold plasma is direct collision of CHx molecule with highly energetic electron. However, as is evident, density of high energy electron is much lower than that of gas molecule resulting in relatively small amount of soot generation. In the case of warm plasmas, soot generation includes thermal dehydrogenation of CHx molecules and results rather larger amount compared to that of cold plasma sources. However, quantitative analysis of soot particle generation is very hard. So in this manuscript, comparison of soot particle generation was not included
The conversion and selectivity of each species were obtained to understand the reaction characteristics. The definitions of conversion and selectivity are given below.
Regarding conversion, AC Spark scored highest. However, as the electric power supplied for the reaction differed along with the reactors, each process should be evaluated on the basis of its efficiency of power usage. For this reason, we defined the specific energy density (SED, kJ/L) to be electric power supplied per unit volume of reactant. The conversion score was then normalized by SED and compared for each reactor. Figure 6 displays both simple conversion and normalized conversion scores. Hollow cathode plasma and rotating arc scored highest for normalized conversion. The tendency in methane conversion is for higher scores in the case of less non-thermal plasma sources.
Plasma pyrolysis is composed of two different kinds of reaction. The first is a plasma (electron impact) reaction, and the second is thermal activation. Their isolation in the overall process is difficult, because the two processes proceed interactively [23]. However, the characteristics of each reaction are well known and can, to some degree, be evaluated separately. From this point of view, we can analyze the results shown in Fig. 6. The stated tendency in methane conversion is due to the fact that the activation of methane is mainly dependent on thermal activation. Typical (thermal) pyrolysis of methane follows sequential dehydrogenation, as given in Eq. (1):
Initiation of the dehydrogenation or the reaction [CH4 → CH3 + H] requires 4.56 eV/molecule [24]. Compared to that, methane decomposition by electron impact requires rather high electron temperatures of above 9 eV, as shown in Eqs. (2)–(5) [25]:
Considering that 9 eV corresponds to the high-energy tail in the electron energy distribution of typical non-thermal plasma, it can be assumed that only a small portion of the electrons contribute to methane activation by generating CHx species. If we recall that the normal electron density is of the order of ppm when considering atmospheric pressure gas reactant systems, it is clear that thermal activation governs the conversion process, and a plasma source that is favorable in generating a thermal environment (high gas temperature) is thus beneficial to the process.
On the other hand, among the CHx species primarily generated by electron impacts with methane molecules, it is the electron energy that determines the relative proportion [13]. The higher the electron energy, the larger the proportion of C and CH; the lower the electron energy, the larger the portion of CH3. Different primary radicals cause different reaction pathways. CH3 tends to dimerize to form C2H6 and C/CH tends to form C2H2 [13, 26]. Generally, the C2H6 formed in a non-thermal environment does not experience further dehydrogenation and remains as the final product. Once the primary radical does not have much C/CH, the main source of C2H2 is the dehydrogenation of C2H6/C2H4. On the other hand, the C2H2 found in the reaction driven by strongly non-thermal plasma is mostly generated from hydrogenation and the dimerization of C2H and CH. This occurs through multiple reaction steps, and remains as the final product [27]. The opposite selectivity behavior of C2H6 and C2H2, shown in Fig. 7, comes from these different reaction pathways.
From the results illustrated in Fig. 7, we can see that AC DBD and Pulse DBD can be considered the most non-thermal plasma sources, whereas the other sources have somewhat thermal characteristics, which we call warm plasma [12]. The electron energy of AC DBD and Pulse DBD is not strong enough to generate C2H2 as the most abundant final product.
The decomposition of CH4 can be indirectly indicated by the H2 selectivity. For this reason, a simultaneous comparison between the selectivity of C2H2 and H2 can give us another insight into the function of each plasma source. As shown in Fig. 8, conditions for higher conversion tend to show a high selectivity of C2H2. According to Eq. (6), the ideal conversion and selectivity conditions for the generation of C2H2 have a ratio of approximately 1.33.
With the exception of AC DBD and Pulse DBD, the slope given by the results in Fig. 8 has a value of around 1.3. However, for non-thermal plasma sources, the slope is much less than 1. This suggests an important point: between the two different reaction pathways of C2H2 formation (strong non-thermal plasma that produces C2H2 via hydrogenation of C2/CH and the dehydrogenation of C2H6/C2H4), a higher yield of C2H2 is only possible when methane conversion is guaranteed. This would imply that a thermal environment is much more favorable. Hence, the strongly non-thermal characteristics of a plasma source cannot give a high yield of C2H2.
Let us define a process parameter, the ‘degree of reaction,’ to be the ratio of methane conversion to the selectivity of the target species. If H2 and C2H2 are the final products of methane activation, the ideal condition is 100 % conversion and 100 % selectivity, resulting in the degree of reaction being unity. We can then define the slope of a [conversion—degree of reaction] plot to be a ‘direction parameter.’ The slope becomes steeper as the plasma source for the reaction approaches the characteristics of thermal plasma. Figure 9 shows the characteristics of this direction parameter for H2 and C2H2 for each methane activation method. In the case of H2, there does not appear to be a meaningful difference in the direction parameter among the plasma sources. This is because H2 is produced throughout all of the reaction steps experienced in both the thermal and non-thermal (purely plasma-generated) reaction environment. However, in the case of C2H2, the direction parameter clearly differs with the characteristics of the plasma sources. This result again proves the above reasoning that a thermal environment, or the characteristics of warm plasma, is of considerable benefit to the process.
From the above comparisons, it is possible to give a relative comparison for the degree of thermal characteristics of each plasma source. However, it is not possible to give a direct comparison within specific reactor configurations, except for rotating arc. Rotating arc can be understood as the three-dimensional extension of gliding arc, where both thermal and non-thermal characteristics can be observed with the progress of the arc string [7]. Considering this, we use rotating arc to investigate the reaction characteristics according to the change in the degree of warmness. In the case of gliding arc, an increase in flow rate and corresponding heat transfer causes a thermal to non-thermal transition [16]. Likewise, in rotating arc, the rotating arc string constitutes a high temperature environment. However, changes in flow rate can cause heat transfer, which can also change the degree of warmness. This reasoning was confirmed in an experiment on the effect of flow rate change, the results of which are shown in Fig. 10. The selectivity of H2 and C2H2 was compared under different flow rate conditions. A 5 % volumetric concentration of N2-balanced reactant was supplied to a rotating arc reactor with a diameter of 25 mm. The flow rate was varied to be 10, 20, and 30 lpm, and different SED conditions were compared. For fixed electric power, as the flow rate increased, or as the non-thermal characteristics became stronger, the selectivity of C2H2 increased. In comparison with a fixed flow rate condition, an increase in SED or an increase in thermal energy due to more electric power reduced the difference between the selectivity of H2 and C2H2.
Figure 11 illustrates that the parametric effect of flow rate on the ratio of C2H2 selectivity to H2 selectivity confirms the above reasoning. While the flow rate was fixed, an increase in SED reduced the ratio, and with a fixed SED, an increase in flow rate magnified the ratio.
Based on this understanding of the thermal characteristics of the plasma sources, we can compare the process efficiency or cost effectiveness. The best plasma source for a specific process should be evaluated from the viewpoint of cost effectiveness. Conversions normalized by input energy for each plasma source are compared in Fig. 12. As shown in the figure, the plasma sources can be classified into three different groups according to their conversion and normalized conversion performance. AC DBD and Pulse DBD give the lowest conversion and energy efficiency (conversion/SED), with Pulse Spark and Gliding arc constituting the second group. In view of conversion itself, AC Spark, hollow cathode plasma, and rotating arc, which make up the third group, give the best score.
If the process is aimed towards the production of specific chemicals, such as H2 or C2H2, the evaluation should be based not only on the conversion of CH4, but also on the yield of the target species. Considering this, the specific input energy density (SIED), or input power per unit volume of product was compared for each plasma source. As shown in Fig. 13a, a rather larger amount of energy was required for AC and pulsed (여기 그림보고 확인해 볼 것) DBD to produce H2 than for other plasma sources that generate a better thermal environment. This is the same in the case of C2H2, as shown in Fig. 13b. Rotating arc exhibited the lowest energy cost in this comparison.
What is remarkable is that, among the plasma sources corresponding to classes II and III in Fig. 12, the order of the Conv./Sel. values for H2 and C2H2 does not change, although the SIED values do. Namely, they follow the order [Gliding arc > Hollow cathode plasma > Rotating arc > Pulse spark > AC spark]. The Conv./Sel. value indicates the characteristics of the chemical path. Once a reaction is triggered, the reaction follows kinetic pathways defined by thermal equilibrium. However, differences can come about based on the characteristics of the plasma or the degree of thermal activation. This is illustrated by Fig. 14. Characteristics of the plasma determine where the reaction is triggered. Different characteristics of the plasma sources produce a different composition of primary radicals, and these can induce different reaction pathways [13]. The degree of warmness regulates successive dehydrogenation.
Conclusion
We have investigated the properties of methane activation by a diverse range of plasma sources. Each plasma source has its own characteristics of electron energy and degree of thermal activation. The reactions were evaluated by measuring the H2, C2H6, and C2H2 products. Among the tested plasma sources, those that provide a somewhat thermal environment, such as AC spark, hollow cathode plasma, and rotating arc, have a rather high degree of warmness, resulting in higher methane conversion and lower operational costs. Plasma sources that have strong non-thermal characteristics show higher C2H6 selectivity, reflecting the formation of C2H6 by the direct collision of CH4 with high-energy electrons. Meanwhile, in the other plasma sources, as the degree of warmness becomes higher, the selectivity of H2 and C2H2 increases. In addition, the effect of the degree of warmness in non-thermal plasma was evaluated using different plasma sources. Among the warm plasma sources, it was confirmed that different product compositions were obtained from the various plasma characteristics. Most importantly, compared to typical thermally activated processes, plasma can be used to control the selectivity of a specific product. The plasma characteristics, i.e., electron energy and the degree of warmness, were determined to be the parameters affecting this selectivity. The results provided in this study reveal that the proper selection of plasma source can be used as a tool to control the reaction pathway. Process optimization should be based on this concept of selectivity control. All of the above results can guide further research on plasma applications for chemical processes.
References
Fincke JR, Anderson RP, Hyde TA, Detering BA (2002) Plasma pyrolysis of methane to hydrogen and carbon black. Ind Eng Chem Res 41(6):1425–1435
Muradov N (2001) Hydrogen via methane decomposition: an application for decarbonization of fossil fuels. Int J Hydrogen Energ 26(11):1165–1175
Oumghar A, Legrand JC, Diamy AM, Turillon N (1995) Methane conversion by an air microwave plasma. Plasma Chem Plasma P 15(1):87–107
Yao SL, Suzuki E, Nakayama A (2001) The pyrolysis property of a pulsed plasma of methane. Plasma Chem Plasma P 21(4):651–663
Yao S, Nakayama A, Suzuki E (2001) Methane conversion using a high-frequency pulsed plasma: Important factors. AIChE J 47(2):413–418
Goujard V, Nozaki T, Yuzawa S, Agiral A, Okazaki K (2011) Plasma-assisted partial oxidation of CH4 at low temperatures: numerical analysis of gas-phase chemical mechanism. J Phys D Appl Phys 44:274011
Lee DH, Kim K-T, Cha MS, Song Y-H (2007) Optimization scheme of rotating glid arc for CH4 reforming. Proc Comb Inst 31:3343–3351
Bromberg L, Cohn DR, Rabinovich A (1998) Plasma reforming of CH4. Energy Fuels 12:11–18
Rusu I, Cormier J-M (2003) On a possible mechanism of the methane steam reforming in a gliding arc reactor. Chem Eng J 91(1):23–31
Hwang N, Song Y-H, Cha MS (2010) Efficient use of CO2 reforming of CH4 with an arc-jet plasma. IEEE Trans Plasma Sci 38(12):3291–3299
Fridman AM (2008) Plasma chemistry. Cambridge University Press, New York
Gutsol A, Rabinovich A, Fridman A (2011) Combustion-assisted plasma in fuel conversion. J Phys D Appl Phys 44:274001
Lee DH, Kim K-T, Song Y-H, Kang WS, Jo S (2013) Mapping plasma chemistry in hydrocarbon fuel processing processes. Plasma Chem Plasma Process 33:249–269
Kim JH, Choi YH, Hwang YS (2006) Electron density and temperature measurement method by using emission spectroscopy in atmospheric pressure non-equilibrium nitrogen plasmas. Phys Plasmas 13:093501
Bye CA, Scheeline A (1993) Saha-Boltzmann statistics for determination of electron temperature and density in spark discharges using an Echelle/CCD system. Appl Spectrosc 47(12):2022–2030
Fridman A, Nester S, Kennedy LA, Savdliev A, Mutaf-Yardimci O (1999) Gliding arc gas discharge. Prog Energy Comb Sci 25:211–231
Chun YN, Song HW, Kim SC, Lim MS (2008) Hydrogen-rich gas production from biogas reforming using plasmatron. Energy Fuels 22(1):123–127
Napartovich AP (2001) Overview of Atmospheric Pressure Discharges Producing Nonthermal Plasma. Plasmas Polym 6:1–14
Kang WS, Park JM, Kim Y, Hong SH (2003) Numerical study on influences of barrier arrangements on dielectric barrier discharge characteristics. IEEE Trans Plasma Sci 31(4):504–510
Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P (2006) Atmospheric pressure plasmas: a review. Spectrochim Acta Part B 61(1):2–30
Phillips DM (1976) Determination of gas temperature from unresolved bands in the spectrum from a nitrogen discharge. J Phys D: Appl Phys 9:507
De Bie C, Verheyde B, Martens T, van Dijk J, Paulussen S, Bogaerts A (2011) Fluid modeling of the conversion of CH4 into higher hydrocarbons in an atmospheric pressure dielectric barrier discharge. Plasma Process Polym 8(11):1033–1058
Hwang N, Lee J, Lee DH, Song Y-H (2012) Interactive phenomena of a rotating arc and a premixed CH4 flame. Plasma Chem Plasma Process 32(2):187–200
Kuo KK (1986) Principles of combustion. Wiley, New York
Nozaki T, Hattori A, Okazaki K (2004) Partial oxidation of CH4 using a microscale non-equilibrium plasma reactor. Catal Today 98:616
Liu C, Mallinson R, Lobban L (1998) Nonoxidative methane conversion to acetylene over zeolite in a low temperature plasma. J Catal 179(1):326–334
Kado S, Urasaki K, Sekine Y, Fujimoto K, Nozaki T, Okazaki K (2003) Reaction mechanism of CH4 activation using non-equilibrium pulsed discharge at room temperature. Fuel 2:2291–2297
Acknowledgments
The authors wish to acknowledge the financial support from MKE (Ministry of Knowledge Economy) and ISTK (Korea Research Council for Industrial Science and Technology) of the Republic of Korea, Grant Number B551179-11-03-00.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, D.H., Song, YH., Kim, KT. et al. Comparative Study of Methane Activation Process by Different Plasma Sources. Plasma Chem Plasma Process 33, 647–661 (2013). https://doi.org/10.1007/s11090-013-9456-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-013-9456-6