Abstract
The kinetics of methane decomposition in low frequency (60 Hz) AC arc plasmas was investigated using on-line mass spectrometry and optical emission spectroscopy (OES) in a batch reactor configuration at pressures up to 3 bar absolute. Plasma conversion of CH4 results largely from thermal dissociation and was seen to follow first-order kinetics up to high conversions (> 90%) without observing any rate impedance from reverse hydrocracking. H– and C-atom selectivities for H2, C2H2, and C2H4 were 78% (1.56 mol H2/mol CH4 reacted), 36% (0.18 mol C2H2/mol CH4), and 30% (0.15 mol C2H4/mol CH4), respectively, at 3 bar. In other experiments, H2 diluent concentration played an important role in CH4 dissociation and final product distributions; H abstraction reactions increased the rate of CH4 decomposition at low H2 (yH2 < 0.6) while high H2 (yH2 > 0.6) impeded CH4 decomposition due to hydrocracking of C2 products. The rate of CH4 dissociation was seen to increase with pressure, up to 0.11 mol/m3/s, and the specific energy requirement (SER) decreased with pressure to 365 kJ/mol CH4 at 3 bar. The latter suggests that even higher operating pressures may improve the efficiency of plasma conversion of CH4, and ultimately that plasma pyrolysis may be a viable and energy efficient route to clean (turquoise) H2 and further implementation of chemical process electrification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The transition from fossil fuels to more sustainable energy sources has become a focus to combat climate change and achieve a sustainable and low carbon economy. Hydrogen, as an energy carrier, has been promoted as a promising solution to transitioning away from CO2-emitting fuels. H2 is an attractive energy carrier that can be derived from fossil fuels such as natural gas, with minimal to no CO2 emission in the process, provided that some of the H2 produced is used to power the conversion. Unfortunately, 95% of industrially produced H2 is via steam methane reforming (SMR), Eq. 1, which emits 9–12 kg of CO2 per kg of H2 produced [1, 2]. Hydrogen can also be produced through water electrolysis, Eq. 2, but the process is energetically expensive as it requires more energy for H2 production than the energy yielded from the H2 product itself. Methane pyrolysis however, Eq. 3, has recently regained interest due to its potential for net zero CO2 emission, while theoretically requiring significantly lower energy compared to water hydrolysis and SMR [2].
To be economically competitive with SMR, the reaction rates and energy efficiency of methane pyrolysis must be investigated and optimized. The (thermal) dissociation of CH4 requires high operating temperature due to high C–H bond energy, 438.8 kJ/mol [3]. Moreover, conversion of CH4 is significantly limited by a kinetic ‘pseudo-equilibrium’ at low temperature (< 1500 K) due to hydrocracking of products back to CH4 [4]. This kinetic limitation is further exacerbated by increasing pressure [5], which is usually mandated to obtain industrially relevant reaction rates and throughput. The carbon product also causes collateral issues such as coking and catalyst poisoning, whose removal results in additional CO2 emissions. However, plasma–based conversion of methane to hydrogen and carbon (as well as other hydrocarbons) is an attractive process due to a plasma’s ability to generate non-thermal reactive species, improve conversion and utilize renewable energy resources easily.
Plasmas can be categorized as non-thermal, where electrons and ions/neutrals are not in equilibrium (high energy electrons and low temperature ions and neutrals), or thermal, where all species are in thermal equilibrium and gas temperatures are high. Both thermal and non-thermal plasmas have been extensively studied for the conversion of hydrocarbons, mainly CH4 to H2, high-valued carbon and/or higher hydrocarbons. In general, non-thermal plasmas such as dielectric barrier discharges (DBD), have lower conversion and higher selectivities for C2H6 and C3–C5 hydrocarbons [6,7,8]. Other plasmas that may transition between thermal and non-thermal states, such as pulsed discharges, microwave (MW) plasmas, and gliding arc discharges, tend to have higher conversion and higher selectivities for C2 hydrocarbons and H2 [6, 7, 9,10,11,12,13,14,15]. It should be noted that MW plasmas can achieve > 90% conversion below atmospheric pressure; however, throughput is low due to the low pressures involved. In addition, thermal plasma conversion of CH4, using high current (> 1 A) DC/AC thermal arcs, has shown high conversion and demonstrated commercial viability for the production of carbon black and acetylene [16,17,18,19]. In particular, Monolith utilized a three-phase AC plasma jet/arc to convert CH4 to high-valued carbon and hydrogen and achieved a specific energy requirement (SER) of 360 kJ/mol CH4. They reported conversions higher than 95%, with 95% carbon and 99% H2 yields [20]. Similarly, Polak and co-workers have shown up to 86% conversion, and 88.4% selectivity for C2H2, with a reported SER of 362 kJ/mol CH4 using a DC plasma jet [18].
The specific energy required to dissociate CH4 in plasmas can vary widely depending on the type of discharge, gas feed conditions, power input and discharge volume. Understanding the mechanism and kinetics behind the dissociation of CH4 in thermal and non-thermal plasmas is therefore a crucial step to evaluating the efficacy of plasma conversion of methane and other hydrocarbons to H2 and valuable products. Conventional kinetics measurements using integral and/or differential flow reactors are not practical for plasma-based reactions. These measurements generally assume homogeneous reactivity throughout the reactor, which may not be true in the case of plasma. Furthermore, Kado et al. noted that not all gases flow through the discharge region in their reactor configuration [7]. This leads to lower conversion and can further complicate kinetics measurements.
In the present work, we investigate the kinetics, product distribution and SER of CH4 dissociation using a low current AC arc discharge in a batch reactor configuration. The impact of pressure on the overall reaction rate and SER were examined from 0.5 to 3.0 bar absolute at different electrode gaps. In addition, the influence of H2 partial pressure on the dissociation of CH4 and C2H4 were investigated. The product distributions under different gas conditions are examined and the dominant reaction pathways are summarized. The carbon products were also evaluated using Raman spectroscopy, CH analysis, and microscopy.
Experimental Methodology
Reactor Design
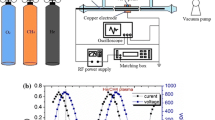
Experiments were conducted in a batch reactor as shown in Fig. 1. The reactor body was constructed out of a stainless-steel cube with an opening of 64 mm internal diameter on each side. The total reactor volume was 923 mL. The electrode configuration consisted of a high voltage (HV) thoriated tungsten tip electrode and a stainless-steel plate ground electrode. The ground electrode was attached to a stepper motor that rotated at 0.5 Hz to break conductive carbon bridges that may form between the electrodes during the discharge. A fan was placed inside the reactor to induce circulation and ensure the reactor was well-mixed.
Plasma Source and Characterization
A 140 V variable transformer was used to supply power to a nominally 18 kV, neon light step-up transformer (Jefferson Transformer No. 721–111), which provided high voltage 60 Hz AC to the electrode. A series of 1500 Ω resistors were placed in series with the drive electrode to limit the current flow and protect the transformer. The voltage and current were measured using a Tektronix P6015 high voltage probe and a Pearson 411 current transformer (Regowski coil). Thorlabs PDA 36A and APD430A Si photodetectors were used to measure optical emission transients. The probes and detectors were connected to an oscilloscope (Tektronix TDS 2004B) for data acquisition. Optical emission spectra were measured using an Ocean Optics USB 4000 spectrometer and the spectral intensity was corrected using an Ocean Optics Cal-2000 calibration light source as a reference.
Experimental Conditions and Gas Analyses
All experiments were carried out with 10 mol% helium gas as an internal reference unless specified otherwise. To compare the kinetics of methane conversion with different gas compositions and pressure, all experiments were carried out at the same root mean squared current (37 mA). Gas composition was analyzed through on-line mass spectrometry with Balzers QMS 200 and SRS RGA 300 mass spectrometers using a custom-built automated sampling system. During each experiment, a small volume of gas was extracted from the reactor and expanded into a sampling chamber at 6–7 Torr every 30 s. The sample gas was then leaked through a variable leak valve into a high vacuum (HV) chamber and analyzed by mass spectrometry. Supporting information S1 discusses the influence of this sampling methodology on the reaction kinetics measurement. C– and H-atom based product selectivities (Si) are defined in Eqs. 4–6. A pressure transducer (Omega PX319–1KG5V) was used to monitor the reactor pressure throughout experiments.
Here, Si, ni(t) are selectivity of species i and moles of species i at time t, respectively.
Solid Carbon Analyses
The carbon product was examined using SEM (Thermo Fisher Scientific Apreo C at 5 kV/0.1 nA), CH analysis, and Raman (Horiba Jobin Yvon T64000 spectrometer with 647 nm excitation).
Results and Discussion
Optical and Electrical Characteristics of AC Arc Discharge
The current and voltage waveforms of the AC arc plasma demonstrate two distinct discharge regimes as shown in Fig. 2. The first regime constitutes initial breakdown of the gas where an unstable intermittent arc or spark discharge occurs. This can be identified by a significant rise in voltage until the field intensity reaches the breakdown threshold, after which there is a sharp drop in voltage, followed by an increase in current flow as shown in Fig. 2b. The optical emission transient of the discharge corresponds to the current trace with pulse widths ranging from 500 ns to roughly 800 ns. The number of intermittent arcs tends to increase with pressure. The second discharge regime is characterized by a continuous and stable arc; the discharge voltage decreases as current increases due to increase in charge carriers and decrease in the plasma impedance.
Optical emission intensity, current and voltage characteristics of a He discharge at 1 bar. a Transient characteristics over two AC cycles, which show intermittent (yellow shaded) and stable (blue shaded) arc regimes. Panel b focuses on transient characteristics during the intermittent discharge (yellow shaded regions in panel (a)). The optical emission intensity is the integral over the visible spectrum (Color figure online)
A sequence of methane plasma images at different times is shown in Figs. 3a–d. As the arc discharge progresses, carbon is deposited on both the high voltage and ground electrodes, which effectively decreases the discharge gap. Over time, the deposited carbon can grow and form a conductive bridge between the electrodes, leading to a short circuit and extinguishing the arc discharge entirely within 20–30 s. To address this problem and extend the duration of each experiment, the ground electrode was attached to a motor, rotating at 0.5 Hz. A wedge was also placed on the ground electrode that removes carbon off the high voltage electrode. As a result, the discharge gap fluctuates periodically during the experiment due to carbon deposition and this mechanical carbon removal mechanism. The reported electrode gap length in this work is defined as the initial distance between the high voltage electrode and the ground plate electrode without considering carbon deposits. Figure 3e shows optical emission intensity, current and voltage transients during an arc discharge in CH4. In contrast to the pure He discharge case (Fig. 2a), the optical emission from the CH4 discharge fluctuates between each cycle. This demonstrates that the discharge volume fluctuates throughout the discharge process, which is attributed to carbon deposition on the electrode and changes in fluid dynamics resulting from gas expansion and reactions within the plasma.
a–d Temporal evolution of a typical (10% He/90% CH4) discharge at 2 bar. Time series images show carbon growth on the high voltage (HV) electrode tip. e Optical emission intensity, current and voltage corresponding to the discharge in panels (a–d). f Optical emission spectra of different CH4 and H2 mixtures (with 10% He) as noted in the figure
The optical emission spectrum from CH4 plasma is dominated by blackbody emission from soot particles as shown in Fig. 3f. An approximation based on the blackbody emission indicates that the plasma temperature is > 3000 K. Formation of diatomic carbon (C2) is also observed based on its primary (Swan band) emission lines around 516 nm (v = 0), which suggests that some CH4 likely dissociates into atomic carbon and hydrogen. As H2 composition increases, less soot particles are formed as suggested by the decrease in blackbody emission intensity, which reveals Hα, Hβ, and CH primary ro-vibrational lines at 656 nm, 486 nm, and 431 nm, respectively.
Plasma-Based Decomposition of Methane in a Batch Reactor
Figure 4 shows the pressure trace and gas composition during a discharge in a 10:90 mol% He:CH4 mixture at 2 bar initial pressure. As the reaction progresses, there is a rise in the reactor pressure corresponding to an increase in the overall gas moles. On-line mass spectrometry measurements in Fig. 4b show increases in the C2H4, C2H2, and H2 mole fractions as CH4 is decomposing. No mass peak associated with C2H6 was detected. The He mole fraction appears to be decreasing because the total number of gas moles in the reactor is increasing, while the amount of helium remains the same. It is important to note that mass spectrometry measurement can only determine the mole fraction of gas species in a batch reactor, but it cannot provide information regarding the concentration. As such, we specifically used He as an internal reference to determine the actual concentrations of CH4 and major products (further information in supporting information S2). Figure 4c shows the decay of methane concentration normalized to its initial concentration during the discharge.
Pressure and gas analyses of (10% He/90% CH4) discharge at 2 bar and 2.8 mm electrode gap length. a Reactor pressure increases as a function of time during the discharge. b Time evolution of CH4, He and major gaseous product mole fractions during the reaction. c Concentration of CH4 decays exponentially as a function of time, which follows a first-order rate of reaction. d Reaction rate constant increases linearly with electrode gap
There are two main pathways for the initial dissociation of methane in a discharge. In a non-thermal plasma with high energy electrons and high enough electron density, methane is more likely to decompose through electron impact dissociation, Eq. 7, which has an energy threshold of 9 eV [11]. Further dissociation into methylene, methylidyne, and carbon atom is also possible if the density of energetic electrons is high enough. As the electron energy decreases (< 10 eV), the cross-sections for electron impact dissociation reactions in CH4 plasma decrease, while the cross sections for vibrational excitation through electron impact increase [21]. In this scenario, most of the energy transferred from electron collisions goes into exciting CH4 molecules to a higher vibrational state, CH4(v). In nanosecond pulsed discharges, the internal vibrational energy of CH4 can exceed the required bond dissociation energy, 4.5 eV, which leads to dissociation into CH3 radicals, shown in Eq. 8 [12]. As for discharges with longer pulse duration, the vibrational excited states are quenched and the vibrational temperature of CH4 molecules equilibrate with their rotational and translational temperatures. Then, the dissociation of CH4 occurs similarly to a thermal process.
In either case, the rate of methane dissociation can be modeled as pseudo first-order in a batch reactor as shown in the following:
where \([C{H}_{4}]\) and \({k}_{1}\) are CH4 concentration and reaction rate constant, respectively. This model neglects the contribution from reverse reactions of H2 and higher hydrocarbon products, which is generally valid at low H2 concentrations. A batch reactor model also assumes the system is homogeneous with uniform concentration and reactivity throughout the entire reactor volume, which is not true in the case of a plasma reactor. The plasma volume, or the active volume for reaction, is therefore significantly smaller compared to the reactor vessel, especially in our case. In order to account for this, a simple ideal dilution assumption was made where the reactor mixing timescale was assumed to be faster than the relevant reaction timescale. Further information regarding the derivation is in supporting information, S3. Equation 9 can be modified into the following:
where \({V}_{P}\), \({V}_{R}\), \({\left[C{H}_{4}\right]}_{0}\), and \({k}_{batch}\) are effective plasma volume, reactor volume, initial CH4 concentration, and reaction rate constant measured in the batch reactor, respectively. In the mass transport-limited regime, the measured reaction rate constant can be a function of the Peclet number, which is the ratio of convective mass transport rate to diffusive mass transport rate inside of the plasma volume. Equation 11 shows that the reaction rate constant measured in the batch reactor is proportional to the true reaction rate constant with the proportionality constant being the volume fraction of plasma in the reactor. Experimental data show that CH4 concentration decays exponentially with time, which follows a first-order reaction rate well. The rate constant can then be determined by linearizing Eq. 12 and fit to a semi–log dataset of normalized CH4 concentration as shown in Fig. 4c.
To further validate Eq. 11, rate constants were measured at different electrode gaps with the same gas mixture. The reaction rate constant measured increased linearly with electrode gap length as demonstrated in Fig. 4d. This is expected if we assume the effective arc volume is a cylinder, which means the active volume increases linearly with the electrode gap (Lgap), Eq. 13. The effective cross-sectional area of the arc is estimated to be 0.44 mm2 based on a series of images of the discharge, similar to Figs. 3a–d. The real rate constant (k1) of CH4 dissociation is estimated to be \(\text{9.44} \bullet {10}^{2}\) s–1, which corresponds to a gas temperature of 1990 K according to the rate parameters from Kevorkian et al. [22].
AP is the effective cross-section area of the plasma arc.
Based on thermodynamic calculation (supporting information, section S4), methane dissociation from 1 to 10 bar pressure primarily yields solid carbon and H2 at temperatures below 2300 K and C2H2 formation becomes more favorable above 2300 K. The calculation shows that no significant amount of C2H6 should form, which agrees with the lack of C2H6 species detected via mass spectrometry. The majority products of methane conversion in low current arcs are C2H4, C2H2, amorphous carbon and H2. The scanning electron micrograph of the carbon shows there is a distribution of carbon aggregates ranging from nanometers to microns, Fig. 5a. Product selectivities, as defined by Eqs. 4–6, are presented in Fig. 5b. C2H4 constitutes a significant portion of the product distribution in the experiment, which differs from the thermodynamic calculation. Further investigation into the temporal behavior of the product species shows that the product distribution of methane conversion has two distinct regimes. Initially, the selectivities for C2H4 and H2 decrease, while the selectivities for C2H2 and carbon increase. As the reaction progresses, the product distribution reaches a state of pseudo-equilibrium as evidenced by the constant selectivities over time. Kado et al. examined the reaction pathway of methane conversion in a non-equilibrium pulsed spark discharge through isotope experiments [13]. They showed that C2H2 is likely formed from hydrogenation of atomic carbon or C2, while C2H4 is simultaneously formed from hydrogenation of C2H2 and dehydrogenation of C2H6. On the other hand, it is demonstrated that thermal plasmas (e.g., arcs, some spark discharges, and microwave plasmas), follow Kassel’s reaction scheme [23]. C2H6 is first formed from CH3 radical recombination. Subsequently, C2H4, C2H2 and carbon are formed through a series of dehydrogenation steps. To understand the behavior of the product distribution observed in Fig. 5b, it is important to determine the significance of electron impact reactions and the reaction pathways in the low current AC arc plasma used in this study.
Effect of Different Inert Gases
A series of experiments with different Ar and He concentration ratios with a constant CH4 partial pressure (0.4 bar) was conducted at 2 bar. Figure 6a shows the normalized concentration of CH4 over time under different concentrations of Ar and He. The measured rate of CH4 dissociation does not show any dependence on Ar and He ratio. This suggests that electron impact dissociation reactions may not play an important role in methane dissociation in low current AC arc discharges. Further investigation into the gas products shows that C2H4 selectivity is not affected by Ar concentration. However, the selectivity for C2H2 decreases from 52.5 to 40.6%, which is compensated by an increase in carbon selectivity, with increase in Ar concentration in the plasma as shown in Fig. 6b. The temperature in the plasma arc is estimated based on the blackbody emission from the soot particles using a reference blackbody source at 3100 K (Ocean Optics Cal-2000). The temperature increases with increasing Ar concentration, shown in Fig. 6c. This possibly leads to an increase in the conversion of C2H2 to carbon, which corresponds with the increase in H2 selectivity as well.
Analyses of CH4 conversion in 80% (Ar + He) and 20% CH4 mixture discharges at 2 bar and 2.8 mm electrode gap length. a Decay of normalized CH4 concentration with time during discharges at different Ar and He compositions. b Product selectivities corresponding to different Ar and He concentration ratios. c Gas temperature estimation from a rough fit of the soot particle blackbody emission, which shows an increase in temperature with Ar concentration
Impact of Hydrogen Partial Pressure on Methane Decomposition
To analyze the kinetics of methane dissociation and resultant product distribution, the influence of H2 on methane dissociation was investigated through a series of experiments with different initial H2 partial pressures ranging from 0.0 to 1.6 bar, corresponding to H2 to CH4 ratios of 0–8. The initial reactor pressure was held at 2 bar for all experimental conditions. The results show that the measured rate constant (kbatch) of CH4 dissociation increases with H2 partial pressure as evidenced by the increase in slope in Fig. 7a. This trend contrasts with experimental results shown in non-catalytic thermal CH4 pyrolysis, which are typically conducted at temperatures between 950 and 1100°C [4, 5, 24]. H2 partial pressure has been shown to impede the conversion rate of CH4 due to the reverse reaction with H2, which hydrocracks (hydro)carbon products back into CH4.
In this study, the increase in rate constant results from the increase in H radical concentration from the additional H2 species, which leads to an increase in the reaction rate of H abstraction from CH4, following Eq. 14. In addition, the rate constant for H abstraction reaction increases significantly with temperature, which means this radical dissociation reaction pathway becomes more prominent in a high temperature process. The rate of CH4 dissociation can be modified to account for the contribution from the H abstraction reaction as shown in Eq. 15 (Derivation is shown in supporting information S5).
\(\alpha\), \({k}_{2}\), \({\left[{H}_{2}\right]}_{0}\) are the degree of dissociation of H2, the rate constant of Eq. 14, and the initial concentration of H2. Equation 15 shows that the rate constant of CH4 dissociation increases linearly with H2 partial pressure. This relationship is in agreement with the experimental data up to a H2 partial pressure of 0.6 bar, which corresponds to a H2 to CH4 molar ratio of 1.8, shown in Fig. 7b. As H2 partial pressure increases further, the rate constant falls below the predicted values based on Eq. 15. This deviation from the model is attributed to an increase in the reverse reaction rate with H2 concentration, which decreases the overall CH4 dissociation rate. As shown in the case where the molar ratio of H2 to CH4 is 8, Fig. 7a, the kinetics of CH4 dissociation deviates from the first-order rate law, which demonstrates significant contribution from the reverse reaction.
The presence of H2 in a low current arc CH4 plasma thus increases the reaction rate constant of CH4 dissociation. A similar effect of H2 on CH4 conversion rate has been previously reported in a pulsed microwave plasma and pulsed DC discharges [25, 26]. This increase in the rate constant with H2 is enabled by the H abstraction reaction, which is thermally activated. At high H2 partial pressures, the benefits of this rate enhancement are unfortunately offset by H2 hydrocracking reactions. This increase in the reverse reaction rate can therefore limit CH4 conversion as the process becomes kinetically limited.
Figure 8 shows the selectivities of CH4 conversion with different initial H2 concentrations. In general, the selectivity for carbon decreases significantly because high H2 concentration can hinder reaction channels and limit the formation of soot precursors from acetylene [27]. H2 selectivity shows no clear trend with respect to the increase in H2 concentration. The increase in H2 concentration favors the formation of hydrogenated products as indicated by the increase in both C2H4 and C2H2 selectivities. However, when the molar ratio of H2 to CH4 increases above 1.0, the selectivity for C2H4 continues to increase, while C2H2 selectivity decreases.
Influence of Ethylene Conversion
To investigate the pseudo-equilibrium product selectivity at long reaction time observed in Fig. 5b, the reaction rate and products of C2H4 dissociation were examined under various H2 partial pressures to simulate the environment during CH4 conversion. The dissociation rate of C2H4 follows a first order reaction rate at low H2 concentrations as shown in Fig. 9a. Similar to the case of CH4 conversion, the rate constant of C2H4 dissociation increases with H2 partial pressure due to the contribution from H abstraction reaction, shown in Eq. 16. As the molar ratio of H2 to C2H4 increases above 1.0, the rate of C2H4 dissociation starts to deviate from a first-order rate law at longer reaction times, as shown by the nonlinear relationship between the semi-log of normalized C2H4 concentration and time. Furthermore, the adverse influence of high H2 concentration due to hydrogenation and hydrocracking reactions in C2H4 dissociation appears to be more significant compared to CH4 dissociation. As shown in the case of a molar ratio of H2 to C2H4 of 8.0, the ethylene dissociation reaches a pseudo-equilibrium limit, where the conversion starts to plateau around 72%. In essence, the upper limit of C2H4 conversion is dependent on the H2 concentration in the system.
a Normalized C2H4 concentration over time with different initial H2 partial pressures at a total pressure of 2 bar and an electrode gap length of 2.8 mm. b Product selectivities of C2H4 conversion under different initial H2 partial pressures. c Raman spectra of carbon products from CH4 and C2H4 conversion
Figure 9b shows the product selectivities of C2H4 conversion with different H2 partial pressures. In pure C2H4 decomposition, amorphous carbon is the main product with a selectivity of 65%, which is higher compared to the selectivity of carbon obtained from CH4 (~ 30%). As the H2 partial pressure increases, the selectivity for carbon decreases, while C2H2 selectivity increases. This suggests that the presence of H2 suppresses reaction channels that lead to conversion of acetylene to carbon and poly-cyclic aromatic hydrocarbons (PAHs). The conversion of C2H4 also produces CH4, whose selectivity increases with H2 partial pressure as well. This demonstrates that there is likely a hydrogen facilitated reaction pathway that converts C2H4 to CH4.
The contents of the amorphous carbon product were analyzed via CH analyses shown in Table 1. The molar ratio of C–H in amorphous carbon converted from C2H4 is 12.1. As H2 partial pressure increases, the carbon product becomes more hydrogenated, which is reflected in the decrease of C–H ratio to 9.73. For comparison, the C–H ratio of carbon converted from CH4 is 6.31. This shows that carbon formed directly from C2H4 is less hydrogenated as compared to the carbon formed from CH4. As might be expected, carbon products become more hydrogenated as H2 concentration increases.
To further evaluate the carbon products, Raman analysis from 1200 to 3000 cm–1 were carried out (Fig. 9c). In general, there are three peaks at 1318, 1584 and 2633 cm–1, which are the standard D, G, and 2D peaks, respectively. The G peak corresponds to in-plane vibration of carbon atoms within the graphite layers, while the presence of defects in graphitic carbon gives rise to the D and 2D peaks [28]. The D and G peak intensity ratio is a qualitative indicator of the degree of graphitization in the carbon products [29]. Table 1 shows D and G peak intensity ratios after subtracting the photoluminescence background using a polynomial fit. Increasing H2 gas composition from 0 to 75% increases the D and G peak ratio of C2H4-produced carbon from 1.24 to 1.67. The increase in H2 concentration leads to formation of less graphitic carbon, which agrees well with the increase in overall H content. Interestingly, the D and G peak ratio of CH4 carbon is 1.67, which is similar to that of carbon obtained from a 25% C2H4 and 75% H2 gas mixture. The latter further supports the aforementioned notion that carbon formation from CH4 likely occurs through conversion of C2H4 and C2H2.
Impact of Pressure on Methane Decomposition
The effect of operating pressure on CH4 decomposition was investigated from 0.5 to 3.1 bar. Figure 10a shows the concentration of CH4 as a function of time at different pressures. The instantaneous rate of CH4 dissociation (\({r}_{C{H}_{4}}\)), as calculated by Eq. 17, increases from 0.02 to 0.11 mol/m3/s from an initial operating pressure of 0.5–3.0 bar. Further investigation shows that the rate constants (kbatch) initially decrease between the pressure range of 0.5 bar and 1.0 bar, but show no further dependence on pressure up to 3.0 bar, as demonstrated in Fig. 10b. As a result, the instantaneous rate of CH4 decomposition increases almost linearly with pressure. To evaluate the efficacy of high-pressure operation, the specific energy requirement (SER) for CH4 conversion was estimated based on the instantaneous rate and the corresponding plasma power input, Eq. 18. This approximation serves as the upper limit for SER calculation. The SER decreases monotonically from 672 to 365 kJ/\({\text{mo}}{\text{l}}_{{\text{C}}{\text{H}}_{4}}\) as the pressure increases from 0.5 bar to 3.0 bar as shown in Fig. 10c. Although the input power (Prms) generally increases with operating pressure, the increase of CH4 dissociation rate with pressure outweighs the increase in energy input. This suggests that increasing operating pressure can serve as another important parameter in maximizing the energy efficiency of plasma conversion of CH4.
a Concentration of CH4 (based on total reactor volume) during the discharge as a function of time at different pressures with 2.8 mm electrode gap. The corresponding instantaneous rate of methane decomposition is estimated based on the initial CH4 concentration and reaction rate constant, denoted by black dashed lines. b Dependence of instantaneous rate of CH4 dissociation on reactor pressure. c Specific energy requirement (SER) for CH4 conversion decreases with increasing pressure. The legend indicates the corresponding electrode gap lengths. d Product selectivities at different pressures with an electrode gap of 2.8 mm
The impact of operating pressure on the product distribution is shown in Fig. 10d. The H2 selectivity ranges from 76 to 80% and shows no clear trend with pressure. For pressures from 0.5 to 1.0 bar, the selectivities of C2H4 and C2H2 slightly decrease, which is compensated by the increase in selectivity for carbon. As the pressure increases above 1.0 bar, the selectivity of C2H2 continues to decrease from 46 to 41%, while the selectivity of C2H4 increases from 24.2 to 30.8%. It is likely that hydrogenation reactions become more favorable at higher pressure, which leads to the increase in C2H4 products. The selectivity of carbon does not show significant change with pressure from 1.0 to 3.0 bar. The corresponding Raman spectra of the carbon product do not show any significant difference as well, (Figure in supporting information S6).
Summary of Reaction Pathways in CH4 Arc Plasma
The dominant reaction pathways corresponding to low current AC arc plasma conversion of CH4 are demonstrated in Fig. 11, according to the results presented in the previous sections. Initially, CH4 dissociates into methyl and hydrogen radicals through thermal dissociation channels. Once there is enough accumulation of H atoms, H abstraction reactions contribute significantly to CH4 decomposition. The resultant CH3 radicals can dissociate into CH2 and CH, as supported by the presence of the CH emission line in Fig. 3f. In addition, CH3 radicals can recombine to form ethane, which undergoes a series of dehydrogenation steps. The conversions of ethane to ethylene and ethylene to acetylene are facilitated by both dehydrogenation and H abstraction reactions. Similarly, acetylene can be converted into C2 through H abstraction and dissociation reactions. Both acetylene and C2 can then undergo a series of dimerization and dehydrogenation reactions to form carbon and polycyclic aromatic hydrocarbons (PAHs).
As the reaction progresses and H2 concentration increases, hydrogenation reactions become more prominent, which creates a pathway for C2H2 and C2H4 regeneration as designated by the purple arrows in Fig. 11. This competition between dissociation and regeneration rates of C2 products establishes a pseudo-equilibrium between the C2 hydrocarbon products and H2 in the system, which is consistent with the constant selectivities over time observed for C2H4 and C2H2 in Fig. 5b. Furthermore, high H2 concentration enables reaction pathways for regenerating CH4, which presumably occurs through some reaction channels involving C2H4. Although the current study does not provide enough information to determine this mechanism, the back conversion of C2H4 into CH4 is likely facilitated by H atoms. C2H5 can be formed through H addition to C2H4, which can then dissociate into CH3 radicals through a reaction with another H atom. CH4 is then regenerated by recombination of a methyl radical with a hydrogen atom. An increase in pressure up to 3 bar does not appear to influence the dissociation pathway of methane, as suggested by the observed pressure-independent rate constant. However, hydrogenation reactions become more favorable at higher pressure.
Conclusion
To summarize, the kinetics and influence of pressure on CH4 conversion in a low current AC arc discharge were examined. By developing a kinetics measurement methodology in a batch reactor, the dissociation rate of CH4 under different pressure and gas conditions was evaluated. The results demonstrate there is little to no detrimental effect on the kinetics of CH4 dissociation as the operating pressure increases up to 3.0 bar, which leads to a decrease in SER for CH4 conversion from 672 to 365 kJ/molCH4. This finding emphasizes the potential of operating pressure as an important parameter in improving the efficiency of plasma conversion of CH4 into H2 and higher valued hydrocarbons.
Further investigation into the kinetics of CH4 plasma reveals that CH4 conversion occurs through thermal dissociation, and that H abstraction reactions play an important role in the dissociation of hydrocarbons in plasma conversion systems. Increase in H2 concentration during the reaction leads to an increase in the activity of hydrogenation reactions, which impede the conversions of CH4 and its C2 hydrocarbon products. This leads to a pseudo-equilibrium between CH4, H2 and other gaseous products. As such, the presence of H2 largely influence the kinetics of CH4 dissociation, and the product distribution from the plasma. This provides valuable insights into plasma conversion of CH4 to C2 hydrocarbons and can inform the design of a more efficient plasma process that can be tuned towards higher selectivity for specific C2 hydrocarbons while potentially minimizing the required energy input for their production.
The current study has mainly probed the influence of gas conditions on the kinetics of plasma conversion. Other plasma parameters such as input power, discharge gap, and different types of discharge, both thermal and non-thermal, can largely influence both the kinetics and the product distribution. The investigation of CH4 conversion kinetics with these different parameters should be the subject of future studies.
Data availability
All data generated or analyzed during this study are included in this published article and supporting information documents.
References
Parkinson B, Balcombe P, Speirs JF, Hawkes AD, Hellgardt K (2019) Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ Sci 12(1):19–40. https://doi.org/10.1039/C8EE02079E
Patlolla SR, Katsu K, Sharafian A, Wei K, Herrera OE, Mérida W (2023) A review of methane pyrolysis technologies for hydrogen production. Renew Sustain Energy Rev 181:113323. https://doi.org/10.1016/j.rser.2023.113323
Ruscic B (2015) Active thermochemical tables: sequential bond dissociation enthalpies of methane, ethane, and methanol and the related thermochemistry. J Phys Chem A 119(28):7810–7837. https://doi.org/10.1021/acs.jpca.5b01346
Olsvik O, Rokstad OA, Holmen A (1995) Pyrolysis of methane in the presence of hydrogen. Chem Eng Technol 18(5):349–358. https://doi.org/10.1002/ceat.270180510
Holliday GC, Exell HC (1929) CXLI—the thermal decomposition of methane. Part I. Decomposition in silica bulbs. J Chem Soc (Resumed). https://doi.org/10.1039/JR9290001066
Li X-S, Zhu A-M, Wang K-J, Xu Y, Song Z-M (2004) Methane conversion to C2 hydrocarbons and hydrogen in atmospheric non-thermal plasmas generated by different electric discharge techniques. Catal Today 98(4):617–624. https://doi.org/10.1016/j.cattod.2004.09.048
Kado S, Sekine Y, Nozaki T, Okazaki K (2004) Diagnosis of atmospheric pressure low temperature plasma and application to high efficient methane conversion. Catal Today 89(1–2):47–55. https://doi.org/10.1016/j.cattod.2003.11.036
Xu C, Tu X (2013) Plasma-assisted methane conversion in an atmospheric pressure dielectric barrier discharge reactor. J Energy Chem 22(3):420–425. https://doi.org/10.1016/S2095-4956(13)60055-8
Scapinello M, Delikonstantis E, Stefanidis GD (2018) Direct methane-to-ethylene conversion in a nanosecond pulsed discharge. Fuel 222:705–710. https://doi.org/10.1016/j.fuel.2018.03.017
Scapinello M, Delikonstantis E, Stefanidis GD (2019) A study on the reaction mechanism of non-oxidative methane coupling in a nanosecond pulsed discharge reactor using isotope analysis. Chem Eng J 360:64–74. https://doi.org/10.1016/j.cej.2018.11.161
Nozaki T, Muto N, Kado S, Okazaki K (2004) Dissociation of vibrationally excited methane on Ni catalyst. Catal Today 89(1–2):57–65. https://doi.org/10.1016/j.cattod.2003.11.040
Lotfalipour R, Ghorbanzadeh AM, Mahdian A (2014) Methane conversion by repetitive nanosecond pulsed plasma. J Phys D: Appl Phys 47(36):365201. https://doi.org/10.1088/0022-3727/47/36/365201
Kado S, Urasaki K, Sekine Y, Fujimoto K, Nozaki T, Okazaki K (2003) Reaction mechanism of methane activation using non-equilibrium pulsed discharge at room temperature. Fuel 82(18):2291–2297. https://doi.org/10.1016/S0016-2361(03)00163-7
Kreuznacht S, Purcel M, Böddeker S, Awakowicz P, Xia W, Muhler M, Böke M, von Keudell A (2023) Comparison of the performance of a microwave plasma torch and a gliding arc plasma for hydrogen production via methane pyrolysis. Plasma Processes Polym 20(1):2200132. https://doi.org/10.1002/ppap.202200132
Indarto A, Choi J, Lee H, Song H (2006) Effect of additive gases on methane conversion using gliding arc discharge. Energy 31(14):2986–2995. https://doi.org/10.1016/j.energy.2005.10.034
Fincke JR, Anderson RP, Hyde T, Detering BA, Wright R, Bewley RL, Haggard DC, Swank WD (2002) Plasma thermal conversion of methane to acetylene. Plasma Chem Plasma Process 22(1):105–136. https://doi.org/10.1023/A:1012944615974
Gautier M, Rohani V, Fulcheri L (2017) Direct decarbonization of methane by thermal plasma for the production of hydrogen and high value-added carbon black. Int J Hydrogen Energy 42(47):28140–28156. https://doi.org/10.1016/j.ijhydene.2017.09.021
Polak LS (1967) Low-temperature plasma in petroleum chemistry. Petrol Chem USSR 7(2):136–152. https://doi.org/10.1016/0031-6458(67)90032-9
Bilera IV, Lebedev YuA (2022) Plasma-chemical production of acetylene from hydrocarbons: history and current status (a review). Pet Chem 62(4):329–351. https://doi.org/10.1134/S0965544122010145
Fulcheri L, Rohani V-J, Wyse E, Hardman N, Dames E (2023) An energy-efficient plasma methane pyrolysis process for high yields of carbon black and hydrogen. Int J Hydrogen Energy 48(8):2920–2928. https://doi.org/10.1016/j.ijhydene.2022.10.144
Ohmori Y, Kitamori K, Shimozuma M, Tagashira H (1986) Boltzmann equation analysis of electron swarm behaviour in methane. J Phys D: Appl Phys 19(3):437–455. https://doi.org/10.1088/0022-3727/19/3/013
Kevorkian V, Heath CE, Boudart M (1960) The decomposition of methane in shock waves1. J Phys Chem 64(8):964–968. https://doi.org/10.1021/j100837a002
Kassel LS (1932) The thermal decomposition of methane1. J Am Chem Soc 54(10):3949–3961. https://doi.org/10.1021/ja01349a019
Palmer C, Gordon MJ, Metiu H, McFarland EW (2022) Influence of hydrocarbon feed additives on the high-temperature pyrolysis of methane in molten salt bubble column reactors. React Chem Eng 7(5):1199–1209. https://doi.org/10.1039/D1RE00517K
Heintze M, Magureanu M, Kettlitz M (2002) Mechanism of C2 hydrocarbon formation from methane in a pulsed microwave plasma. J Appl Phys 92(12):7022–7031. https://doi.org/10.1063/1.1521518
Kado S, Sekine Y, Fujimoto K (1999) Direct synthesis of acetylene from methane by direct current pulse discharge. Chem Commun 24:2485–2486. https://doi.org/10.1039/a906914c
Slovetskii DI, Mankelevich YA, Slovetskii SD, Rakhimova TV (2002) Mathematical modeling of the plasma-chemical pyrolysis of methane. High Energy Chem 36:44–52
Ferrari AC, Basko DM (2013) Raman spectroscopy as a versatile tool for studying the properties of graphene. Nature Nanotech 8(4):235–246. https://doi.org/10.1038/nnano.2013.46
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61(20):14095–14107. https://doi.org/10.1103/PhysRevB.61.14095
Funding
This material was based upon work supported by the Robert G. Rinker Endowment for Chemical Engineering at UCSB, with auxiliary funding provided by C-Zero, Inc and the National Science Foundation Graduate Research Fellowship under Grant No. 2139319. The results presented made use of the MRL Shared Experimental Facilities of UCSB supported by the MRSEC program (NSF DMR 1720256), a member of the Materials Research Facilities Network (www.mrfn.org), as well as the UCSB Nanofabrication Facility, an open access laboratory.
Author information
Authors and Affiliations
Contributions
NL and MJG conceived and planned experiments. NL carried out experiments, analyzed data, and wrote the draft manuscript. YW assisted with experiments and supplemental information. MJG managed the project and edited the manuscript. All authors reviewed the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, N., Wu, Y. & Gordon, M.J. Impact of Pressure and Hydrogen Dilution on the Kinetics of Methane Decomposition in AC-Excited, High Pressure Plasmas. Plasma Chem Plasma Process 44, 47–64 (2024). https://doi.org/10.1007/s11090-023-10416-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10416-w