Abstract
This study focuses on the oxidation behavior of commercially available HR120 in air at 1,050 °C from 30 min to 100 h. The oxidation kinetics were first studied by thermogravimetry and isothermal exposure. The oxidation products were fully characterized using ex and in situ X-ray diffraction (XRD) and FEG-SEM observations. HR120 experienced at 1,050 °C a non protective transient stage and formed a multilayered oxide scale (SiO2–Cr2O3–XCr2O4 with X = Mn and/or Fe, Ni). A series of complementary characterization methods (gold and isotopic marker experiments, photoelectrochemistry (PEC)) were implemented to elucidate the oxidation mechanism. The study identified a n-type semi-conductivity accompanied by an inward growth of the scale. Thus, assuming that diffusion in the oxide scale controlled chromia-scale growth, the oxygen vacancy was the major point defect governing the solid state transport. This result was attributed to the presence of a MnCr2O4 spinel layer at the top of chromia that strongly decreased the oxygen pressure at the interface spinel/chromia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
HR120 is an austenitic Fe–Ni–Cr solid-solution-strengthened alloy that conserves high strength levels at elevated temperature combined with high resistance to oxidizing, carburizing and sulfidizing environments [1]. HR120, containing a high chromium content (~25 wt%), is a chromia forming alloy which can be used up to 1,100 °C [2]. The alloy contains also elements that, even in minor amounts, can have considerable influence on the oxidation resistance (aluminum 0.1 wt%, manganese 0.7 wt% and silicon 0.6 wt%). The oxidation behavior of chromia forming alloys has been extensively studied by the past [3–6] but the specific behavior of such multi-elementary industrial alloys is generally difficult to anticipate and thus, justifies a specific study.
The required chromium content for high temperature applications should be between 22 and 28 wt% to obtain an optimum oxidation resistance through the formation of a protective Cr2O3 scale [3]. The selective oxidation of chromium can nevertheless be difficult in the first times of exposure [7] leading to a transient period of fast oxidation before the establishment of the protective steady-state. Notably, the co-oxidation of some alloying elements can hinder the establishment of the protective layer [8, 9] and may significantly influence lifetime.
Once the protective layer is established, the solid state diffusion of species through Cr2O3 should control the oxidation rate at long term. Microstructure characterization evidenced the development of multilayered oxide scales [10, 15, 22] on such industrial alloys. However, this aspect was generally neglected in the determination of the mechanism controlling growth rate despite the fact it could have significant influence on oxidation kinetic.
As will be presented in the present paper, the commercially available HR120 experienced at 1,050 °C a non protective transient stage and formed a multilayered oxide scales. So, the objectives of the present study were (i) to study the oxidation behavior of HR120 in the first moment of exposure in air at 1,050 °C, (ii) to characterize the oxide scale that established at the surface of the alloy after the transient step (iii) to determine the mechanism of oxidation of this alloy and the role of different alloying elements.
The study concerns the oxidation behavior of HR120 in air at 1,050 °C from 30 min to 100 h. The oxidation behavior was first studied by thermogravimetry and by isothermal exposure. The oxidation products were fully characterized using ex and in situ X-ray diffraction (XRD) and field emission gun scanning electron microscope (FEG-SEM) observations. A series of complementary characterization methods (gold and isotopic marker experiments, photoelectrochemistry (PEC)) were implemented to evaluate the oxidation mechanism.
Experimental Procedures
HR120 is a Fe–Ni–Cr commercially available alloy that contains 25 wt% Cr. Its main alloying elements from the oxidation point of view, are Al, Si and Mn. The nominal composition is given in Table 1. The samples were cut from a plate to the dimension 10 × 10 × 0.5 mm3.
Before oxidation test, the surface of the alloy was ground and the corners were rounded on SiC paper down to grit 800. Then, the samples were cleaned ultrasonically in ethanol. Thermogravimetry was performed at 1,050 °C in dry air flow (1.5 L h−1) using a SETSYS thermobalance (Setaram Instrumentation) with an accuracy of ±5 μg. Some short time exposures (20 min, 4 and 16 h) were performed in a tubular furnace under lab atmosphere.
For gold marker experiment, specimens were first slightly oxidized at 750 °C (2 h) to avoid the diffusion of gold markers into the bulk and then coated using a gold sputter coater. Then the samples were oxidized for 4 h at 1,050 °C.
Isotopic marker experiments processed by oxidizing HR120 specimen at 1,000 °C firstly under 200 mbar 16O2 pressure (4 h) and secondly under 200 mbar of 18O2 pressure (2 h) (isotopic enrichment was about 90 %). The samples were not cooled between the two steps of the oxidation test to avoid thermal shocks which could induce cracks and spallation of the oxide scales. Secondary ion mass spectroscopy (SIMS) was used to determine oxygen 16 and 18 elementary profiles in the oxide layer after oxidation treatment. The instrument was a Cameca IMS SIMS ion microprobe 7F using a primary ion beam of Cs+.
The oxidation products were identified by XRD, using Kα (0.154 nm) copper radiation, on a Philips X’PERT pro diffractometer. The in situ experiments were performed under a dedicated furnace in air at 1,050 °C. The heating was done under argon.
The electronic properties of oxide phases were characterized by PEC, after 20 min oxidation at 1,050 °C and cooling down, by illuminating oxidized samples immersed in sodium sulphate 0.1 mol L−1. The photocurrent was recorded versus light energy and applied potential (referred to the K2SO4-saturated Hg2SO4/Hg electrode: E0 = +650 mV/SHE throughout the rest of this paper). The experimental set-up used has been described elsewhere [11].
After XRD and surface observations, the samples were sputtered with a gold film using sputter coater, then, coated by electroplating nickel to protect the oxidation layers from spallation during metallographic preparation. Surface and cross sections of the samples were observed using a FEG-SEM equipped with an energy dispersion spectrometer of X-ray (EDX) to perform elemental analysis.
Results
Kinetic Results
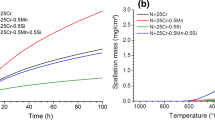
Figure 1a presents the mass change of HR120 specimen recorded during 100 h oxidation in air at 1,050 °C. The mass gain was initially rapid and evolved, after 2 h, according to a parabolic-like kinetic up the 100 h of isothermal exposure. The parabolic constant (kp) for HR120 at 1,050 °C was evaluated ploting Eq. 1; kp is ca. 3 × 10−12 g2 cm−4 s−1:
Figure 1b displays the mass change during the first 3 h of oxidation, including the heating period. The test was reproduced three times with three samples taken from different places in the plate. The mass gain was clearly linear during the first hour at 1,050 °C and slowed down transiting towards the parabolic regime. The results obtained for the three specimens demonstrated the repeatability of the transient oxidation phenomena.
X-Ray Diffraction Results
The in situ diffraction patterns of the specimen oxidized in air at 1,050 °C are presented in Fig. 2. The substrate and two crystalline oxide phases were identified: a spinel compound (noted XCr2O4 with X = Fe or Mn) and the chromia Cr2O3 with the corundum structure. Before 34 min of exposure at 1,050 °C, only chromia and the MnCr2O4 spinel were observed. Then, a second FeCr2O4 spinel grew between 34 and 42 min. Those oxides were exactly the same than those identified by XRD (not shown here) after 100 h of exposure at 1,050 °C.
SEM Characterizations
The surface morphology of HR120 specimen oxidized 16 h at 1,050 °C in air is displayed in Fig. 3b along with two EDX spectra (Fig. 3a, c) which correspond to the analyzed areas. Cross section micrographs for both region (line one Fig. 3b) are given in Fig. 3d, e.
The surface was mainly covered by a uniform oxide layer presenting oxide outgrowths of various sizes (from few micrometers to a hundred micrometer). The uniform part was composed of Cr, Mn and O whereas the outgrowths were particularly enriched in Fe. Low levels of spallation were also observed (Fig. 3b). The corresponding cross sections show that a dense chromia scale, overlaid by a thin MnCr2O4 layer constituted the uniform part (Fig. 3d). The outgrowths corresponded to the FeCr2O4 spinel identified prior by XRD. These Fe-rich oxides were localized on the surface of HR120 from 30′ of isothermal exposure at 1,050 °C. It should be mentioned that the transient oxidation stage was avoided performing oxidation in Ar gas [21] and that in such conditions, the iron containing oxides were not present. Therefore the transient stage can be allotted to the formation of the FeCr2O4 spinel.
Figure 4 displays the X-ray map of the elements constitutive of the oxide scale in the uniform part after 100 h of exposure at 1,050 °C. The reader should take into account the interferences existing between Cr (Kβ) and Mn (Kα) as well as the low concentration of silicon in the alloy (0.6 wt%).
Manganese and iron (in low amount) accumulated at the external interface whereas silicon accumulated at the metal-oxide interface. It can be suggested that a thin and continuous layer of SiO2 formed at the bottom of the layer. Internal oxidation of aluminum (0.1 wt% initially) was also evidenced.
Results of Marker Experiments
Figures 5a, b present the cross section of HR120 respectively through an outgrowth and through the uniform part of the oxide scale. Markers were located in both cases at the surface of the chromia layer. In fact, gold markers were located between chromia and the iron spinel FeCr2O4 in outgrowths and some small gold particles were also localized at the interface between Cr2O3 and the spinel MnCr2O4.
These results being not in agreement with the admitted mechanism of growth of chromia scale, isotopic marker experiments were also performed at 1,000 °C (first 4 h 16O2 and then 2 h 18O2).
The oxygen 16 and 18 profiles determined by SIMS analyses trough the oxide scale are reported in Fig. 6. No particular accumulation of O18 was noticed both in the outer or internal part of the scale. The profile of O18 exhibited a classical diffusion profile in the two outer micrometers of the scale. Considering the interface oxide-air as immobile and a semi-infinite system, the diffusion coefficient at 1,000 °C \( \left( {D_{O*}^{{Cr_{2} O_{3} }} } \right) \) was estimated from these data equal to 2.1 × 10−13 cm2 s−1.
PEC Results
Figures 7a, b present respectively (a) the photocurrent \( \left( {I_{ph} } \right) \) versus potential curve and (b) the \( (I_{ph} \times h\nu )^{1/2} {\text{ versus }}h\nu \) classical transform, hν being the energy of the chromatic light, related to HR120 oxidized for 20 min in air at 1,050 °C. The \( I_{ph} = f(E) \) curve, and mainly the more cathodic part, indicated that the photocurrent value increased slightly when the potential increased. Such a behavior was generally associated to a n-type semiconductor. The transform \( (I_{ph} \times h\nu )^{1/2} {\text{ versus }}h\nu \) was used to evaluate the gaps of the semi-conductors presents in the oxide scale. Two values of gaps at 2 and 2.8 eV can be deduced from Fig. 7b. The obtained values of gap can respectively be associated to haematite Fe2O3 and to the rhombohedral solid solution (Fe, Cr)2O3 according to previously reported values [12–15]. The current phase being constant with hv, the semiconductivity of the oxides did not change versus light energy.
Discussion
The HR120 alloy investigated in the present study is an austenitic Fe–Ni–Cr alloy containing ca. 28 at.% Cr (EPMA results). It is characterized by low contents in aluminum (0.1 wt%), silicon (0.6 wt%) and manganese (0.7 wt%).
Oxidation Rate and Protective Layer
From the kinetic point of view, the general behavior corresponded to those observed in literature for chromia forming alloys [6]. The value of kp (3 × 10−12 g2 cm−4 s−1) is in the order of magnitude of the more oxidation resistant chromia forming alloys [16]. This excellent behavior can surely be associated to the multilayered structure of the oxide scale. Indeed, protection of the HR120 alloy was due mainly to the formation of chromia but also of the spinel MnCr2O4, at the top of the oxide scale, and to SiO2, at the interface metal-oxide. The formation of the spinel MnCr2O4 was regularly observed on industrial chromia-forming alloys containing manganese [17]. The formation of this oxide reveals both the strong affinity of manganese for oxygen and its rapid diffusion through chromia [18]. These observations were in good agreement with those of Couture [19] and Deodeshmuk et al. [2] who observed also that silica forms a continuous layer at the base of the chromia layer.
The mass gain changed strongly and linearly during the first hours of oxidation, showing that the access of oxygen to the metal was not limited. This stage was characterized by the development of un-protective FeCr2O4 which delayed from a few hours the establishment of the protective layer. This oxide was clearly identified at the top of the oxide scale after ca. 30 min of oxidation by in situ XRD analyzes. This implies that the layer of Cr2O3 that developed during the first minutes remained porous or “open” [20] and that oxygen had access to the substrate. In the first minutes, the need in chromium was high and the quality of the oxide layer was poor so that the chromia scale brokes in many locations. As the substrate below the oxide layer was depleted in chromium, the flow of chromium was not sufficient to oxidize Cr selectively. Then the spinel FeCr2O4 formed. This mechanism was supported by oxidation experiments performed in argon containing 10−6 atm. of O2 [21]. In these conditions, the oxidation of iron did not occur anymore and the corresponding thermogravimetric curve consequently evolved according to a parabolic like kinetic [21].
Oxidation Mechanism
The identification of the type of semiconductivity and of the growth direction of the oxide scale allowed the determination of the major point defect governing the oxide growth kinetic, on the assumption that the oxidation rate was controlled by lattice diffusion in the oxide structure.
The measurements performed by PEC on surfaces oxidized for 20 min at 1,050 °C consistently show a n-type semi-conductivity. Two gap values were obtained by plotting the linear transforms of the photocurrent according to incident light energy. The first one was in the range 2.2–2.6 eV and was associated, according to literature [12], to the solid solution (FexCr1−x)2O3. However Marchetti et al. [22] mentioned that these values can also be attributed to Ni(OH)2 or to the spinel Ni1−yFe2yO4. This value may also correspond to the oxide MnO [23], but at this stage, further analyzes are needed to attribute the gap to one or another oxide. The second value was located at 3 eV. This gap value was attributed to the chromia n-type semiconductor [14, 15].
The signature of the spinel MnCr2O4 (or Mn1,5Cr1,5O4), identified in this work during microstructure characterization, was not observed. It can be noted that a similar remark can be made regarding the results of Marchetti et al. [22] conducted on the alloy HR230. These authors reported also the presence of spinel at the surface but did not identify it by photoelectrochemical measurements. As the gap of Ni1−xFexCr2O4 was estimated in literature [22] at 4.1 eV, MnCr2O4 should be expected also at high energy values.
Markers experiments (gold or isotopic oxygen marker) converged to demonstrate the inward growth of the oxide scale. The gold grains were systematically located at the top of the chromia layer. The oxygen 18 profile depicted a diffusion profile without any accumulation of oxygen in the outer part of chromia. As a consequence, the growth of the oxide scale takes place at the metal-oxide interface.
Assuming that the growth rate is limited by the lattice diffusion through the more stoichiometric oxide among those identified, namely the chromia, the growth of the layer can be controlled by the diffusion of the oxygen vacancy \( \left( {V_{\text{O}}^{2 \bullet } } \right) \). The following equilibriums reflect its formation at the metal-oxide interface (Eq. 2) and its annihilation at the interface oxide-gas (Eq. 3):
This result was not in agreement with what is commonly accepted, i.e. that the diffusion takes place by the cationic network in Cr2O3. However, the inward growth of chromia has been already reported on chromia forming alloys [20], including external development of a layer of spinel (Fe or Ni)Cr2O4 at the outer surface. In fact, there is no contradiction with the mechanism mentioned above if the formation of spinel MnCr2O4 is taken into account. Indeed, the formation of the spinel imposed an oxygen pressure at the interface chromia-spinel interface of 10−13 atm. at 1,000 °C [24] for an activity of chromium and manganese in the order of magnitude of 0.2–0.001 respectively. Then the chromia scale growing at the surface of HR120 developed under low oxygen pressure. In such conditions, it is established that oxygen vacancy and interstitial chromium are the predominant point defects [25].
From the O18 profile, the self-diffusion coefficient of oxygen in chromia \( D_{{{\text{O}}*}}^{{{\text{Cr}}_{2} {\text{O}}_{3} }} \) was estimated to 2.1 × 10−13 cm2 s−1. This value was in good agreement with that determine by Tsai et al. [26] (10−14 cm2 s−1) in polycrystalline Cr2O3. However these authors attributed this value to the diffusion of oxygen at grain boundaries since the self-diffusion coefficient of oxygen in chromia should be of five orders of magnitude lower [27]. It can then be proposed that oxygen vacancies were generated at the bottom of the oxide scale and that these point defects diffused mainly through grain boundaries.
Conclusions
Oxidation of HR120 at 1,050 °C led to the formation of a multilayered oxide scale constituted of the following layers SiO2–Cr2O3–XCr2O4 (with X = Mn and/or Fe, Ni) from the metal-oxide interface to the top of the oxide scale. Its long time oxidation behaviour was characterized by a parabolic like kinetic, oxidation being mainly limited by the diffusion through the chromia scale.
Before the establishment of this protective layer, the short time oxidation period was characterized by the development of local outgrowths of spinel FeCr2O4 that were associated to fast and linear oxidation rate.
The study of growth mechanism of the chromia layer allowed the identification of a n-type semi-conductivity and an inward growth of the scale. Thus, assuming that diffusion in the oxide scale controlled chromia growth, the oxygen vacancy was the major point defect governing the solid state transport. This can be the result of the presence of a MnCr2O4 spinel layer at the top of chromia that strongly decreased the oxygen pressure at the interface spinel/chromia. At this stage, the characterization of oxygen diffusion at grains boundaries is required to support the proposed mechanism; nano-SIMS experiments will be attempt to this end.
References
HAYNES, http://www.haynesintl.com.
V. P. Deodeshmukh, S. J. Matthews, and D. L. Klarstrom, International Journal of Hydrogen Energy 36, 4580 (2011).
C. Wood, I. G. Wright, T. Hodgkiess, and D. P. Whittle, Werkstoffe und Korrosion 21, 900 (1970).
A. de S. Brasunas, J. T. Gow, O. E. Harder, 30th Annual Convention of the American Society for Metals, Proceeding Vol. 46 p. 870 (1946).
J. E. Croll and G. R. Wallwork, Oxidation of Metals 4, 1972 (121).
Kofstad, High Temperature Corrosion, (Elsevier New York, 1988).
B. Chattopadhyay, and G. C. Wood, Oxidation of Metals 2, 373 (1970).
F. H. Stott, P. K. N. Bartlett, and G. C. Wood, Material Science Engineering 8, 163 (1987).
D. Renusch, B. Veal, K. Natesan, and M. Grimsditch, Oxidation of Metals 46, 365 (1996).
J. Zurek, D. J. Young, E. Essuman, M. Hansel, H. J. Penkalla, L. Niewolak, and W. J. Quadakkers, Materials Science and Engineering. A 477, 259 (2008).
Y. Wouters, A. Galerie, and J-P. Petit, Journal of Electrochemical Society 154, C587 (2007).
A. Srisrual, S. Coindeau, A. Galerie, J-P. Petit, and Y. Wouters, Corrosion Science 51, 562 (2009).
A. Galerie, S. Henry, Y. Wouters, M. Mermoux, J-P. Petit, and L. Antoni, Materials at High Temperature 22, 105 (2005).
J.-P. Petit, M. Mermoux, Y. Wouters, A. Galerie, and C. Chemarin, Materials Science Forum 461–464, 681 (2004).
S. Guillou, C. Cabet, C. Desgranges, L. Marchetti, and Y. Wouters, Oxidation of Metals 76, 193 (2011).
H. Hindam, and D. P. Whittle, Oxidation of Metals 18, 245 (1982).
D. L. Douglass, and J. S. Armijo, Oxidation of Metals 2, 207 (1970).
R. E. Lobnig, H. P. Schmidt, K. Hennesen, and H. J. Grabke, Oxidation of Metals 37, 81 (1992).
L. Couture, PhD Thesis, INP Grenoble (2011).
G. C. Wood, T. Hodgkiess, and D. P. Whittle, Corrosion Science 6, 1966 (129).
X. Ledoux, PhD Thesis, Université de Lorraine (2012).
L. Marchetti, S. Perrin, Y. Wouters, F. Martin, and M. Pijolat, Electrochimica Acta 55, 5384 (2010).
F. Tran, P. Blaha, K. Schwarz, and P. Novák, Physical Review B 74, 155108 (2006).
D. Young, High Temperature Oxidation and Corrosion of Metals, (Elsevier Corrosion Series, Amsterdam, 2008).
A. Holt, and P. Kofstad, Solid State Ionics 69, 127 (1994).
S. C. Tsai, A. M. Huntz, and C. Dolin, Material Science and Engineering A 212, 6 (1996).
A. C. S. Sabioni, B. Lesage, A. M. Huntz, J. C. Pivin, and C. Monty, Philosophical Magazine A 66, 333 (1992).
Acknowledgments
Authors thank Ludovic Mouton and Sandrine Mathieu from SCMEM of the University of Nancy for Microscopic observations, Sylvain Weber from CCMEM of the Jean Lamour Institute for SIMS analyses, Sébastien Chevalier and Nathalie Roudergues from University of Dijon for the 18O marker analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ledoux, X., Mathieu, S., Vilasi, M. et al. Oxide Growth Characterization During Short-Time Oxidation of a Commercially Available Chromia-Forming Alloy (HR-120) in Air at 1,050 °C. Oxid Met 80, 25–35 (2013). https://doi.org/10.1007/s11085-013-9367-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-013-9367-1