Abstract
To explore the controlling factors of the hydrochemistry and hydraulic connections of groundwater in three mining districts, 45 water samples from three mining districts (Nos. 31, 32 and 42) in the Peigou Coal Mine are analyzed. The hydrogeochemical compositions of the three mining districts are analyzed, and the water–rock interaction and controlling factors of the hydrogeochemistry of the groundwater are discussed by examining the ionic composition (ion ratio) and using a factor analysis. Then, the hydraulic connection is determined by conducting cluster and discriminant analyses. Finally, a model that identifies the source of the water inrush of the three mining districts is provided. The results show the controlling factors of the hydrogeochemistry in the three mining districts. And it is speculated that the hydraulic connection between the Nos. 31 and 42 mining districts is higher than that between the Nos. 31 and 32 mining districts. It is hypothesized that there may be an obscured tunnel between the Nos. 31 and 42 mining districts, which is connected through the Fushanzhai fault. Based on the water source identification model of mining districts, the groundwater recharge relationship of three mining districts is inverted by analyzing the causes of misjudgment and comparing with groundwater pulse in different years. The proposed method provides a new idea for correctly recognizing the groundwater circulation conditions under the influence of mining.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Inrush events in mines have always been a safety concern. After the inflow of water into a mine, there are substantial financial loss and repercussions (LaMoreaux et al. 2014; Sammarco and Eng 1986; Gui et al. 2017; Sun et al. 2017a; Odintsev and Miletenko 2015; Wu et al. 2015). Hydrogeochemistry has an important role in studies on water management in mines. The most typical application of hydrogeochemistry is to identify the source of the water inrush (Dong et al. 2012; Sun 2014; Sun et al. 2016, 2017b; Gurarslan and Karahan 2015; Wang et al. 2017a). The basis of identifying the source of the water inrush is to determine the factors that control the hydrogeochemistry because the primary hydrogeochemical composition of groundwater in the runoff process is based on contact with minerals in the surrounding rocks (Helstrup et al. 2007; Sun 2017; Chen and Gui 2017; Li et al. 2018). Therefore, the lack of understanding of the mineral composition of the aquifer rocks and water–rock interaction in examining the factors that control the hydrogeochemistry will obviously affect identifying the source of the water inrush (Ismail et al. 2016; Wang et al. 2017b; Qaisar et al. 2018). This study therefore examines the water–rock interaction and how this interaction affects the hydrogeochemistry of the groundwater, as well as the characteristics of the source and factors that control the hydrogeochemical composition and thereby provide a better understanding for the establishment of a model that identifies the source of water inrush (Gao et al. 2018; Drollette et al. 2015; Lei et al. 2014). For coal mines that have complex hydrogeological conditions, determining the hydraulic connection between different mining districts in advance can contribute to the prevention of inrush events (Gui and Lin 2016; Qian et al. 2018). Pumping tests are usually conducted to determine the hydraulic connectivity. However, with the rapid developments in hydrogeochemical models in recent years, they have been favored by researchers because they can solve simple to complex problems, and can be used for site-specific assessments or predict the behavior of groundwater systems or design issues. These models can be ultimately used to improve the understanding of the relationship between hydrologic transport and biogeochemical reactions. In this case, a hydrogeochemical model can be used to examine the strength of the hydraulic connections between different regions by comparing the impacts of water–rock interaction on the groundwater in the different regions.
In this paper, 45 water samples are taken from three mining districts—Nos. 31, 32 and 42, in the Peigou Coal Mine in Henan, China, for analysis. Then, a Gibbs diagram is drawn for the ion analysis. In addition, examining the ionic composition (ion ratios) and carrying out factor and cluster analyses are done to determine a hydrogeochemical method that would provide details on the hydrogeochemical composition of the groundwater in the three mining districts. Moreover, the controlling factors of the hydrogeochemistry of the three mining districts are discussed. Finally, the hydraulic connections among the three mining districts are determined and a model that identifies the sources of water inrush for the different mining districts is provided. This study has great significance for examining the changes in the hydrogeochemical composition of the water found in the Peigou Coal Mine and acting as a guideline to prevent and control water inrushes.
2 Background of research area
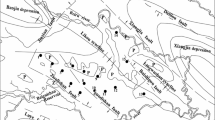
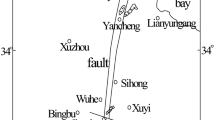
The Peigou Coal Mine is located in Xinmi City of the Henan Province in China (Fig. 1a). The mine is situated 35 km to Zhengzhou City in the northeast and 8.5 km to Xinmi City in the northwest. The Peigou Coal Mine is southeast of the Xinmi Coalfield and considered to be a part of this coalmine. The area consists of a transitional terrain from hilly to an area of plains. A continental semiarid climate is found in the Peigou Coal Mine area with four distinct seasons. The summers are hot and rainy, while the winters are cold and dry. The annual average temperature is 14.3 °C, with an annual average precipitation of 658.4 mm that mostly falls between June and September. The sedimentary strata consist of (from old to new) Archean, Proterozoic, Cambrian, Ordovician, Carboniferous, Permian, Mesozoic (Triassic) and Cenozoic (Tertiary).
The Xinmi Coalfield mainly consists of faults, mostly in the approximate east–west direction, with a strata formation also approximately in the east–west direction. The strata inclination toward the south is a monocline between 4° and 26° and in general 8° and 17°. The mine contains three main aquifer formations in descending order: Permian strata of fissured sandstone saturated with water on the roof and coal seam floor in the No. II1 coal seam, four aquifers (L5 to L8) in the Taiyuan formation (Carboniferous) and four aquifers (L1 to L4) in Ordovician limestone (which also includes the Taiyuan formation). The fault structure of the Peigou Coal Mine is well developed, especially near the east–west strike. The primary mining districts in the study area are Nos. 31, 32 and 42 as shown in Fig. 1b. The relationship profile of the main aquifers in the mining district is shown in Fig. 2. The main rock types of aquifer are fine sandstone and medium sandstone with abundant water reserves. Among them, the Nos. 31 and 42 mining districts are separated by the Fushanzhai fault, which is located in the southern part of the minefield. The strike of the fault is approximately along the east–west axis with a drop of 50–360 m and a dip of 70°. This fault greatly impacts the mining of the coal seams in this area.
3 Hydrogeochemical composition
In the Peigou Coal Mine, water samples were taken from an Ordovician limestone aquifer based on the mining districts, and the mean value (MV), standard deviation (SD) and coefficient of variation (CV) of the water samples were determined (Table 1). As shown in Fig. 1b, three mining districts were sampled, including 11 water samples in the No. 31 mining district, 29 water samples in the No. 32 mining district and five water samples in the No. 42 mining district. It was found that the mass concentration of cations in the water samples taken from the Nos. 31 and 42 mining districts is in the order of K+ + Na+ > calcium ions (Ca2+) > magnesium ions (Mg2+) and that in the water samples taken from the No. 32 mining district is in the order of Ca2+ > K+ + Na+ > Mg2+. Moreover, the mass concentration of anions in the water samples taken from the Nos. 31, 32 and 42 mining districts is in the order of bicarbonate (HCO3−) > sulfate ions (SO42−) > chloride ions (Cl−). The coefficient of variation denotes the extent of the variability in the groundwater data. A larger coefficient of variation means more variability in the hydrogeochemical composition and more fluctuations in the variability.
Ion concentrations with a coefficient of variation greater than 0.5 include K+ + Na+, Mg2+ and Ca2+ in the No. 31 mining district; K+ + Na+ in the No. 32 mining district; and K+ + Na+, Mg2+ and Ca2+ in the No. 42 mining district. These show that K+ + Na+, Mg2+ and Ca2+ can be used as the main chemical ingredients to examine the changes in the hydrogeochemical composition of the groundwater in the mining districts. Therefore, it is only necessary to discuss the dynamic regime changes for K+ + Na+, Mg2+ and Ca2+ in the three mining districts while neglecting the changes in the other chemical ingredients. Ion change in hydrogeochemistry was a consequence of a pulse in groundwater flow, and the pulse delays and attenuations were a consequence of limited hydraulic connectivity between the mining districts. According to the previous date from Peigou Coal Mine (Fig. 3), the raise in K+ + Na+ average concentration and drop in Mg2+ and Ca2+ average concentrations in the Nos. 31 and 32 mining districts in 2011 was observed. The fluctuation pulse of the No. 32 mining district lasted only 2 years, and that of the No. 31 mining district lasted 4–5 years. The ionic changes in the two mining districts were similar to each other to some extent. The fluctuation pulses of ion change in the Nos. 31 and 42 mining districts are basically the same, and only the phenomenon of pulse delay occurs in some time intervals. The pulses in the Nos. 31 and 42 mining districts initially delayed but later simultaneously real homogenous pulses are likely to imply a change in hydraulic connectivity. The raise/drop pulse in the No. 42 mining district K+ + Na+, Mg2+ and Ca2+ average concentrations was bigger than the subsequent pulse in the Nos. 31 and 32 mining districts. Figure 3 shows the image of regional hydrogeochemical pulses, from which it can be concluded that the pulses originated from the No. 42 mining district and then transferred to the Nos. 31 and 32 mining districts with delay and pulse loss.
As shown in Fig. 4, the cation concentration of K+ + Na+ and anion concentration of HCO3− are higher in the water samples taken from the No. 31 mining district. The distribution of the water sampled points in the No. 32 mining district is mostly concentrated in the No. 5 zone. The concentration of Ca2+ and HCO3− in the water samples is relatively high. Most of the water samples in the No. 42 mining district are taken from the No. 3 zone, which have a high cation concentration of K+ + Na+ and high anion concentration of HCO3−. It is obvious from the comparative analysis in Table 1 that the groundwater in the Peigou Coal Mine is generally alkaline, but there is little difference in the amount of total dissolved solids (TDS) and pH in each mining district. A comparison of the primary ion concentrations shows that the primary cation and anion concentrations in the groundwater of the Nos. 31 and 42 mining districts are comparable, but the concentration of K+ + Na+ and HCO3− in the No. 32 mining district is significantly different from those in the other two mining districts. Moreover, the hydrogeochemical composition of the groundwater in the No. 31 mining district mainly consists of Ca–Mg–HCO3, Na–K–HCO3–Cl and Na–HCO3, in the No. 32 mining district is mainly Ca–Mg–HCO3 and in the No. 42 mining district is primarily Ca–Mg–HCO3, Na–K–HCO3–Cl and Na–HCO3. In summary, the groundwater in the Nos. 31 and 42 mining districts has a similar hydrogeochemical composition.
4 Water–rock interaction
4.1 Gibbs diagram for ion analysis
The Gibbs diagram has been widely used in the study of natural surface water but has also been applied in studies of the sources that contribute to the hydrogeochemical composition of groundwater. The Gibbs diagram is used here to establish the relationship between the groundwater composition and the characteristics of the source that controls the hydrogeochemistry of the groundwater by considering three conditions: evaporation, rock and precipitation dominance. Figure 5 shows the Gibbs diagram of the hydrogeochemical composition of the groundwater of the three mining districts, and it can be observed that almost all the water samples are taken from a rock dominance area, which indicates that rock primarily characterizes the hydrogeochemical composition of the groundwater in the three mining districts.
4.2 Ionic composition ratio
The ion composition ratio can be used to examine the hydrogeochemical composition and ion sources. The source of Cl− is mainly from the dissolution of rock salt with a relatively constant concentration in the groundwater (see Eq. 1). Generally, Na+/Cl− (sodium chloride coefficient) can be used to determine the source of the sodium ions (Na+). Since potassium (K) is a nutrient element and easily absorbed by plants, the concentration of K+ in groundwater is very low. Therefore, the source of Na+ can approximately indicate the concentration of K+ + Na+/Cl−. With increases in the TDS, the concentration of K+ + Na+/Cl− in the water samples is higher than 1 as shown in Fig. 6a, thus indicating that the dissolution of rock salt is not the only source of the Na+ in the groundwater in the Peigou Coal Mine. More Na+ probably originates from the weathering of silicate minerals. This is further confirmed in Fig. 6b, since the weathering of silicate minerals not only causes an increase in Na+ relative to Cl−, but also increases the concentration of HCO3−. Moreover, the chloro-alkaline indices (Cl–Na+)/Cl− of the three mining districts are all less than 0, which indicates that there might be ion exchange during water–rock interaction, and is validated by Fig. 6c (see Eq. 2). All of the plotted ion compositions in the three mining districts are near the line of (Ca2+ + Mg2+–HCO3−–SO42−)/(K+ + Na+–Cl−) = 1, which indicates that ion exchange is an important process in all of the mining districts. (Ca2+ + Mg2+)/(SO42− + 0.5HCO3−) = 1, which indicates that the concentration of Ca2+ and Mg2+ in the groundwater is mainly derived from the dissolution of carbonate and sulfate (see Eqs. 3–6). Figure 6d shows that relatively few water samples in Peigou Coal Mine fall near the line (Ca2+ + Mg2+)/(SO42− + 0.5HCO3−) = 1 and its vicinity, which indicates that the concentration of Ca2+ and Mg2+ is not only from the dissolution of carbonate and sulfate in the groundwater. (Ca2+ + Mg2+)/(SO42− + 0.5HCO3−) in the No. 32 mining district is significantly greater than 1, thus indicating that desulfurization occurred (see Eq. 7). (Ca2+ + Mg2+)/SO42− = 1, which means that the concentration of Ca2+, Mg2+ and SO42− in the groundwater is mainly derived from the dissolution of sulfate (see Eqs. 3 and 4). Figure 6e shows that some of the water samples are found at the (Ca2+ + Mg2+)/SO42− = 1 line and its vicinity, thus indicating that sulfate dissolution is not the only source of Ca2+, Mg2+ and SO42−. When (Ca2+ + Mg2+)/SO42− is greater than 1, the concentration of Ca2+ and Mg2+ comes from other sources, such as the dissolution of carbonate (see Eqs. 5 and 6). When (Ca2+ + Mg2+)/SO42− is less than 1, the concentration of SO42− comes from other sources, such as pyrite redox reactions (see Eq. 8). However, almost all of the groundwater has a (Ca2+ + Mg2+)/SO42− greater than 1, thus indicating that there is primarily the dissolution of carbonate. (Ca2+ + Mg2+)/0.5HCO − 3 = 1, which means that the concentration of Ca2+, Mg2+ and HCO3− in the groundwater is mainly derived from the dissolution of carbonate. Figure 6f shows that (Ca2+ + Mg2+)/0.5HCO3− in the No. 32 mining district is significantly greater than 1, which means that the concentration of Ca2+ and Mg2+ comes from other sources, such as the dissolution of sulfate. To sum up, the controlling factors of the hydrogeochemistry of the groundwater in the Peigou Coal Mine mainly include ion exchange, rock salt, dissolution of sulfate and carbonate, weathering of the silicate minerals and desulfurization among which the dissolution of sulfate and desulfurization in the No. 32 mining district are more stronger controlling factors. The chemical reaction equations (Eqs. 1–8) are as follows:
4.3 Factor analysis
Factor analysis is a statistical method that extracts the common variance from all variables. The purpose of a factor analysis is to reflect most of the multivariate data by simplifying the data structure. In geochemistry studies, factor analysis has been used to determine the source of pollutants and now used here to determine the source of the ions found in the groundwater. Factor analyses were carried out for the three mining districts based on two factors: The eigenvalue is greater than 1 and the cumulative percentage of the number of extracted factors exceeds 75%. The hydrogeochemical compositions of the Nos. 31, 32 and 42 mining districts are all represented by using these two factors. The cumulative contribution rate of the two factors for the Nos. 31, 32 and 42 mining districts after orthogonal rotation is 91.081%, 75.544% and 90.577%, respectively. The specific results are shown in Fig. 7. The concentration of Ca2+, Mg2+ and SO42− in the Nos. 31 and 42 mining districts has a higher positive load on FA1, which is hypothesized to be due to the dissolution of sulfate and carbonate. The concentration of K+ + Na+, HCO3− and Cl− in the Nos. 31 and 42 mining districts also has a positive load on FA2, which is hypothesized to be due to the influence of the dissolution of rock salt and weathering of the silicate minerals. The concentration of K+ + Na+ and Cl− in the No. 32 mining district has a strong positive load on FA1, which is presumed to be the result of the dissolution of rock salt. The concentration of K+ + Na+ and HCO3− in the No. 32 mining district also has a positive load on FA2, and SO42− shows a strong negative load on FA2, which is hypothesized to be the effect of the dissolution of carbonate, weathering of the silicate minerals and desulfurization. The result of factor analysis is consistent with that of ionic composition ratio, which proves the similarity in the Nos. 31 and 42 mining districts.
5 Cluster analysis
Cluster sampling is a method used to classify samples based on their affinity and sparseness and divides the sample into two groups, that is, similar samples into one group and different samples into another group. In this study, the water samples are divided into C1 and C2; see Fig. 8. Note that C2 contains mostly the water samples from the Nos. 31 and 42 mining districts, except for one from the No. 32 mining district. This indicates that there might be a hydraulic connection between the Nos. 31 and 42 mining districts. The cluster analysis results also show that there is similarity between these two mining districts.
6 Discussion
6.1 Discriminant analysis
In this study, a discriminant analysis is carried out for classification purposes to determine group membership of the predictor variables by using SPSS to establish discriminant equations. After the analysis, the following discriminant equations are obtained:
where C1, C2, C3, C4, C5 and C6 are the concentrations of HCO3−, SO42−, Cl−, Ca2+, Mg2+ and K+ + Na+, respectively, expressed in milligram equivalent, and the data are not standardized against the original concentration. The first discriminant function has the strongest discriminant ability, with a variance contribution of 89.0%. The hydrogeochemical composition of all of the water samples can be determined by adding a second discriminant function. Meanwhile, the range and average of the F1 and F2 scores in the Nos. 31, 32 and 42 mining districts are given in Table 2. If it is difficult to differentiate between the different water samples with the first discriminant function, the mining district can be used together with the second discriminant function. Since the first discriminant function can provide most of the information on the hydrogeochemical composition of the mining districts, only the range of the F1 scores is considered to determine the hydraulic connectivity. Table 2 shows that the range of the F1 scores of the No. 42 mining district falls within that of the No. 31 mining district, which indicates that the hydrochemistry of these two mining districts is similar, and demonstrates that the hydraulic connection between the Nos. 31 and 42 mining districts is high. At the same time, the discriminant analysis results of the water samples in the mining district (Table 3) showed an accuracy of 27.27%, 93.10% and 60.00% for the Nos. 31, 32 and 42 mining districts, respectively, with an overall accuracy of 75.56%. The discriminant model in this study can also provide guidance for identifying the mining districts. Here, the model shows that there is a strong hydraulic connection between the No. 31 mining district and the other mining districts, while the hydraulic connection of the No. 32 mining district was incorrectly identified which attributed all of the connection to the No. 31 mining district. However, this indicates that there may be a hydraulic connection between these two mining districts. All of the hydraulic connections of the No. 42 mining district were incorrectly identified which attributed all of the connection to the No. 31 mining district and neglected the No. 32 mining district, thus indicating that there is a strong hydraulic connection between the Nos. 31 and 42 mining districts, with no hydraulic connection between the Nos. 32 and 42 mining districts. Two water samples in the No. 42 mining district were misjudged to be from the No. 31 mining district, indicating that water–rock interaction occurred sufficiently after the No. 42 mining district recharges the No. 31 mining district, so that part of the water in the No. 42 mining district presented hydrochemical characteristics of the No. 31 mining district. Two water samples in the No. 32 mining district were misjudged to be from the No. 31 mining district, which also indicated that the No. 32 mining district recharges the No. 31 mining district. In the No. 31 mining district, three water samples were misjudged to be from the No. 32 mining district and four water samples were misjudged to be from the No. 42 mining district. The result shows that the hydraulic connection between the No. 31 mining district and No. 42 mining district is higher than that between the No. 31 mining district and No. 32 mining district. In summary, it is speculated that the No. 42 mining district recharges the No. 31 mining district and the No. 32 mining district recharges the No. 31 mining district. These results are further confirmed by the previous judgment that the pulse variation originated in the No. 42 mining district and then transferred to the Nos. 31 and 32 mining districts with delay and pulse loss.
Figure 9 shows the scatter plot of the three mining districts that takes the first and second discriminant functions into consideration. This plot also uses a drop zone to identify the source of the water inrush. On this basis, we can determine which group to allocate a new sample by comparing the distance between the value function of a water sample and the centroid of the area of the three mining districts. Figure 9 shows that the Nos. 31 and 42 mining districts are located in proximity to one another, thus indicating that there is a hydraulic connection between them, and at the same time, also provides an explanation for the incorrect identification of the hydraulic connections.
6.2 Overall analysis
By examining the hydrogeochemical composition, using a Gibbs diagram, examining the ionic composition (with ion ratios) and conducting factor, cluster and discriminant analyses, it is found that the hydraulic connection between the Nos. 31 and 42 mining districts is higher than that between the Nos. 31 and 32 mining districts. A map of the three mining districts as shown in Fig. 1b illustrates that the Nos. 31 and 32 mining districts are relatively close in distance, while the Fushanzhai fault is found between the Nos. 31 and 42 mining districts. The lithological differences in the area separated by faults are greater than those in the adjacent area. However, the analyses in this paper have shown that the mining districts separated by faults are similar to a large extent, while adjacent mining districts are quite different. It is hypothesized that there is a hydraulic connection between the Nos. 31 and 42 mining districts which are separated by the Fushanzhai fault (Fig. 10). However, there is still no direct evidence to prove this hypothesis and show that there is a channel that connects these two mining districts, and its specific location would still require further studies in the future.
7 Conclusions
Different mathematical statistics analyses have been systematically carried out in this study to determine the hydrogeochemical composition of water samples taken from three mining districts of the Peigou Coal Mine, and the following conclusions are made accordingly. A model that identifies the source of the water inrush is established, which examines the strength of the hydraulic connections between different regions by comparing the impacts of water–rock interaction on the groundwater in the different regions. The model validates that:
-
(1)
The hydraulic connection between the Nos. 31 and 42 mining districts is higher than that between the Nos. 31 and 32 mining districts. It is hypothesized that there may be an obscured tunnel between the Nos. 31 and 42 mining districts, which is connected through the Fushanzhai fault.
-
(2)
The controlling factors of the hydrogeochemistry in the three mining districts mainly include ion exchange, rock salt, dissolution of sulfate and carbonate, weathering of the silicate minerals and desulfurization among which the dissolution of sulfate and desulfurization in the No. 32 mining district are more stronger controlling factors.
-
(3)
It is speculated that the No. 42 mining district recharges the No. 31 mining district and the No. 32 mining district recharges the No. 31 mining district. The proposed method provides a new idea for correctly recognizing the groundwater circulation conditions under the influence of mining.
This study has significance for examining the changes in the hydrogeochemical composition of the water in mines and acting as a guideline to prevent and control water inrushes.
References
Chen S, Gui HR (2017) Hydrogeochemical characteristics of groundwater in the coal-bearing aquifer of the Wugou coal mine, northern Anhui province, China. Appl Water Sci 7(4):1903–1910
Dong DL, Sun WJ, Xi S (2012) Water-inrush assessment using a GIS-based Bayesian network for the 12-2 coal seam of the Kailuan Donghuantuo coal mine in China. Mine Water Environ 31(2):138–146
Drollette BD, Hoelzer K, Warner NR et al (2015) Elevated levels of diesel range organic compounds in groundwater near Marcellus gas operations are derived from surface activities. Proc Natl A Sci India A 112(43):13184–13189
Gao R, Yan H, Ju F et al (2018) Influential factors and control of water inrush in a coal seam as the main aquifer. Int J Min Sci Technol 28(2):187–193
Gui HR, Lin ML (2016) Types of water hazards in China coalmines and regional characteristics. Nat Hazards 84(2):1501–1512
Gui HR, Lin ML, Song XM (2017) Features of separation water hazard in China coalmines. Water Pract Technol 12(1):146–155
Gurarslan G, Karahan H (2015) Solving inverse problems of groundwater-pollution-source identification using a differential evolution algorithm. Hydrogeol J 23(6):1109–1119
Helstrup T, Jorgensen NO, Banoeng-Yakubo B (2007) Investigation of hydrochemical characteristics of groundwater from the cretaceous-eocene limestone aquifer in southern Ghana and southern Togo using hierarchical cluster analysis. Hydrogeol J 15(5):977–989
Ismail A, Toriman ME, Juahir H, Zain SM et al (2016) Spatial assessment and source identification of heavy metals pollution in surface water using several chemometric techniques. Mar Pollut Bull 106(1–2):292–300
LaMoreaux JW, Wu Q, Zhou WF (2014) New development in theory and practice in mine water control in China. Carbonate Evaporite 29(2):141–145
Lei M, Qian JZ, Zhao WD (2014) An approach for quickly identifying water-inrush source of mine based on GIS and groundwater chemistry and temperature. Coal Geol Explor 42(2):49–53
Li PY, Wu JH, Tian R et al (2018) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi coal mine, Northwest China. Mine Water Environ 37(2):222–237
Odintsev VN, Miletenko NA (2015) Water inrush in mines as a consequence of spontaneous hydrofracture. J Min Sci 51(3):423–434
Qaisar FUR, Zhang F, Pant RR et al (2018) Spatial variation, source identification, and quality assessment of surface water geochemical composition in the Indus River Basin, Pakistan. Environ Sci Pollut R 25(5):1–15
Qian JZ, Tong Y, Ma L et al (2018) Hydrochemical characteristics and groundwater source identification of a multiple aquifer system in a coal mine. Mine Water Environ 37(3):528–540
Sammarco O, Eng D (1986) Spontaneous inrushes of water in underground mines. Int J Mine Water 5(3):29–41
Sun LH (2014) Statistical analysis of hydrochemistry of groundwater and its implications for water source identification: a case study. Arab J Geosci 7(9):3417–3425
Sun LH (2017) Statistical analyses of groundwater chemistry in coalmine and its hydrological implications. J Appl Sci Eng 20(3):335–344
Sun LH, Chen S, Gui HR (2016) Source identification of inrush water based on groundwater hydrochemistry and statistical analysis. Water Pract Technol 11(2):448–458
Sun CY, Zhang JQ, Ma QY et al (2017a) Polycyclic aromatic hydrocarbons (PAHs) in water and sediment from a river basin: sediment–water partitioning, source identification and environmental health risk assessment. Environ Geochem Health 39(1):63–74
Sun J, Hu Y, Zhao G (2017b) Relationship between water inrush from coal seam floors and main roof weighting. Int J Min Sci Technol 27(5):873–881
Wang Y, Zhou MR, Yan PC et al (2017a) Identification of coalmine water inrush source with PCA-BP model based on laser-induced fluorescence technology. Spectrosc Spectr Anal 37(3):978–983
Wang Y, Zhou MR, Yan PC et al (2017b) Rapid identification model of mine water inrush sources based on extreme learning machine. Int J Wirel Mob Comput 13(4):286–290
Wu Q, Liu YZ, Luo LH et al (2015) Quantitative evaluation and prediction of water inrush vulnerability from aquifers overlying coal seams in Donghuantuo coal mine, China. Environ Earth Sci 74(2):1429–1437
Acknowledgements
This research is supported by the Fundamental Research Funds for the Central Universities (2017XKQY057) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The authors are grateful to the anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhu, S. & Xiao, S. Discussion on controlling factors of hydrogeochemistry and hydraulic connections of groundwater in different mining districts. Nat Hazards 99, 689–704 (2019). https://doi.org/10.1007/s11069-019-03767-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11069-019-03767-1