Abstract
When metals and alloys are used at high temperatures, especially in combustion processes, deposits often accumulate on the metal surfaces and affect the oxidation processes. This paper is concerned with deposit-induced accelerated corrosion, or hot corrosion, of metals and alloys. Initially, the characteristics of hot corrosion are identified for Na2SO4 deposits in terms of the factors that influence the reaction process. It is shown that hot corrosion consists of initiation or incubation and propagation stages. During the initiation or incubation stage, the deposit is shown to not have a significant effect on the corrosion processes, but it is causing conditions to develop whereby the propagation stage characteristics are determined with attendant large increases in the corrosion rates. Type I, high temperature hot corrosion and Type II, low temperature hot corrosion are then described in terms of historical mechanistic perspectives. The dependence of Type I and Type II hot corrosion on temperature and SO3 partial pressure is discussed along with future work that is needed in order to more completely understand these hot corrosion processes along with the effects of some elements such as Cr, Al, Mo, Co and Pt.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
When metals and alloys are used at elevated temperatures, especially in equipment that involves combustion, deposits, usually liquid, can accumulate on component hardware whereby the deposits can have a profound influence on the corrosion process involved in the degradation of the alloys. This process of deposit-induced accelerated oxidation is called hot corrosion. The origin of the name comes from the similarity of these conditions to aqueous corrosion in which a liquid, aqueous medium plays a significant role in the degradation process.
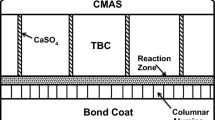
The degradation of alloys due to hot corrosion has been a serious problem for at least 80 years, and there are numerous papers and reviews [e.g., 1–4] on this process. There are a number of different deposits that have been shown to cause hot corrosion of alloys. Such deposits include sulfates of Na, Ca, and K, as well as vanadates and carbonates. In fact, as illustrated in Fig. 1, deposits on the surfaces of alloys separate the alloys from the gas environments and providing that transport through the deposit is slow, some reaction between elements in the alloy and components in the deposit are likely. Therefore, most deposits can exert some influence on the oxidation characteristics of alloys.
In gas turbines a common deposit is Na2SO4 where the sodium and sulfur may exist as impurities in the fuel, or NaCl as well as sulfates may be ingested in the air required for combustion and the NaCl is converted to Na2SO4 via reaction with sulfur in the fuel. In this paper the effects of Na2SO4 deposits will be considered, however, what is discussed is relevant to other deposits that are liquid or can react to form a liquid under the exposure conditions.
The objectives of this paper are to provide a consistent description of the hot-corrosion process emphasizing the important mechanisms and their dependence on temperature and SO3 partial pressure in the gas. Initially the important characteristics of the hot corrosion process will be identified and then the mechanisms by which hot corrosion occurs will be discussed. It will be shown that the characteristics of hot corrosion, as well as the effects of different elements, depend upon the particular mechanism by which the hot corrosion is occurring.
The Characteristics of Hot Corrosion
The characteristics of hot corrosion attack are numerous. The Na2SO4 deposit causes a non-protective scale to form on the alloy or metal, whereas a protective scale is formed by reaction with the gas in the absence of the deposit. It is important to emphasize that the attack may not be self-sustaining in that a protective scale eventually forms unless additional deposit arrives upon the surface of the alloy, Fig. 2.
In Fig. 2 the weight change versus time data for nickel with and without a Na2SO4 deposit are compared. The Na2SO4 deposit causes an initial large weight gain followed by much smaller subsequent weight gains and, during the period of large weight gains, a nonprotective NiO scale develops on the nickel specimen
The hot corrosion attack is influenced by the exposure time, as shown in Fig. 3, where the attack consists of an initiation or incubation stage and a propagation stage. In the initiation stage the alloy is behaving much as it would by reacting with the gas in the absence of the deposit whereas, in the propagation stage, the deposit exerts a profound effect on the attack.
In Fig. 3 data are presented for uncoated superalloys and for aluminide coated superalloys. Both types of specimens undergo hot corrosion attack but the aluminide coated specimens require many cycles at temperature before the propagation stages are reached
Most cases of deposit-induced accelerated attack occur when the deposit is a liquid; although, some examples of attack caused by solid deposits are available [5], but no attack has been reported when the substance comprising the deposit exists as a gas [6]. Such effects of the physical state of the deposit are related to conditions that can develop as the alloy reacts with the deposit in the absence of effects caused by reaction with the gas. As shown in Fig. 4, a liquid Na2SO4 deposit can result in the Na2SO4 developing basic (excess Na2O) or acidic (excess SO3) characteristics. By applying the law of mass action to Eq. 1,
the acidic and basic characteristics of the Na2SO4 can be related to one another. Such effects can have substantial influence on the hot-corrosion process, as will be discussed subsequently. The hot corrosion of alloys is dependent upon the amount of the deposit as shown in Fig. 5 where the weight gain of a Ni–8Cr–6AlFootnote 1 specimen is substantially greater as the amount of Na2SO4 is increased. However, exceptions do exist, for example, when the effects of the Na2SO4 are induced by an element in the alloy, in this case Mo in the Ni–8Cr–6Al–3Mo, since a thin layer of Na2SO4 causes attack after a shorter exposure time than a thicker layer, Fig. 5.
A thermodynamic stability diagram for sodium, sulfur and oxygen that shows how Na2SO4 can change in composition due to reaction. The basic conditions arise via the removal of oxygen and sulfur from the Na2SO4 by the alloy. The acidic conditions can arise due to the formation of refractory metal oxides resulting from the oxidation of refractory metals in the alloy. The basic and acidic characteristics of Na2SO4 are related by applying the law of mass action to Eq. 1. The dashed lines indicate sulfur isobars
The composition of the deposit plays a significant role in the hot corrosion process. This is shown in Fig. 6 where Ni–30Cr and Ni–25Cr–6Al specimens are exposed to Na2SO4–NaCl deposits at 900 °C in air. In the absence of such deposits an alumina layer is formed on the Ni–25Cr–6Al specimen whereas a Cr2O3 scale is formed on the Ni–30Cr specimen. The attack of both alloys increases as the amount of NaCl in the deposit is increased but the attack of the Ni–30Cr alloy is more severe compared to that of the Ni–25Cr–6Al alloy.
The hot corrosion process is influenced by the temperature. This is shown schematically in Fig. 7. As the temperature is increased the attack changes from what is called low temperature attack, Type II, to high temperature attack, Type I. These two types of hot corrosion are also affected by the gas composition which will be discussed subsequently. Type I hot corrosion was first encountered in aircraft gas turbines. Type I degradation microstructures could be reproduced in laboratory experiments at 900–950 °C using Na2SO4 deposits and exposure in air or oxygen. As gas turbines began to be used for marine propulsion, another type of hot corrosion was observed and it was determined that in order to reproduce the Type II microstructure, it was necessary to have SO3 present in the gas [7]. As a result of the observation that SO3 should be present to cause hot corrosion attack a number of experiments were performed over the temperature range from 650 °C to 950 °C using oxygen–SO2 gas mixtures. At temperatures from 650 °C to 750 °C, low temperature, Type II, attack was only observed when SO3 gas pressures were established in the gas mixture, Fig. 8. However, high temperature, Type I, hot corrosion could occur at temperatures of 900 °C to 950 °C in gas mixtures of air or pure oxygen, Fig. 9. When low temperature hot corrosion was first observed and efforts were made to describe why it occurred, NaCl deposits on alloys were considered as possible causes. As can be seen in Fig. 6, NaCl does cause attack of alloys but the microstructure of degraded specimens is not the same as that for low temperature hot corrosion. The NaCl reacts with chromium and aluminum in alloys to form gaseous chlorides that result in porous chromia and alumina scales upon reaction with oxygen.
The hot corrosion of alloys is dependent upon alloy composition as shown in Fig. 10. The test conditions to initiate the hot corrosion of the alloys in Fig. 10 consisted of Na2SO4 applied every 20 h. The test is at 900 °C in air where the specimens were cycled from the test temperature to room temperature every hour. It can be seen that the CoCrAlY specimens are much more resistant to hot corrosion under these Type I conditions compared to the NiCrAlY specimens. It should also be noted that depending upon the alloy composition relatively long test times can be required to initiate the propagation mode of hot corrosion attack.
The hot corrosion process is also dependent upon other factors such as the fabrication condition of the alloys which influences their microstructures, the gas velocity in the test, cyclic versus isothermal conditions, erosive particles and specimen geometry. These factors will not be discussed in this paper. All of these factors are relevant because they affect the protectiveness of the oxide scales that form on alloys during the initiation stage of hot corrosion, or the availability of certain elements in the alloy to the Na2SO4 deposit.
Some of what has been presented in the previous paragraphs may seem to be inconsistent. This occurs because Type I and Type II hot corrosion occur via different, but related, mechanisms. In order to develop consistency it is necessary to consider the mechanisms by which these two types of hot corrosion take place.
High Temperature, Type I, Hot Corrosion
Hot corrosion attack was first observed in boilers and gas turbines in the period between 1930 and 1960 [8]. In the case of boilers the temperatures were about 540 °C with liquid deposits of Na3Fe(SO4)3. In the 1960s Type I attack was observed in aircraft gas turbines at temperatures of 900 °C with Na2SO4 deposits. As shown in Fig. 11, this attack appeared to be related to the oxidation of sulfides in the alloys where the sulfur came from the Na2SO4 deposits and consequently this type of hot corrosion was frequently called “sulfidation” [9]. Bornstein and DeCrescente [6, 10] performed experiments using the alloy B-1900 and showed that when the amount of sulfur in 1 mg/cm2 of Na2SO4 was introduced into this alloy no hot corrosion attack occurred upon subsequent oxidation, but substantial attack of this alloy took place with deposits (1 mg/cm2)of Na2SO4 or Na2NO3. They concluded that the hot corrosion attack of this alloy occurred because of the Na2O component in the Na2SO4 or Na2NO3. It was proposed that the Na2O destroyed the normally protective oxide scale that usually developed on this alloy due to a basic fluxing reaction as indicated in the following for a NiO scale
Rapp and Goto [11] examined the hot corrosion fluxing process and proposed that a negative solubility gradient was necessary whereby the oxide on the alloy dissolved in the molten deposit at the scale/deposit interface, but precipitated out in the deposit where the solubility was lower as a discontinuous scale, as illustrated in Fig. 12a. Rapp and his students determined the solubility of a number of oxides in Na2SO4 as a function of the activity of Na2O in the Na2SO4 and showed that the solubilities could be rationalized via reactions with the Na2O or SO3 components in the Na2SO4, Fig. 12b [12].
In considering how the Na2O activity in a Na2SO4 deposit on nickel could increase to levels whereby NiO dissolved into the Na2SO4, Goebel and Pettit [13] observed that at 900 °C Na2SO4 did not decompose rapidly in air or oxygen via the reaction
It was proposed that the liquid Na2SO4 layer separated the nickel specimen from the oxygen gas, and consequently an oxygen gradient was established across the Na2SO4 from the gas interface to the nickel interface. As a result of this gradient in the Na2SO4, as shown in Fig. 13, the sulfur activity was increased to levels such that sulfur could penetrate the oxide scale formed on the nickel to form nickel sulfide in the nickel. The removal of oxygen and sulfur from the Na2SO4 caused the activity of Na2O in the Na2SO4 to increase as shown in the following reaction,
Stability diagram showing the phases of nickel that are stable in the Na2SO4 region of Fig. 4 at 1000 °C. The dashed lines are sulfur isobars and the arrows indicate how the composition of the Na2SO4 can change due to oxygen and sulfur removal from the initial composition. X indicates the as deposited composition of the Na2SO4
The activity of Na2O was greatest at the NiO/Na2SO4 interface and decreased in the Na2SO4 layer towards the Na2SO4/gas interface. The Rapp-Goto solubility criterion was satisfied and NiO dissolved at the NiO/Na2SO4 interface and precipitated as discontinuous particles in the outer regions of the Na2SO4. The reaction that occurred at the oxide/salt interface was
with the reverse of this reaction occurring out in the Na2SO4 deposit. The reaction by which the NiO precipitates provides Na2O to the Na2SO4 and eventually the negative solubility gradient is removed and the fluxing reaction stops. In particular, this type of hot corrosion is not self sustaining.
Much of the experimental work to study Type I attack was performed in air or pure oxygen, but with the advent of studies on Type II attack the importance of SO3 in the gas became apparent. A question that became relevant was,” how important is SO3 in the gas for the case of Type I attack”? In order to answer this question, Rapp and Otuska [14] performed experiments with preoxidized nickel with a Na2SO4 deposit in an oxygen–SO2 gas mixture and measured the Na2O activity and oxygen pressure at the NiO/Na2SO4 interface as a function of time. The results are presented in Fig. 14 where a stability diagram is used to show the phases of nickel that are stable as a function of the Na2O activity and oxygen partial pressure. The times at which these measurements were made are also indicated on this stability diagram. It can be seen that as the oxygen pressure is decreased substantially due to reaction with nickel and the region of sulfide stability is entered, the activity of Na2O increases due to oxygen and sulfur removal from the Na2SO4. The point to be emphasized is that the effects due to reaction of the Na2SO4 with the nickel override any influence of the gas environment. Consequently the attack is not dependent on the composition of the gas in the case of nickel.
Sketches using a nickel–oxygen–sulfur thermodynamic stability diagram to show the oxygen and Na2O activity values at the NiO/Na2SO4 interface as a function of time. The dashed line indicates the change in the dissolution process from basic to acidic. Exposure times are in minutes and hours (not labeled)
In considering the Type I mechanism it appears that both basic fluxing and sulfide formation followed by oxidation of these sulfides take place concomitantly. In Fig. 15 cyclic oxidation data are presented for a Ni–25Cr–6Al alloy where this alloy was coated with Na2SO4 every 5 h up to 20 h and then after every 10 h. Other specimens were sulfidized in an H2S/H2 gas mixture at the same time intervals where the amount of sulfur added to the specimens was the same as that in the Na2SO4 deposits. Inspection of Fig. 15a shows that the degradation of both specimens is similar showing, in contrast to the results of Bornstein and DeCrescente, that the sulfur in the Na2SO4 can cause substantial attack of alloys in cyclic oxidation. As shown in Fig. 15b, the microstructures of the exposed specimens are similar. These results are not consistent with the results of Bornstein and DeCrescente because those investigators used the alloy B-1900 in their studies. As will be shown this alloy undergoes catastrophic hot corrosion due to the Mo in the alloy reacting with Na2SO4, or NaNO3, to form Na2MoO4 with a high activity of MoO3. Protective scales such as alumina undergo alloy-induced acidic fluxing involving the following reaction
There is a negative solubility gradient in the Na2MoO4 because MoO3 is removed from the liquid solution at the gas interface due to vaporization. This process can occur with Na2SO4 or NaNO3 deposits but not when the sulfur from the Na2SO4 is added to this alloy via an H2S–H2 gas mixture.
Some investigators have studied the attack of alloys with Na2SO4 deposits in O2–SO2 gas mixtures where the SO2 concentrations were high (~4%) which established SO3 gas pressures on the order of 10−2 atm [15–17]. Table 1 presents the SO3 partial pressures in different O2–SO2 gas mixtures as well as the SO3 pressures to form Na2SO4 and CoSO4 at different temperatures. The results of such studies show that at both 700 °C and 900 °C a substantial number of sulfides is formed in alloys and the degradation consists of oxidation of these sulfides. It therefore appears that there are regimes involving degradation of alloys at temperatures and SO3 pressures where the hot corrosion process consists predominantly of this sulfidation mode as shown in Fig. 7.
In some cases of hot corrosion, one application of Na2SO4 can result in catastrophic attack of the alloys whereby the alloy is completely converted to corrosion products. In such cases the hot corrosion process involves the oxides of certain elements in the alloy, usually refractory metals such as Mo or W whereby the Na2SO4 is converted to a molybdate or tungstate and SO3 is evolved as shown in the following reaction,
In such cases the molten deposit can be considered to have two acidic components, namely SO3 and MoO3, and the solubility of the protective oxide is different depending upon the acidic oxide as shown in Fig. 16. Moreover the Rapp-Goto solubility criterion is satisfied because the acidic component formed by oxidation of an element in the alloy is usually volatile. This form of hot corrosion, which has been called alloy-induced acidic fluxing, is observed at temperatures between 900 °C and 1000 °C. A comparison of this type attack to basic fluxing-sulfidation is shown in Fig. 17 where weight change versus time measurements are presented for Na2SO4 deposits on Ni–25Cr–6Al and Ni–25Cr–6Al–6Mo. Both alloys show increased attack via basic fluxing-sulfidation but the alloy with Mo has a catastrophic amount of attack due to the conversion of the Na2SO4 to Na2MoO4 and the alloy-induced attack. Mishra [15] has shown that the alloy-induced acidic fluxing process is influenced by the SO3 pressure in the gas phase, Fig. 18. Rapid attack of the alloy U-700 was observed with small concentrations of SO2 in the gas but the attack was found to decrease as the SO2 concentration approached 1 and 2%. This can be explained by applying the law of mass action to Eq. 7 and assuming the Na2SO4–Na2MoO4 solution is ideal. One obtains
It can be seen that the \( {\text{a}}_{{{\text{MoO}}_{ 3} }} \) in the Na2SO4–Na2MoO4 solution is dependent upon the mole fraction of Na2SO4 in the solution and the SO3 pressure. When the SO3 pressure is low the Na2SO4 is converted to Na2MoO4 and \( {\text{N}}_{{{\text{Na}}_{ 2} {\text{SO}}_{ 4} }} \) approaches zero whereby the activity of MoO3 is high. However, for high values of SO2 the mole fraction of Na2SO4 in the Na2SO4–Na2MoO4 solution is significant and consequently the activity of MoO3 is low. Hence alloy-induced acidic fluxing does not occur. In this latter case, Mishra [15] has shown the attack occurs via a sulfide formation and oxidation of sulfide mode.
Type I hot corrosion attack has been shown to be induced via liquid Na2SO4 deposits whereby oxygen and sulfur removal from the Na2SO4 causes the Na2O activity in the Na2SO4 to be increased to levels at which oxides such as NiO and Al2O3 can be fluxed from the alloy surface and precipitated out in the Na2SO4 as discontinuous particles. This process has been shown to be unaffected by the composition of the gas phase but more work is required especially with alloys resistant to this type of attack, to further examine gas effects on Type I hot corrosion.
Low Temperature Type II Hot Corrosion
The observation of Type II hot corrosion was made in the 1970s in gas turbines used for marine propulsion. The degradation microstructure was much different than those caused by basic fluxing-sulfidation and alloy-induced acidic fluxing as can be seen in Fig. 19. The Type II microstructure often consisted of pits that extended into the alloy, Fig. 20. At the corrosion product-gas interface the pit was enriched in cobalt or nickel depending upon the base element of the alloy. However, the concentrations of these two elements decreased as the pit was entered going toward the alloy. The aluminum and chromium concentrations in the pit had the distributions similar to those in the alloy which suggested that these two elements were converted to oxides in situ with little or no diffusion. In the case of CoCrAlY coatings, which were very susceptible to this type of attack, no sulfides were evident in the alloy adjacent to the corrosion product, Fig. 20. However, as the nickel content of the alloy was increased sulfides at such locations were evident, Fig. 21. Furthermore, in the case of CoCrAlY alloys sulfur was detected in the corrosion product adjacent to the alloy, Figs. 20 and 21, but no sulfur containing compounds were detected.
A number of mechanisms have been proposed to account for the Type II degradation process [18–20]. All of these mechanisms propose that at temperatures of 650 °C to 750 °C solid Na2SO4 is converted to a liquid solution of Na2SO4–CoSO4 or Na2SO4–NiSO4, Fig. 22, via the conversion of CoO or NiO to sulfates due to reaction with SO3 in the gas. In the case of CoCrAlY alloys, the mechanism proposed by Luthra [18] is probably the most accurate. In this mechanism a liquid Na2SO4–CoSO4 solution develops upon the alloy surface. The compositional limits of this sulfate solution are shown in Fig. 23 where the cobalt–oxygen–sulfur phase stability diagram is used to describe its limits. It can be seen that this sulfate solution has a composition where CoO, Co3O4 and CoSO4 can be stable. In the CoO region the SO3 minimum pressure is obtained by determining the activity of CoSO4 necessary to form a liquid phase. This activity is 0.03 at 750 °C [18] and the SO3 pressure is calculated from the following reaction,
This SO3 pressure exists over a range of oxygen pressures up to the CoO/Co3O4 equilibrium. Above this oxygen pressure the following reaction must be used to calculate the SO3 pressure,
It can be seen, Fig. 23, that the SO3 pressure increases with the oxygen pressure. The total pressure in the gas phase cannot exceed 1 atm since laboratory experiments at 1 atm total pressure caused Type II hot corrosion attack. This limits the oxygen pressure that can be in equilibrium with Co3O4 to approximately 1 atm. At this oxygen limit the SO3 pressure will be determined by the amount of sulfur in the gas and can be sufficient to form CoSO4 at unit activity. In the case of these higher SO3 pressures the oxygen pressure can vary from 1 atm down to values at which the SO2 pressure approaches 1 atm where the SO2 pressure can be calculated from the reaction,
Luthra proposes that SO3 is transported from the gas through the Na2SO4–CoSO4 liquid via S2O7 2− ions involving an exchange reaction with SO4 2−. The reaction at the salt/gas interface consists of
The following reaction provides oxygen and sulfur at the scale/sulfate interface
It is also proposed that Co ions are diffusing in the liquid sulfate, namely, Co2+ outward and Co3+ inward. The important species and reactions are presented in Fig. 24. Cobalt goes into the liquid sulfate at the scale/sulfate interface as Co2+ and Co3O4 is precipitated out in the liquid sulfate near the gas interface. (If the SO3 pressure is sufficiently high, CoSO4 can be precipitated rather than Co3O4.) Oxygen and sulfur are supplied to the alloy whereby oxides and sulfides of cobalt, chromium and aluminum are formed. The dissolution of cobalt from the alloy causes any Cr2O3 or Al2O3 that are formed to be discontinuous and no protective oxide barrier can be developed.
The Type II mechanism that has been presented is applicable to some alloys but perhaps not to all alloys. Moreover at high SO3 pressures it has been observed that extensive sulfide formation is part of the attack [15–17]. As shown in Fig. 25, there is a need to examine the transition from Type II attack to sulfide formation and oxidation at 700 °C and from Type I attack to sulfide formation at 900 °C.
Summary diagram showing the regions where Type I and Type II hot corrosion, as well as alloy induced acidic fluxing hot corrosion, predominate as a function of temperature and SO3 partial pressure. This diagram also indicates the regions where extensive sulfide formation followed by oxidation of these sulfides is proposed to be a significant form of degradation
Concluding Remarks
This paper has attempted to use the results that are available in the literature to explain the phenomena of Type I and Type II hot corrosion and to identify research that is necessary to further clarify the interrelationships between these two deposit-induced degradation processes as well as the effects produced by some of the important alloying elements. It is probable that all may not agree with what has been presented, but it is hoped that such disagreement will drive research to further clarify these two important forms of deposit-induced degradation at elevated temperatures. Research topics that are inferred to be important for elaboration of certain aspects of hot corrosion processes are identified in the following:
-
An important aspect of the Type II degradation mechanism involves the counter diffusion of Co3+ and Co2+ ions through the liquid sulfate solution. The degradation of other alloys such as NiCrAlY under Type II conditions should be examined since counter diffusion of nickel ions may be less favorable than cobalt. Such studies should also examine the influence of Cr, Al, Ni and Co on Type II attack.
-
The proposed transition from Type I and Type II attack to a degradation mechanism involving sulfide formation and oxidation of these sulfides should be confirmed at 700 and 900 °C at high SO3 pressures.
-
The major amount of experimental data on hot corrosion induced by Na2SO4 is at 700 and 900 °C. The attack of CoCrAlY and NiCoCrAlY at 800 °C in an oxygen–SO2 gas mixture that will produce a liquid sulfate solution should be studied to examine the effect of temperature in more detail.
-
The effect of Mo on the hot corrosion of alloys at 900 °C is not totally clear. It is well documented that Mo causes alloy-induced acidic fluxing in gas mixtures with low SO3 partial pressures. However, there are also results that indicate Mo may cause less hot corrosion attack. This may be due to a change in the degradation mechanisms at low and high SO3 pressures. It is necessary to perform experiments to clarify the effect of Mo on hot corrosion attack at 900 °C.
-
While not discussed in this paper, the addition of platinum to alloys has been shown to improve cyclic oxidation resistance as well as resistance to Type I and Type II hot corrosion attack [21]. Work to examine the effects of platinum on the hot corrosion of alloys is also recommended.
Notes
All compositions are in weight percent unless stated otherwise.
References
J. Stringer, Annual Review of Materials Research 7, 477 (1976).
Y. S. Zhang and R. A. Rapp, Journal of Metals 46, 47 (1994).
F. S. Pettit and C. S. Giggins, in Superalloys II, eds. C. T. Sims, N. S. Stoloff, and W. C. Hagel (John Wiley and Sons, New York, NY, 1987).
P. Hancock, Corrosion of Alloys at High Temperatures in Atmospheres Consisting of Fuel Combustion Products and Associated Impurities, (Her Majesty’s Printing Office, London, 1968).
K.-Y. Jung, F. S. Pettit, and G. H. Meier, Materials Science Forum 595–598, 805 (2008).
N. S. Bornstein and M. A. DeCrescente, Transactions of the Metallurgical Society of AIME 245, 1947 (1969).
L. F. Aprigliano, Burner Rig Simulation of Low Temperature Hot Corrosion. David W. Taylor Naval Ship Research and Development Center, Report MAT-77-68, November, 1977.
W. T. Reid, External Corrosion and Deposits in Boilers and Gas Turbines, (Elsevier, New York, 1971).
A. U. Seybolt, Transactions of the Metallurgical Society of AIME 242, 1955 (1968).
N. S. Bornstein and M. A. DeCrescente, Metallurgical Transactions 2, 2875 (1971).
R. A. Rapp and K. S. Goto, in Molten Salts, eds. J. Braunstein and J. R. Selman (The Electrochemical Society, 9, Pennington, NJ, 1981), p. 81.
Y. S. Zhang and R. A. Rapp, Corrosion 43, 348 (1987).
J. A. Goebel and F. S. Pettit, Metallurgical Transactions 1, 1943 (1970).
N. Otsuka and R. A. Rapp, Journal of the Electrochemical Society 137, 46 (1990).
A. K. Mishra, Journal of the Electrochemical Society 133, 1038 (1986).
K. P. Lillerud and P. Kofstad, Oxidation of Metals 21, 233 (1984).
P. Kofstad and G. Akesson, Oxidation of Metals 14, 301 (1980).
K. L. Luthra, Metallurgical Transactions, 13A, 1982, 1647 and 1843.
R. H. Barkalow and F. S.Pettit, On the oxidation mechanisms for hot corrosion of CoCrAlY coatings in marine gas turbines. Proceedings of the 14th Conference on Gas Turbine Materials in a Marine Environment (Naval Sea Systems Command, Annapolis, MD, 1979), p. 493.
K. T. Chiang, F. S. Pettit, and G. H. Meier, in High Temperature Corrosion, NACE-6, ed. R. A. Rapp (National Association of Corrosion Engineers, Houston, TX, 1983), p. 519.
J. Schaeffer, G. M. Kim, G. H. Meier, and F. S. Pettit, in The Role of Active Elements in the Oxidation Behavior of High Temperature Metals and Alloys, ed. E. Lang (Elsevier, London, 1989), p. 231.
Acknowledgments
G. H. Meier and B. Gleeson are thanked for helpful discussions and assistance in preparing this paper. The Office of Naval Research (ONR) is gratefully acknowledged for support in preparing this paper as well as for many research efforts dealing with hot corrosion attack.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pettit, F. Hot Corrosion of Metals and Alloys. Oxid Met 76, 1–21 (2011). https://doi.org/10.1007/s11085-011-9254-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-011-9254-6