Abstract
The electric activities of neurons are dependent on the complex electrophysiological condition in neuronal system, and it indicates that the complex distribution of electromagnetic field could be detected in the neuronal system. According to the Maxwell electromagnetic induction theorem, the dynamical behavior in electric activity in each neuron can be changed due to the effect of internal bioelectricity of nervous system (e.g., fluctuation of ion concentration inside and outside of cell). As a result, internal fluctuation of electromagnetic field is established and the effect of magnetic flux across the membrane should be considered during the emergence of collective electrical activities and signals propagation among a large set of neurons. In this paper, the variable for magnetic flow is proposed to improve the previous Hindmarsh–Rose neuron model; thus, a four-variable neuron model is designed to describe the effect of electromagnetic induction on neuronal activities. Within the new neuron model, the effect of magnetic flow on membrane potential is described by imposing additive memristive current on the membrane variable, and the memristive current is dependent on the variation of magnetic flow. The dynamics of this modified model is discussed, and multiple modes of electric activities can be observed by changing the initial state, which indicates memory effect of neuronal system. Furthermore, a practical circuit is designed for this improved neuron model, and this model could be suitable for further investigation of electromagnetic radiation on biological neuronal system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The neuronal system consists of a large number of neurons and gliocytes, and complex behaviors in electric activities of neurons have been investigated extensively [1–8]. For example, Buschman et al. [1] found evidences that oscillatory synchronization of local field potentials (LFPs) formed neural ensembles representing the rules: There were rule-specific increases in synchrony at “beta” frequencies (19–40 Hz) between electrodes. Wig et al. [2] presented some principles to analyze the complex behaviors in brain. Seely and Crotty [3] discussed the effect of leakage current on action potential on squid giant axon. Postnov et al. [4] studied the dynamical behavior of calcium signal based on functional model of neuron–astrocyte networks. Volman et al. [5] set up a model of neuron–astrocyte interaction to explain the emergence of epilepsy [6] associated with gap junction. Channel noise also can change the dynamics of electric activities of neuron. Ozer and Ekmekci [7] discussed the effect of channel noise on the time course of recovery by blocking the ion channels of sodium. Barthélemy [8] argued that spatial networks could provide enough guidance to understand the collective behaviors of neurons and possible mechanism for neuronal disease. Some theoretical models [9–14] have been established for computational neuroscience, and some biological neuron model could be helpful to understand plasticity, mode transition in electric activities. For example, the Hodgkin and Huxley [11] and Morris and Lecar [12] neuron models are useful to describe the effect of ion channels on membrane potential of neurons. Izhikevich [13] ever gave short comments for most of the neuron model. Ibarz et al. [14] suggested that map-type model could also be effective to produce the dynamical properties in neuronal activities. Perc and Marhl [15] confirmed that excitable neurons that reside in a steady state near a bifurcation point to elliptic bursting oscillations, and it was also found that in addition to the resonant frequency of damped oscillations around the stable focus, another frequency exists that resonantly enhances large amplitude bursts and thus amplifies the information transfer in the system. These results could be helpful to understand the amplification of information transfer in excitable systems. In fact, the three-variable Hindmarsh–Rose (HR) neuron model [16] simplified from the original Hodgkin–Huxley neuron model could be feasible to reproduce main properties of neuronal activities and effective for bifurcation analysis [17–19]. Indeed, some researchers suggested that a four-variable HR neuron [20–22] could be better to model the bifurcation behaviors of neurons and this model can show chaos in large parameter region and this model is also verified by experimental results [23]. To our knowledge, the HR neuron model is classified as a mathematical neuron model because ion channel effect could not be described. Surely, extensive studies could be carried out on isolate neuron and network of neurons that the effect of ion channels could be considered as mentioned in Refs. [24, 25]. In fact, the electric activity of neurons in neuronal system is much too complex and many factors should be considered as well. According to the Faraday’s law of induction, the fluctuation or changes in action potentials in neurons can generate magnet field in the media; thus, the electrical activities of neurons will be adjusted under feedback effect. That is to say, the fluctuation of membrane potentials of neurons can change the distribution of electromagnetic field inner and external of neurons; thus, the magnetic flux across membrane and electromagnetic effect should be considered. However, the presented neuron models seldom consider the effect of electromagnetic induction on membrane potential of neurons. In this case, it is important to set more reliable neuron model so that the effect of electromagnetic induction in neurons could be considered; furthermore, external electromagnetic radiation could also been calculated and the author of this paper suggested that magnetic flux could be used to finish this task.
More often, it is claimed that neuronal system can be in good memory to keep normal activities and the memory effect is often described by using time delay term in the model. Indeed, magnetic field or magnet flux storage could be associated with the memory effect. In fact, the neuronal system can be used as a reliable signal processor because slight stimuli on the neuron can be give sensitive response by analyzing the sampled time series. For example, Aggarwal et al. [26] presented an optimal design of two-dimensional finite impulse response (2D FIR) filter with quadrantally even symmetric impulse response, and the presented scheme did show improved design accuracy and flexibility with varying values of FDCs. Kumar and Rawat [27] proposed the use of power function and least squares method for designing of a fractional-order digital differentiator. The input signal can be transformed into a power function by using Taylor series expansion, and the fractional-order digital differentiator was described by a finite impulse response (FIR) system that yields fractional-order derivative of the G–L type for a power function. Wang et al. [28] investigated the propagation of the firing rate and synchronous firings in a 10-layer feed-forward neuronal network and found that these abilities in information processing due to synchrony can be modulated by noise and the operating mode of neurons. Suffczynski et al. [29] developed a computational model of thalamo-cortical circuits based on relevant (patho) physiological data, and these results can provide more insight into the dynamics of the neuronal networks leading to seizure generation in a rat experimental model of absence epilepsy. Cullheim and Thams [30] investigated the role for microglia in interplay with synapses, and the development of various disorders of the central nervous system (CNS) was also discussed. To discern the complex functional role of brain, the dynamic brain network was constructed from human functional magnetic resonance imaging data based on the sliding window method, and then the eigenvalues corresponding to the network are calculated. Wang et al. [31] analyzed the global properties of eigenvalues by using eigenvalue analysis, and the local properties were measured based on the random matrix theory (RMT).
In this paper, a new four-variable HR neuron model is established by introducing additive variable as magnetic flux which adjusts the membrane potential via a memristor [32–34], so that the effect of electromagnetic induction could be considered by calculating the magnetic flux on membrane. Most of the previous neuron models can generate a variety of modes in electric activities by changing the external forcing current and/or other bifurcation parameters; for example, the HR neuron model can generate quiescent state, spiking, bursting even chaotic state by increasing the intensity of external forcing current. It is also confirmed that chaotic and bursting state in electric activities makes neuron keep lower Hamilton energy [35, 36], and it could be important to understand the potential mechanism for emergence of epilepsy. This model could be reliable for further investigation on effect of electromagnetic radiation on biological tissue.
2 Model descriptions
The dynamical equations for the improved HR neuron model are described by
where the variables x, y, z represent the membrane potential, slow current for recovery variable, and adaption current, respectively. \(I_\mathrm{ext}\) denotes the external forcing current, and the fourth variable \(\phi \) describes the magnetic flux across membrane. The \(\rho (\phi )\) is the memory conductance of a magnetic flux-controlled memristor [32, 33] and here used to describe the coupling between magnetic flux and membrane potential of neuron. The memory conductance of memristor is often described by \(\rho (\phi ) = \alpha +3 \beta \phi ^{2}\), and \(\alpha , \beta \) are fixed parameters [34]. Similar to the previous works, the parameters could be selected with the same values as \(a=1,b=3, c=1,d=5,r=0.006, s=4\). \(k_{1}\) and \(k_{2}\) are parameters that describe the interaction between membrane potential and magnetic flux. The term \(k_{1} \rho (\phi )x\) describes the suppression modulation on membrane potential, and it is dependent on the variation in magnetic flux by generating additive faradic current. According to the Faraday law of electromagnetic induction and description about memristor, the term \(k_{1} \rho (\phi )x\) could be regarded as additive induction current on the membrane as follows
The ion currents of sodium, potassium contribute the membrane potential and also the magnetic flux across the membrane; thus, a negative feedback term \(-k_{2}\phi \) is introduced in the fourth formula. It is believed that time delay could be introduced into neuron model and memory effect could be estimated in the time-delayed neuron model. In fact, a specific synapse called as autapse which the synapse connects to its body via a close loop is found in some intermediate neurons, and the effect of autapse [36, 37] on membrane potential of neuron is often described by applying a time-delayed feedback current along the close loop. The autapse connection can change the dynamics of electric activities of neuron, collective behaviors of neuronal networks [38–40]; particularly, it can account for the self-adaption response and selection in electric modes of neurons to external forcing [41]. Not all the neurons need autapse connection even the autapse can play important biological functions in neuronal system by generating continuous pacemaker, so the collective behaviors of neurons could be regulated. That is to say, autapse connection provides evidence for intrinsic time delay or response delay, and it is also believed that another time delay (propagation time delay) exists when signals are propagated among nodes or neurons. Here, we argue that memristor could be suitable to describe the memory effect by remembering the magnetic flux across the membrane of neurons or cells, and the corresponding parameters for the memory conductance of the memristor could be dependent on the media indeed. With respect to memristor, a variety of nonlinear circuits and dynamical models have been established for dynamical analysis [42–44]; furthermore, memristor-based neuronal network [45, 46] is also paid much attention to signal processing, pattern recognition, associative memory to artificial intelligence. It is interesting to find that memristor-coupled resonator can produce chaotic, bursting phenomena [47] under appropriate parameter region. Therefore, it is important to introduce the memristor into the neuron model because electric activities in neuron can generate quiescent, spiking, bursting and chaotic properties in series for membrane potentials. Furthermore, the memory effect could be even considered. In the following studies, numerical calculation and PSpice verification will be carried out according to the dynamical model shown in Eq. (1).
3 Numerical and experimental results on PSpice
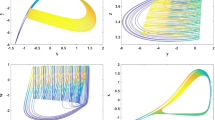
In the numerical studies, the fourth-order Runge–Kutta algorithm is used and the time step is selected as 0.01. The time series for membrane potential are calculated by selecting different parameters and gains for \(k_{1}, k_{2}, \alpha , \beta \); thus, different electric modes could be observed. For example, \(k_{1}=1, k_{2}=0.5,\alpha =0.1, \beta =0.02\), the electric activities in neuron can show different modes by changing the external forcing current, and the results are plotted in Fig. 1.
It is found in Fig. 2 that quiescent state, spiking, bursting and periodical states could be observed by selecting appropriate external forcing current; furthermore, the bifurcation diagram is plotted and shown in Fig. 2.
The bifurcation diagram in Fig. 2 confirms that multiple modes in electrical activities of neuron can be selected by applying appropriate external forcing current. Furthermore, periodical type of external forcing current \(I_\mathrm{ext} =A \cos \omega t\) is also considered, and the numerical results are plotted in Figs. 3 and 4.
The results in Fig. 3 show that the mode of electrical activities is much dependent on the periodical forcing current; particularly, the discharge mode could be dominated by the angular frequency when low amplitude (intensity) is used in the external forcing current. Multiple modes of electrical activities are also observed at \(k_{1}=1, k_{2}=0.5, \alpha =0.1, \beta =0.02\) by selecting other parameters and gains as \(A=0.1, \omega =0.03\) (periodical); \(A=0.7, \omega =0.01\) (spiking); \(A=3.0, \omega =0.04\) (bursting). Furthermore, we also check the model independence by selecting another group of parameters and gains in Eq. (1); for example, it selects \(k_{1}=0.4, k_{2}=0.5, \alpha =0.4, \beta =0.02\), and it can also generate multiple modes in electrical activities, and the results for time series are shown in Fig. 4, and bifurcation diagram is plotted in Fig. 5.
Figure 4 shows that electrical activity can select different discharge modes by applying external forcing current with appropriate values; furthermore, the inter-spike interval (ISI) is calculated by changing the external forcing current slightly, and the results are shown in Fig. 5.
It differs from the bifurcation diagram shown in Fig. 2, and neuron can select its oscillating state with different discharge modes; indeed, quiescent state is removed by selecting the parameters and gains as \(k_{1}=0.4, k_{2}=0.5, \alpha =0.4, \beta =0.02\). We also detected the time series for membrane potentials by applying periodical forcing currents, and the results are shown in Figs. 6 and 7.
Comparing the results in Figs. 6 and 7, it finds that multiple modes of electrical activity emerge in the time series for membrane potentials by applying external forcing current with periodical type. Bursting states are observed when larger amplitude and lower angular frequency in the periodical forcing current are considered as well. Furthermore, the rhythm of the output time series is mainly dominated by the angular frequency when lower intensity of forcing current is used and bursting states are more likely to occur under higher intensity of periodical forcing. Indeed, the output series for membrane potentials of neuron can be regulated by the external forcing current but seldom keep pace with the periodical forcing with the same rhythm because the neuron can give appropriate response to external forcing due to self-adaption and nonlinearity. Extensive numerical studies confirm that the neuron model can reproduce multiple modes of electrical activity in a wide parameter region because three new controllable parameters are introduced into the new neuron model. Furthermore, the chaotic parameter regions could also be detected by calculating the Lyapunov exponent spectrum beyond zero, and the distribution for the largest Lyapunov exponents in the parameter region could be calculated in Fig. 8.
It is found in Fig. 8 that positive Lyapunov exponent could be detected; thus, the improved model still produces chaotic electrical mode, and as a result, this modified neuron model can give appropriate response to external forcing and selects appropriate electrical mode if possible. Extensive numerical results for time series analysis can verify the chaotic properties by selecting gains in the chaotic region as shown in Fig. 8.
As a result, this model could be useful for further studies about transition of discharge modes in electrical activity. Finally, it is interesting to verify the effectiveness of the designed neuronal circuit by using PSpice. The neuron circuit is plotted in Fig. 9.
Circuit diagram for the four-variable neuron model (a) and additive magnet flux current \(I\phi =- \rho ( \phi )x (k_{1}=1)\) (b). X_Analog denotes the output variable for membrane potential, and \( I_\mathrm{ext}\) represents the external forcing current. The circuit is designed for Eq. (1) by selecting parameters and gains as \(k_{1}=1, k_{2}=0.5, \alpha =0.1, \beta =0.02\)
In the experiments, the external forcing current is selected to drive this circuit, and the voltage outputs associated with membrane potentials of neuron are monitored, and the time series for membrane potentials are shown in Fig. 10.
It is found in Fig. 10 that bursting states can be generated from the neuronal circuit by applying external forcing current as \(I_\mathrm{ext} =300\,\upmu \hbox {A}\). Other modes of electrical activity can still be generated by changing the external forcing current carefully. As a result, the neuronal model could be effective for further study about transition of electrical activity induced by time-varying forcing currents, noise and other bifurcation parameters.
In a summary, the presented neuron model in this paper can be effective to reproduce the main properties of electric activities of neurons; particularly, the effect of electromagnetic induction is considered by introducing the additive variable as magnetic flux; furthermore, the feedback from magnetic flux on membrane potential is realized by using memristor. Based on the proposed neuron models, different topologies of networks have been constructed to study the synchronization problems and spatial pattern selection so that the phase transition for collective behaviors could be discerned [48, 49]. It is observed that some specific spatial patterns such as target wave and spiral wave can emerge in the two-dimensional neuronal network [48, 49]. These target waves can be induced by local periodical forcing, or external forcing with diversity, and also heterogeneity as well; furthermore, spiral wave can be developed from the broken target waves. The potential mechanism for the emergence of spatial pattern keeps open; it could be associated with self-adaption and memory effect. As a result, it is believed that autapse connection could make a neuron become self-adaptive by imposing time-delayed feedback, and the local pacing from autapse in the network can develop stable target wave or pulse, so that the collective behaviors of network could be regulated [38, 40].
Surely, it is challengeable to update the neuron models so that the effect of electromagnetic induction and radiation on neuronal tissue could be considered, and as a result, our proposed model may work well because the magnetic flux effect is considered in feasible way. The improved neuron model can response to external forcing with different modes in electrical activity in a large parameter and gain region, it indicates some self-adaption in neuron, and it could be useful for further study on the effect of electromagnetic radiation.
4 Open problems and further suggestion
Finally, it is interesting to discuss some possible open problems on the relevant neuron model when ion channels are considered. For the Hodgkin–Huxley (HH) [11, 13] neuron model, it could also be improved by adding the variable as magnetic flux, and the similar dynamical equations should be described as follows
where the variable \(\phi \) describes the magnetic flux across membrane, V denotes the membrane potential of neuron and \(I_\mathrm{ext}\) is the external forcing current, m, n, h are gate variables to measure open probability for ion channels. The term \(\rho ( \phi \)) still represents the memory conductance of a magnetic flux-controlled memristor that the effect of electromagnetic induction on membrane potential is calculated by magnetic flux coupled with membrane potential which is realized by memristor with appropriate parameters \(k, k_{1},k_{2}\). Extensive investigation could be carried out on the improved model shown in Eq. (3) if the effect of ion channels, channel noise should be considered. Surely, appropriate networks could be designed to study relevant works about collective behaviors of neuronal network, and forthcoming biological experiments should be verified to confirm the exact parameter region for parameters \(k, k_{1},k_{2}\). Furthermore, autapse connection could also be considered on this model, and these interesting problems keep open and are expected to be investigated by readers in this field. The mentioned results in this paper are expected to propose an interesting question and give possible guidance for computational neuroscience.
5 Conclusions
Based on the previous HR neuron model, the effect of electromagnetic induction in the biological system is considered in an improved neuron model by introducing additive variable as magnetic flux across membrane, and the dynamical property of electric activity is also verified in the PSpice tool by generating bursting series from the circuit. Most of the previous neuron models can describe the properties of membrane potential and the response to external forcing current, noise and channel noise well, while the effect of electromagnetic induction is out of the consideration. In fact, the fluctuation in membrane potential and signal propagation in neuronal system can generate induced-electrical field and additive current in the media due to electromagnetic induction. As a result, the membrane potential of neuron can be adjusted slightly by induction field and induced current associated with variation of magnetic flux. Our proposed model still can reproduce the electrical activity with multiple modes, and also the electromagnetic induction is considered by including terms of magnetic flux into the dynamical equation. In addition, the new presented neuron model can expand the parameter region to generate complex modes of electrical activity. For further studies, the external electromagnetic radiation on neuronal activity could be investigated by using this model. Furthermore, similar scheme is used for Hodgkin–Huxley neuron model so that the effect of ion channels could be considered as well. We wish the presented model could be verified by further biological experiments. In addition, the proposed model could also be used to detect the electromagnetic radiation and signal processing like sensors. Furthermore, the network of this improved model could also be used to investigate the collective behaviors of neurons of brain and central nervous system, and the potential mechanism for disease induced by electromagnetic radiation could be explained.
References
Buschman, T.L., Denovellis, E.L., Diogo, C., et al.: Synchronous oscillatory neural ensembles for rules in the prefrontal cortex. Neuron 76, 838–846 (2012)
Wig, G.S., Schlaggar, B.L., Petersen, S.E.: Concepts and principles in the analysis of brain networks. Ann. N.Y. Acad. Sci. 1224, 126–146 (2011)
Seely, J., Crotty, P.: Optimization of the leak conductance in the squid giant axon. Phys. Rev. E 82, 021906 (2010)
Postnov, D.E., Koreshkov, R.N., Brazhe, N.A., et al.: Dynamical patterns of calcium signaling in a functional model of neuron–astrocyte networks. J. Biol. Phys. 35, 425–445 (2009)
Volman, V., Bazhenov, M., Sejnowski, T.J.: Computational models of neuron–astrocyte interaction in epilepsy. Front. Comput. Neurosci. 6, 58 (2012)
Volman, V., Perc, M., Bazhenov, M.: Gap junctions and epileptic seizures-two sides of the same coin? PLoS One 6, e20572 (2011)
Ozer, M., Ekmekci, N.H.: Effect of channel noise on the time-course of recovery from inactivation of sodium channels. Phys. Lett. A 338, 150–154 (2005)
Barthélemy, M.: Spatial networks. Phys. Rep. 499, 1–101 (2011)
Herz, A.V.M., Gollisch, T., Machens, C.K., et al.: Modeling single-neuron dynamics and computations: a balance of detail and abstraction. Science 314, 80–85 (2006)
Gerstner, W., Kistler, W.M.: Spiking Neuron Models Single Neurons, Populations, Plasticity. Cambridge University Press, Cambridge (2002)
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952)
Morris, C., Lecar, H.: Voltage oscillations in the barnacle giant muscle fiber. Biophys. J. 35, 193–213 (1981)
Izhikevich, E.M.: Which model to use for cortical spiking neurons? IEEE Trans. Neural Netw. 15, 1063–1070 (2004)
Ibarz, B., Casado, J.M., Sanjuán, M.A.F.: Map-based models in neuronal dynamics. Phys. Rep. 501, 1–74 (2011)
Perc, M., Marhl, M.: Amplification of information transfer in excitable systems that reside in a steady state near a bifurcation point to complex oscillatory behavior. Phys. Rev. E 71, 026229 (2005)
Hindmarsh, J.L., Rose, R.M.: A model of the nerve impulse using two first-order differential equations. Nature (London) 296, 162–164 (1982)
Gu, H.G., Pan, B.B., Chen, G.R.: Biological experimental demonstration of bifurcations from bursting to spiking predicted by theoretical models. Nonlinear Dyn. 78, 391–407 (2014)
Storace, M., Linaro, D., de Lange, E.: The Hindmarsh–Rose neuron model: bifurcation analysis and piecewiselinear approximations. Chaos 18, 033128 (2008)
Pinto, R.D., Varona, P., Volkovskii, A., et al.: Synchronous behavior of two coupled electronic neurons. Phys. Rev. E 62, 2644 (2000)
Moujahid, A., d’Anjou, A., Torrealdea, F.J., et al.: Efficient synchronization of structurally adaptive coupled Hindmarsh–Rose neurons. Chaos Solitons Fractals 44, 929–933 (2011)
Selverston, A., Rabinovich, M., Abarbanel, H.D., et al.: Reliable circuits for irregular neurons: a dynamical approach to understanding central pattern generators. J. Physiol. 94, 357 (2000)
Rech, P.C.: Dynamics in the parameter space of a neuron model. Chin. Phys. Lett. 29, 060506 (2012)
Gu, H.G., Pan, B.B.: A four-dimensional neuronal model to describe the complex nonlinear dynamics observed in the firing patterns of a sciatic nerve chronic constriction injury model. Nonlinear Dyn. 81, 2107–2126 (2015)
Uzun, R., Ozer, M., Perc, M.: Can scale-freeness offset delayed signal detection in neuronal networks? EPL 105, 60002 (2014)
Ozer, M., Uzuntarla, M., Perc, M., et al.: Spike latency and jitter of neuronal membrane patches with stochastic Hodgkin–Huxley channels. J. Theor. Biol. 261, 83–92 (2009)
Aggarwal, A., Kumar, M., Rawat, T.K., et al.: Optimal design of 2-D FIR filters with quadrantally symmetric properties using fractional derivative constraints. Circ. Syst. Signal Process. (2016). doi:10.1007/s00034-016-0283-x
Kumar, M., Rawat, T.K.: Fractional order digital differentiator design based on power function and least-squares. Int. J. Electron. (2016). doi:10.1080/00207217.2016.1138520
Wang, S.T., Wang, W., Liu, F.: Propagation of firing rate in a feed-forward neuronal network. Phys. Rev. Lett. 96, 018103 (2006)
Suffczynski, p, Kalitzina, S., Lopes Da Silva, F.H.: Dynamics of non-convulsive epileptic phenomena modeled by a bistable neuronal network. Neuroscience 126, 467–484 (2004)
Cullheim, S., Thams, S.: The microglial networks of the brain and their role in neuronal network plasticity after lesion. Brain Res. Rev. 55, 89–96 (2007)
Wang, R., Zhang, Z.Z., Ma, J., et al.: Spectral properties of the temporal evolution of brain network structure. Chaos 25, 123112 (2015)
Bao, B.C., Liu, Z., Xu, J.P.: Steady periodic memristor oscillator with transient chaotic behaviors. Electron. Lett. 46, 228–230 (2010)
Muthuswamy, B.: Implementing memristor based chaotic circuits. Int. J. Bifurc. Chaos 20, 1335–1350 (2010)
Li, Q.D., Zeng, H.Z., Li, J.: Hyperchaos in a 4D memristive circuit with infinitely many stable equilibria. Nonlinear Dyn. 79, 2295–2308 (2015)
Ma, J., Tang, J.: A review for dynamics of collective behaviors of network of neurons. Sci. China Technol. Sci. 58, 2038–2045 (2015)
Song, X.L., Jin, W.Y., Ma, J.: Energy dependence on the electric activities of a neuron. Chin. Phys. B 24, 128710 (2015)
Song, X.L., Wang, C.N., Ma, J., et al.: Transition of electric activity of neurons induced by chemical and electric autapses. Sci. China Technol. Sci. 58, 1007–1014 (2015)
Qin, H.X., Ma, J., Jin, W.Y., et al.: Dynamics of electrical activities in neuron and neurons of network induced by autapses. Sci. China Technol. Sci. 57, 936–946 (2014)
Yılmaz, E., Baysal, V., Perc, M., et al.: Enhancement of pacemaker induced stochastic resonance by an autapse in a scale-free neuronal network. Sci. China Technol. Sci. 59, 364–370 (2016)
Qin, H.X., Ma, J., Wang, C.N., et al.: Autapse-induced target wave, spiral wave in regular network of neurons. Sci. China Phys. Mech. Astron. 57, 1918–1926 (2014)
Ren, G.D., Wu, G., Ma, J.: Simulation of electric activity of neuron by setting up a reliable neuronal circuit driven by electric autapse. Acta Phys. Sin. 64, 058702 (2015). In Chinese
Ma, J., Chen, Z.Q., Wang, Z.L., et al.: A four-wing hyper-chaotic attractor generated from a 4-D memristive system with a line equilibrium. Nonlinear Dyn. 81, 1275–1288 (2015)
Chen, M., Li, M.Y., Yu, Q., et al.: Dynamics of self-excited attractors and hidden attractors in generalized memristor-based Chua’s circuit. Nonlinear Dyn. 81, 215–226 (2015)
Pei, J.S., Wright, J.P., Todd, M.D., et al.: Understanding memristors and memcapacitors in engineering mechanics applications. Nonlinear Dyn. 80, 457–489 (2015)
Li, Q.D., Tang, S., Zeng, H.Z., et al.: On hyperchaos in a small memristive neural network. Nonlinear Dyn. 78, 1087–1099 (2014)
Pham, V.T., Jafari, S., Vaidyanathan, S., et al.: A novel memristive neural network with hidden attractors and its circuitry implementation. Sci. China Technol. Sci. 59, 358–363 (2016)
Wu, H.G., Bao, B.C., Liu, Z., et al.: Chaotic and periodic bursting phenomena in a memristive Wien-bridge oscillator. Nonlinear Dyn. 83, 893–903 (2016)
Ma, J., Tang, J., Zhang, A.H., et al.: Robustness and breakup of the spiral wave in a two-dimensional lattice network of neurons. Sci. China Phys. Mech. Astron. 53, 672–679 (2010)
Ma, J., Wu, Y., Wu, N.J., et al.: Detection of ordered wave in the networks of neurons with changeable connection. Sci. China Phys. Mech. Astron. 56, 952–959 (2013)
Acknowledgments
This work was supported by the National Natural Science Foundation of China under Grant Nos. 11265008 (MJ) and 11365014 (WCN).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lv, M., Wang, C., Ren, G. et al. Model of electrical activity in a neuron under magnetic flow effect. Nonlinear Dyn 85, 1479–1490 (2016). https://doi.org/10.1007/s11071-016-2773-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-016-2773-6