Abstract
The influence of noise on the complete synchronization in a Morris–Lecar (ML) model neuronal system is studied in this work. Two individual ML neurons with different initial conditions can discharge completely synchronously when the noise intensity is large enough, and for a smaller reversal potential (V Ca), the uncoupled neuronal system could be induced to a complete synchronization state under smaller noise intensity. Two coupled ML neurons could be synchronized under very small noise intensity even in the case of weak coupling, the synchronization characteristics of the two coupled neurons are discussed by analyzing the Similarity Function (S(0)) of their membrane potentials, which proves that noise can promote the complete synchronization. The critical noise intensity (D j ) to induce complete synchronization in coupled ML neurons will decrease with the increase of V Ca. This result is helpful to study the synchronization and the code of a neural system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Synchronization is a typical form of group motion rhythm, which means the neurons of a system discharge at the same time, or their discharge rhythms have some kind of relationship [1, 2]. Neural synchrony activities are found not only among coupled neuron groups in the same brain region, but also among uncoupled neuron groups in the same brain region or among different cortical areas; moreover, it can cross over two hemispheres of the brain [3]. Synchronization processes are crucially important for the neural system; and well-coordinated synchrony within and between neuronal populations appears to play an important role in neuronal signaling and information processing [4]. There are many neuroscientists who have been mainly concentrated on the coupling system topology and stability, as well as the parameters for the coupled effects [5–9].
Noise is ubiquitous in linear system and electronic engineering applications; in most cases, noise is considered harmful, especially in signal measurement [10–12], but the phenomenon of Stochastic Resonance (SR) makes us recognize that noise is also beneficial, especially, the noise induced synchronization [13–16]. In different ways, the noise could influence the synchronization of a nonlinear system. The known approaches are noise induced synchronization and noise enhanced synchronization. These are very special aspects that noise could be utilized for a specific object.
Neurons are living in an environment that may be full of noise. The noise comes either from outside or from the inside of nerve cells, for example, from the temperature changes outside, neuronal interactions, various ion fluctuations, and so on [17]. Wang et al. [18] studied the properties of a chemical synaptic coupled neuronal system with exterior noise of a stochastic resonance and synchronization dynamics, the results show that the coupling term can enhance the system’s autonomous stochastic resonance, and when the coupling strength is big enough, the synchronization could be induced. Casado et al. [19, 20] have reported the phase synchronization of two electrically coupled Hodgkin–Huxley (HH) model neurons. They found that the internal noise makes two neurons discharge synchronously, including the discharge frequency and phase synchronization. Generalized synchronization induced by noise or parameter mismatch in two Hindmarsh–Rose (HR) model neurons is studied in [21], which illustrated that the two neurons can get into generalized synchronization by introducing noise and changing another parameter in one of the neurons. The relation between the noise induced synchronization and autonomous stochastic resonance was also found in the coupled mismatching excitable ML neurons with noise intensity as the control parameters [22].
The influence of the complete synchronization affected by noise has attracted particular attention extensively. Many independent excitatory and inhibitory synaptic currents are usually approximated by a Gaussian distribution. Zambrano et al. [23] studied the synchronization of uncoupled excitable systems with common noise both numerically and experimentally. They considered two identical FitzHugh–Nagumo (FHN) systems which display both spiking and non-spiking behaviors in chaotic or periodic regimes. An electronic circuit provides a laboratory implementation of these dynamics. Synchronization is tested with both white and colored noise, showing that colored noise is more effective in inducing synchronization of the system.
The ML neuron model is a model for the electrical activity in the barnacle muscle fiber; it is a simplified version of Hodgkin–Huxley neuron model for describing the discharge and the refractory properties of real neurons [24]. This model contains a Calcium current generating a fast action potential and a delayed potassium current, at the same time, a leakage current is considered to maintain a constant potential at the rest state. Dynamics of the reduced ML neuron is governed by the following differential equations:

where

and t is the time, V is the membrane action potential of the ML neuron model, W is the probability of potassium channel activation, I is a slow variable which could result in rich firing patterns in a single ML neuron model. The related parameters are: g Ca=1.2, g K=2.0, g L =0.5, V K=−1.1, V L =−0.5, V a =−0.01, V b =0.15, V c =0.1, V d =0.05, μ=0.005. Besides the noise intensity D, the reversal potential V Ca also be used as another control parameter.
2 Noise induced complete synchronization
Systems with two uncoupled ML neurons are given as follows:

Here indices 1 and 2 mark separate neurons with different initial conditions. The noises ξ V (t) and ξ I (t) are added to the variable V and variable I of the ML neuron model, respectively. In order to verify the degree of synchronization of the two neurons’ system, one real-time error of response of the two coupled neurons is defined as:
In this work, we propose discussing the synchronous behavior of the system governed by Eq. (4) in terms of the average error 〈e〉, additionally, with the average Maximal Lyapunov Exponent 〈λ 1〉, and the average Interspike Interval 〈ISI〉.
For the two uncoupled ML neurons (shown in Eq. (4)) with different initial conditions and two fixed reversal potentials V Ca, from the view of mathematics, with the passage of time, the behavior of the two neurons does not become the same. However, if the two identical systems use the same noise stimulation, the two nonlinear systems will achieve complete synchronization when the noise intensity is large enough after a period of time, i.e., noise can induce synchronization.
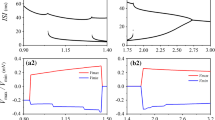
From Fig. 1(a), we can find that the exponent 〈λ 1〉 will change from positive to negative when the noise intensity is large enough; this means that the two uncoupled ML neurons can get completely synchronized. At the same time, compared with V, I is more sensitive to noise because the cross-noise intensity (the value of 〈λ 1〉 from positive to negative) of I is much less than that for V, therefore, white noise is only added to I in the following. The reversal potential V Ca is also a sensitive parameter, which significantly affects the system discharge patterns. Figures 1(b), (c), and (d) show the joint effects of the dynamic behavior with V ca and the white noise. Figure 1(b) indicates that a smaller V Ca yields a smaller 〈λ 1〉 in a certain D, which implies that it is more likely that in an uncoupled system with smaller intensity, noise can induce complete synchronization; moreover, this could be proved by the error 〈e〉 shown in Fig. 1(c). From Fig. 1(d), we can find that noise has a quite obvious relationship with the neuronal discharge frequency; generally, the average interspike interval of a sequence decreased with the increase of noise intensity. What is interesting is that a larger V Ca corresponds to a smaller 〈ISI〉 at the same noise intensity D, however, the conclusion to be drawn is that D is one of the main factors for the discharge frequency, and the strong noise could induce more discharges and activities.
Noise induced complete synchronization: (a) the average Maximum Lyapunov Exponent 〈λ 1〉 vs noise intensity D with noise in the variables V and I under V Ca=0.925; (b) 〈λ 1〉 vs noise intensity D under V Ca=0.925 and V Ca=0.6; (c) the average synchronization error 〈e〉 vs noise intensity D under V Ca=0.925 and V Ca=0.6; (d) the average Interspike Interval 〈ISI〉 vs noise intensity D under V Ca=0.925 and V Ca=0.6
3 Noise enhanced complete synchronization
In this section, we focus on the effect of noise on a system with two coupled ML model neurons, and the coupling scheme is shown in the last part of the first equation of Eq. (6):

Here C is the coupling strength, the other variable being the same as in Eq. (4). In this work, a statistical index, called Similarity Function S(0), is introduced to test complete synchronization. It is given by
where 〈⋅〉 represents the signal average value with respect to time. A smaller S(0) shows greater correlation between two signals. From the standpoint of synchronization, it means that the two coupled neurons’ phase synchronization is strengthened. The value S(0)=0 refers to the complete synchronization for the coupled system under identical parameters with different initial conditions.
Under the absence of noise and with the coupling strength C=0.01, the coupled ML neurons described in Eq. (6) could not be synchronized. Compared with Fig. 1, we can find that there is even a smaller noise intensity D for the system synchronization, shown in Fig. 2(a), due to the coupling with noise ξ(t). A smaller V Ca results in a bigger exponent 〈λ 1〉; this situation is contrary to the case in Fig. 1(b), indicating that the two ML neurons will be synchronized under weak noise and weak coupling, as shown in Fig. 2(b). The results shown in Fig. 2(c) are to examine the influence of synchronization by S(0), setting V Ca=0.925 and with C located in [0,0.065]. The coupled neuronal system could not be synchronized when D=0, but the majority of the section shows complete synchronization for D=10−6, even in the part of above 0.65, which proves that noise has a promoting effect for the synchronization of coupled neurons. We find that the minimum D (named the critical noise intensity D j ) which could induce complete synchronization under different weak coupling strength C shows different situations with the changing of V Ca under noise intensity D=1.0×10−6, seen from Fig. 2(d), while with the value of V Ca≤0.625, the smaller C corresponds to a larger D j . However, the situation is just the opposite when V Ca>0.625; this demonstrates that the activity of ML neurons in the strong coupling system will be reduced when V Ca increases to a certain extent, the stronger noise is needed to achieve complete synchronization.
Noise enhanced complete synchronization: (a) the average Maximum Lyapunov Exponent 〈λ 1〉 vs noise intensity D under V Ca=0.925 and V Ca=0.6 with C=0.01; (b) the average synchronization error 〈e〉 vs noise intensity D under V Ca=0.925 with C=0.01; (c) Similarity Index S(0) vs coupling strength C under noise intensity D=0 and D=1.0×10−6; (d) the minimum value of noise intensity (critical noise intensity) D j in ML neurons cause complete synchronization for different V Ca
4 Conclusions
In summary, we have studied the effects of noise to the complete synchronization of ML neurons. The investigation indicates that the slow variable I is more sensitive to noise. Two uncoupled ML neurons can discharge completely synchronously when the noise intensity is large enough. The smaller the V Ca, the more it is likely that the weak noise can induce complete synchronization. A bigger noise can induce more discharge spikes. For a coupled neuronal system, even in the case of weak coupling, the two ML neurons will be synchronized under very small noise, based on the study of the Similarity Function S(0), which proves that noise has a promoting effect to the synchronization of coupled neurons, and there are different situations for critical noise intensity D j with the change of V Ca under different coupling strength C. Anyhow, the results prove that noise can enhance the complete synchronization in coupled ML neurons with the cooperation of other factors.
References
Peter, D., Abbott, L.F.: Theoretical Neuroscience Computational and Mathematical Modeling of Neural Systems. MIT Press, Cambridge (2001)
Golomb, D., Rinzel, J.: Clustering in globally inhibitory neurons. Physica D 72, 259–284 (1994)
Gray, C.M., Konig, P., Engel, A.K., Singer, W.: Oscillatory response in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338, 334–337 (1989)
Popovych, O.V., Hauptmann, C., Tass, P.A.: Control of neuronal synchrony by nonlinear delayed feedback. Biol. Cybern. 95, 69–85 (2006)
Jin, W.Y., Feng, R.C., Rui, Z.Y., Zhang, A.H.: Effects of time delay on chaotic neuronal discharges. Math. Comput. Appl. 15, 840–845 (2010)
Wang, H.X., Lu, Q.S., Wang, Q.Y.: Complete synchronization in coupled chaotic HR neurons with symmetric coupling schemes. Chin. Phys. Lett. 22, 2173–2175 (2005)
Dhamala, M., Jirsa, V.K., Ding, M.Z.: Enhancement of neural synchrony by time delay. Phys. Rev. Lett. 92, 074104 (2004)
Li, F., Liu, Q.R., Guo, H.Y., Zhao, Y.H., Tang, J., Ma, J.: Simulating the electric activity of FitzHugh–Nagumo neuron by using Josephson junction model. Nonlinear Dyn. 69, 2169–2179 (2012)
Dahasert, N., Öztürk, İ., Kiliç, R.: Experimental realizations of the HR neuron model with programmable hardware and synchronization applications. Nonlinear Dyn. 70, 2343–2358 (2012)
Leigh, J.R.: Functional Analysis and Linear Control Theory. Dover, New York (2007)
Ge, Y.Q.: Analog Electronic Technology. Science Press, Beijing (2005) (in Chinese)
Ivancevic, T., Jain, L., Pattison, J., Hariz, A.: Nonlinear dynamics and chaos methods in neurodynamics and complex data analysis. Nonlinear Dyn. 56, 23–44 (2009)
Benzi, R., Sutera, A., Vulpiani, A.: The mechanism of stochastic resonance. J. Phys. A, Math. Gen. 14, 453–457 (1981)
Benzi, R.: Stochastic resonance in climatic change. Tellus 34, 10–16 (1982)
Zhou, C.S., Kurths, J.: Noise-induced phase synchronization and synchronization transitions in chaotic oscillators. Phys. Rev. Lett. 88, 230602 (2002)
Zhou, C.S., Kurths, J., Kiss, I.Z., Hudson, J.L.: Noise-enhanced phase synchronization of chaotic oscillators. Phys. Rev. Lett. 89, 014101 (2002)
Shou, T.D.: Neurobiology. Higher Education Press, Beijing (2006) (in Chinese)
Wang, W., Perez, G., Cerdeira, H.A.: Dynamical behavior of the firings in a coupled neuronal system. Phys. Rev. E 47, 2893–2898 (1991)
Casado, J.M.: Synchronization of two Hodgkin–Huxley neurons due to internal noise. Phys. Lett. A 310, 400–406 (2003)
Casado, J.M., Baltanas, J.P.: Phase switching in a system of two noisy Hodgkin–Huxley neurons coupled by a diffusive interaction. Phys. Rev. E 68, 061917 (2003)
Wu, Y., Xu, J.X., He, D.H., David, J.D.: Generalized synchronization induced by noise and parameter mismatching in Hindmarsh–Rose neurons. Chaos Solitons Fractals 23, 1605–1611 (2005)
Postnov, D.E., Sosnovtseva, O.V., Han, S.K., Yim, T.G.: Stochastic synchronization of coupled coherence resonance oscillators. Int. J. Bifurc. Chaos 10, 2541 (2000)
Zambrano, S., Mariño, I.P., Seoane, M., et al.: Synchronization of uncoupled excitable systems induced by white and colored noise. New J. Phys. 12, 053040 (2010)
Morris, C., Lecar, H.: Voltage oscillations in the barnacle giant muscle. Biophys. J. 35, 193–213 (1981)
Acknowledgements
We are grateful for the support of the National Natural Science Foundation of China under Grant No. 11072099.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Jin, W. & Hu, C. The complete synchronization of Morris–Lecar neurons influenced by noise. Nonlinear Dyn 73, 1715–1719 (2013). https://doi.org/10.1007/s11071-013-0897-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-013-0897-5