Abstract
Findings on neurocognitive effects of sustained cannabis use are heterogeneous. Previous work has rarely taken time of abstinence into account. In this review, we focus on understanding sustained effects of cannabis, which begin when clinical symptoms of the drug have worn off after at least 14 days. We conducted a search between 2004 and 2015 and found 38 studies with such a prolonged abstinence phase. Study-design quality in terms of evidence-based medicine is similar among studies. Studies found some attention or concentration deficits in cannabis users (CU). There is evidence that chronic CU might experience sustained deficits in memory function. Findings are mixed regarding impairments in inhibition, impulsivity and decision making for CU, but there is a trend towards worse performance. Three out of four studies found evidence that motor function remains impaired even after a time of abstinence, while no impairments in visual spatial functioning can be concluded. Functional imaging demonstrates clear differences in activation patterns between CU and controls especially in hippocampal, prefrontal and cerebellar areas. Structural differences are found in cortical areas, especially the orbitofrontal region and the hippocampus. Twenty studies (57 %) reported data on outcome effects, leading to an overall effect size of r mean = .378 (CI 95 % = [.342; .453]). Heavy use is found to be more consistently associated with effects in diverse domains than early age of onset. Questions of causality―in view of scarce longitudinal studies, especially those targeting co-occurring psychiatric disorders―are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cannabis use – the generic term we use throughout this article for all non-medical prescription forms of hashish, marijuana or synthetic cannabinoid consumption – is widespread in European countries, as it is in the United States and other Western civilizations such as Australia or Canada (Roncero et al. 2015). Over the past 20 years, cultivation and plant-breeding techniques have greatly increased the potency of cannabis products. Thus, the modern cannabis smoker may be exposed to doses of Δ9-tetrahydrocannabinol (THC) many times greater than his or her counterpart in the 1960s and 1970s (Ashton 2001). The consumed THC concentration varies among different sources and preparations of cannabis.
Regular cannabis use is significantly associated with increased health problems, e.g., respiratory symptoms, general indisposition, or neurocognitive impairments (Hall and Degenhardt 2014; Hoch et al. 2015). Furthermore, it is well documented that cannabis use can increase psychosocial difficulties such as academic underachievement and/or school dropout (Brook et al. 2008; Silins et al. 2014). Since most cannabis users (CU) start consuming during adolescence or emerging adulthood, impaired educational attainment plays an especially critical role (Hall and Degenhardt 2014; Johns 2001). THC users also increase their risk of developing psychotic symptoms and disorders (Di Forti et al. 2007), especially if there is a family history of these mental illnesses (Moore et al. 2007). In turn, a number of mental health disorders such as depression, anxiety, schizophrenia, bipolar disorder and obsessive-compulsive disorder are associated with high rates of comorbid substance use (Barnes et al. 2006; Saban et al. 2014, Scott et al. 2014). This association appears to be independent of culture (Wu et al. 2013). Current statistics show that about one of ten chronic CU develops a dependence syndrome (Budney et al. 2007; Hall 2015). Dependency symptoms appear progressively and treatment for cannabis dependence is complex (Danovitch and Gorelick 2012). While loss of control over cannabis use and continued cannabis use against better knowledge emerge earlier, self-reported withdrawal symptoms tend to emerge later and for a much smaller proportion of users (Rosenberg and Anthony 2001).
Cannabis and the Brain
The pharmacokinetics of cannabinoids are reviewed by Agurell et al. (1986) and Khiabani and Mørland (2007). Effects and adverse effects are versatile (Ashton 1999; Maykut 1985), in fact, the use of cannabinoids has been advocated for several medical indications (Radbruch and Nauck 2003; Whiting et al. 2015). Cannabinoids exert their effect by interaction with specific endogenous cannabinoid receptors, discovered by Devane et al. (1988). Neuronal cannabinoid receptors termed cannabinoid type 1 (CB1) receptor have been found in high density both in animal and human cerebral cortices, particularly for the frontal regions, i.e. in regions involved in processing emotional inputs, rewarding stimuli, habit formation, and higher cognitive functions (Herkenham et al. 1990; Pertwee 2005). The response of cannabinoid receptors to THC exposure varies depending on the brain area (Romero et al. 1995). The impact of cannabis on high-density CB1 areas (such the frontal lobe, hippocampal/temporal regions, basal ganglia, and cingulate cortices) and on associated cognitive functions (such as memory and attention) was already observed in early cannabis research (Herkenham et al. 1990).
The endocannabinoid system appears to be functionally linked to the extended dopamine reward pathway involving the ventral tegmental area, nucleus accumbens, prefrontal cortex (including the orbitofrontal and dorsolateral prefrontal cortex), and anterior cingulate, viewed as central to the development of addictive behavior (Volkow et al. 1996). With repeated dosage, high levels of cannabinoids accumulate in the body and continue to reach the brain. Animal studies show reversible downregulation of brain CB1 receptors after chronic exposure to cannabis (Gonzalez et al. 2005; Sim-Selley 2003). Once the adult cannabinoid receptor levels are reached, binding activity in the nervous system neither increases nor declines during the normal aging process in rats (Belue et al. 1995). Animal research also indicates that brain regions that are rich in cannabinoid receptors are more susceptible to the effects of cannabis (Freedland et al. 2002; Pontieri et al. 1999; Zimmer et al. 1999; Schneider 2008). The relative absence of the cannabinoid receptors from brainstem nuclei may account for the low toxicity of cannabinoids when given in overdose (Iversen 2003). Frequently, the onset of THC consumption occurs during adolescence and therefore, in a sensitive period of brain maturing. The prefrontal brain cortex is one of the last regions of maturation during adolescence (Gogtay et al. 2004; Lenroot and Giedd 2006; Sowell et al. 2004a, b) and is one of the densest CB1 parts of the human brain. Therefore, it is likely to be particularly vulnerable to the early effects of heavy marijuana exposure (Egerton et al. 2006; Horti and Van Laere 2008; Lubman et al. 2015; Lorenzetti et al. 2014, Quickfall and Crockford 2006).
Research on Neurocognitive Effects of Cannabis use
Research on cannabis and neurocognitive impairments has studied acute and persistent effects of cannabis use. Acute effects of cannabis reported in studies occurred in diverse areas such as general intellectual function, memory, abstraction ability, sustained attention, verbal fluency, and the ability to learn and recall new verbal and visuospatial information (for an overview see Ranganathan and D’Souza 2006; Iversen 2005; Pope et al. 2001a, b; Earleywine 2002; Gruber et al. 2011). Persistent effects of cannabis use have also been widely studied. Table 1 provides an overview of 31 reviews we found in the time period between 2004 and 2015 that had their main focus on residual/long-term effects of cannabis use on neurocognitive functioning. These reviews report impaired performance on a variety of attention, memory (Solowij and Battisti 2008) and executive function tasks (Wrege et al. 2014) as well as alterations in blood flow and brain tissue density (e.g., Yucel et al. 2007). They also point to a considerable variability of findings (e.g., Crane et al. 2013). A possible link between residual impairments and the duration and quantity of cannabis use is frequently mentioned (e.g., Crean et al. 2011; Hall 2015). Early age of onset is discussed as an especially critical factor in the development of cannabis-related neurocognitive impairments (e.g., Schweinsburg et al. 2008a; Lisdahl et al. 2013). In their systematic review, Batalla et al. (2013) conclude that neuroimaging studies provide evidence of morphological brain alterations particularly in the medial temporal and frontal cortices as well as in the cerebellum both in adolescents and adults. Only few reviews report evidence levels of studies or effect sizes. Through meta-analytic research, Grant et al. (2003; 11 studies) found evidence for deficits in learning (r = .104) and forgetting domains (r = .134) but non-significant confidence intervals for other neurocognitive domains. In a replication to this study (Schreiner and Dunn 2012; 33 studies), a significant overall effect of r = .144 is reported. Rocchetti et al. (2013; 14 studies) observed alterations in structural imaging studies of r = .222 [r-transformations: these authors]. In sum, although evidence is building to suggest that cannabis use in adolescence is associated with cognitive and psychosocial and health impairment (Rubino and Parolaro 2008; Lisdahl et al. 2014; Volkow et al. 2014), the exact effects of long-term cannabis use on cognitive performance as well as the magnitude or reversibility of possible effects remain unclear to date.
The Role of Abstinence Duration
Reviews on long-term effects typically do not make a clear distinction between studies that explore effects of cannabis use in barely abstinent CU (usually starting with a minimum of 12 h of abstinence) and true sustained effects of cannabis, which begin when clinical symptoms of the drug have worn off, usually starting with 14 days (Budney et al. 2004). Instead, these reviews include studies with rather heterogeneous abstinence durations ranging from several hours to many months (see Table 1). Next to other limitations for reviews such as heterogeneity of studies, assessment methods and samples, this variability in abstinence might considerably contribute to the inconsistency of findings mentioned above. As Roten et al. (2015) stated: “Cognitive performance in certain domains […] was significantly better in those with abstinence when compared to those who were not abstinent. Results suggest an improvement in these cognitive performance domains with abstinence from marijuana” (p. 121). While it is important to assess cognitive deficits associated with each stage of abstinence, assessment during short-term abstinence presents interpretive problems. Specifically, decrements in functioning may be due to anything from alterations in the brain, residues of the drug in the brain or the withdrawal symptoms themselves. These symptoms typically appear during the first 1–2 days after cessation of THC consumption and return to baseline within 1–2 weeks (Budney et al. 2004; Wiesbeck et al. 1996). For instance, Kouri and Pope (2000) report that during this time, CU reported greater levels of anxiety, irritability, negative mood, physical symptoms, and decreased appetite. Most symptoms returned to baseline after two weeks. Only few of the 31 reviews presented in Table 1 excluded studies with very short abstinence periods: Schreiner and Dunn (2012) conducted a meta-analysis on the effects of cannabis use after 25 days of abstinence and found no significant effect of cannabis use on global neurocognitive performance or any effect on the eight assessed domains. However, due to their choice of a protracted abstinence period (25 days), they were only able to include 13 studies in their meta-analysis. Van Holst and Schilt (2011) reviewed effects of cannabis and other drugs after 14 days of abstinence but only included three studies on cannabis in their review due to a limited time scope (literature between 2005 and 2010). Earlier reports on cognitive functioning in drug users with longer-term abstinence presented mixed findings (Bolla et al. 2002; Pope et al. 2001a, b).
Objective
In this systematic review, we investigate the long-term effects of cannabis use after a prolonged duration of abstinence (at least 14 days), thus excluding possible effects resulting from drug residues or withdrawal symptoms. We choose a 14 day period to permit a larger data base than previous work along this line (Schreiner and Dunn 2012; van Holst and Schilt 2011). Our research questions are the following:
-
1)
What are the long-term effects of cannabis use on neurocognitive functioning after a prolonged abstinence period of at least 14 days?
-
2)
What magnitude do long-term effects of cannabis use have?
-
3)
Does the early onset (EO) of cannabis use exacerbate long-term effects of cannabis use?
To answer question 1), we report the effects of cannabis use in the neurocognitive domains of attention, executive function, motor function, memory and learning, and visual spatial. We also report results in the areas of functional imaging/EEG and structural imaging. To answer question 2), we provide additional meta-analytic information on effect sizes. To answer question 3), we report our findings for a selected EO sample within the domains mentioned in 1), and consider the role of EO separately within the discussion section.
Even though no formal review protocol exists, the procedures of our systematic review are guided by the PRISMA statement (Moher et al. 2009).
Methods
Search Strategy
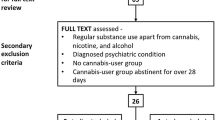
Electronic searches were performed using EMBASE, Ovid MEDLINER, PsycInfo, PSYNDEXplus Literature and Audiovisual Media as well as PSYNDEXplus Tests databases. Search terms used were cannabi* or THC or marijuana or marihuana AND neuro* or cognit* or assess* or abilit* or affect* or process* or function* or impair* AND residual or long-term or abstinen* or abstain* or lasting or non-acute or non-intox* or persist*. In addition, we did reference searching, and reviewed book chapters on substance abuse neuroimaging. We focused on literature published between 2004 and 2015 (a comprehensive overview about the research of cannabis and neurocognitive impairments from 1960s to 2004 can be found in Verdejo-Garcia et al. 2004), thus covering more than ten years of research. Only clinical trials with human subjects were included. This search yielded a total of 1038 studies after removal of duplicates. The title and the abstract of these studies were reviewed by two independent reviewers (FG and SK) for relevance. We included studies only on subjects with regular consumption of cannabis or marijuana. To rule out acute intoxication effects, only studies with a period of at least 14 days of abstinence were included. We excluded studies on subjects with a history of chronic medical and neurological illness or severe psychiatric disorders (like schizophrenia or mania), or with a diagnosis of additional substance use disorders (alcohol, opioids, amphetamines) defined in ICD-10 or DSM-IV as addiction (without nicotine dependence). We also excluded animal studies, case reports, expertises, commentaries, and books. Discrepancies were resolved in discussion. At the end of this process, a total of 38 studies fulfilled all of the abovementioned criteria and were included in this review.
Evidence Level
One of the authors (SB) supervised two trained University students in their 5th term who graded the evidence-level of studies according to the Scottish Intercollegiate Guidelines Network (SIGN) methodology (Harbour and Miller 2001; Baker et al. 2010). Differences were resolved in discussion. Since none of the studies at hand were RCTs, but all studies included control groups and were well-designed, study quality was comparatively similar, for instance with regard to sample size or blinding. Thus, all studies either received grades 2++ or 2+ within level 2 of the SIGN methodology,Footnote 1 depending on whether they had a matched or an un-matched control group. Table 2 provides an overview on study participants and methodology, neurocognitive domain and evidence levels of the studies included in this review. Studies were classified as EO studies if subjects started using cannabis before the age of 18.
Additional Meta-Analytic Information
Effect sizes (ES) were computed in terms of (unweighted) correlations r using Fisher’s Z-transformation (Cohen 1988; Rosenthal and DiMatteo 2001).Footnote 2 To that end, statistical parameters reported (F, t, d, etc.) were converted in correlations r. For these and other meta-analytic computations, Psychometrica software was employed (Lenhard and Lenhard 2015; www.psychometrica.de), furthermore IBM SPSS Statistics 22 (www.ibm.com). For multiple effects studies, one mean r was computed, for zero effect studies, r = .00 was applied by definition, and thus, all zero correlations were included in computations. This could only be done in studies supplying adequate data. Marginal effects (p ≤ .10) were also included (Chang et al. 2006; Pujol et al. 2014; Schweinsburg et al. 2010; Sevy et al. 2008; Verdejo-Garcia et al. 2007) following the reasoning of Grant et al. (2003, p. 683) in “that in investigating the potential toxic effects of a substance such as cannabis, it [is] important to be more ‘permissive’ rather than ‘conservative’.” Via heterogeneity statistic Q and index I 2 we assessed whether the pooled effect sizes of study results measure the same underlying population effect in specific domains and in total (Rosenthal and DiMatteo 2001). Cochrane’s Q, a widely used statistic, is impacted by sample size. Therefore, it is completed by I 2, which is not (Huedo-Medina et al. 2006).
Results
Of the 38 studies reviewed here, nine studies or 24 % were rated with evidence level 2++, while the others received evidence level 2+. This renders the studies fairly comparable with regard to study quality. Study characteristics, however, proved to be heterogeneous with regard to sample size, age, gender ratio, subject acquisition, comorbidities, other substance use, abstinence duration, quantity or frequency of consumption and test methods (see Table 2). Twenty-nine studies were rated as having an adolescent, 22 as having an EO study sample. Table 3 provides an overview over main results within five neurocognitive domains. Twenty-four zero effects out of 13 performance studies (Ashtari et al. 2011; De Bellis et al. 2013; Eldreth et al. 2004; Hooper et al. 2014; Jacobus et al. 2012, 2014; Lyons et al. 2004; Medina et al. 2010; Padula et al. 2007; Schweinsburg et al. 2008b; Schweinsburg et al. 2010; Schweinsburg et al. 2011; Winward et al. 2014) and 2 zero effects out of 2 imaging studies (Hirvonen et al. 2012; Jacobus et al. 2012) were found. All of them were included in further computations. As detailed in Tables 4 and 5 for studies providing sufficient data, neurocognitive performance (I 2 = 55.9 %) and functional and structural imaging studies (I 2 = 62.5 %) are both of similar medium heterogeneity. Their ES (r mean = .305 resp. r mean = .446) differ only by a small ES according to Cohen’s q = .165. In all, impairment ES in CU relative to non-using controls (NC) could be overall qualified as medium to large from both perspectives, and a pooled overall ES would be r mean = .378 (CI 95 % = [.342; .453]).
Attention
Ten studies examined differences in attention between CU and a group of NC, five of them found impairments in CU. Quantifiable data (n = 10 studies) rendered an overall medium ES of r = .273 (CI 95 % = [.109; .423]). Most of the reviewed studies provide evidence for some attention and concentration deficits in CU even after a time of abstinence. In a study by Medina et al. (2007a), CU demonstrated poorer complex attention results even after 23 days of abstinence. Jacobsen et al. (2004) reported impaired attention for CU in a selective attention task and Chang et al. (2006) in an fMRI study on visuospatial attention. Hanson et al. (2010) found similar attention processing speed between CU and NC, but attention accuracy remained deficient in users throughout a 3-week abstinence period (p < .01).
Within the five EO studies on attention, four studies found impairments while one study found no differences between CU and NC.
Executive Functions
Sixteen studies examined differences in executive functions, and ten studies found impairments. Quantifiable data (n = 14 studies) revealed an overall medium ES of r = .294 (CI 95 % = [.109; .423]). Evidence is mixed as to whether inhibition, impulsivity and decision making are impaired in abstinent CU. However, there is a trend towards worse performance even after a period of abstinence in CU groups. Executive functions, and how they are measured, are highly heterogeneous. For example Medina et al. (2007a) found deficits in planning and sequencing ability while Tapert et al. (2007) found impaired verbal fluency. Bolla et al. (2005) identified a dose-related association between increased THC use and a lower decision-making task performance (Iowa gambling task, IGT) and alterations in brain activity. Verdejo-Garcia et al. (2007) administered the IGT twice to 25-day abstinent cocaine and THC users and matched controls. CU performed worse than controls but this effect was not significant. They showed linear dose–response effects on IGT performance. The “number of joints” smoked prior to the period of enforced abstinence was negatively related to IGT performance. CU (abstinent for at least 3 weeks) showed significantly more impulsivity and tolerated a lower level of certainty in their decision-making compared to NC (Clark et al. 2009). On the other hand, five studies found no differences in executive function (e.g., Eldreth et al. 2004; Lyons et al. 2004), for instance in the area of decision making (De Bellis et al. 2013). Medina et al. (2009) reported an interaction effect in the way such that among CU, smaller prefrontal cortex (PFC) total volume was associated with better executive functioning while the opposite pattern was seen among the controls.
Within the seven EO studies on executive functioning, four studies found impairments.
Motor Function
Five studies examined differences in motor function abilities between CU and NC. Quantifiable data (n = 4 studies) gave an almost large ES of .478 (CI 95 % = [.394; .555]). Altogether, four out of five studies found evidence that motor function remains impaired even after a time of abstinence. It remains unclear if this result is due to reaction time (speed), or accuracy, or both. Bosker et al. (2013) assessed psychomotor function in CU and reported that sustained cannabis abstinence moderately improved critical tracking and divided attention performance in chronic, daily cannabis smokers, but that impairments were still observable compared to controls after three weeks of abstinence. Pillay et al. (2008) conducted finger-tapping tests and concluded that residual diminished brain activation in motor cortical circuits is still observed after discontinuing cannabis use for 28 days. In another study, after at least 23 days of abstinence, adolescent CU had impairments in psychomotor performance compared to controls, and there was a negative, dose-dependent association between performance and lifetime cannabis use episodes (Medina et al. 2007a).
In the only EO study on motor function, Medina et al. (2010) uncovered no significant relationships between cerebellar volume and psychomotor speed in adolescents.
Memory and Learning
Sixteen studies examined differences in memory and learning between CU and NC, seven of which found impairments. Quantifiable data (n = 15 studies) gave an overall medium ES of .229 (CI 95 % = [.130; .323]). There is some evidence that chronic CU might experience sustained deficits in the area of information encoding, storage and retrieval. Results for deficits in other types of memory seemed to be more nuanced when considering length of abstinence, age at which CU discontinue their use, amount and duration of cannabis use. For instance, Medina et al. (2007a) found poorer results for story memory, Jacobsen et al. (2007) demonstrated that THC users performed a working memory task less accurately than controls, and Hanson et al. (2010) demonstrated poorer verbal learning and working memory in a longitudinal study. Seven studies found no differences, for instance Lyons et al. (2004) in the area of memory function, Jager et al. (2010) in the area of associative memory or Chang et al. (2006), in whose study abstinent CU even showed a trend for better performance on one of the verbal memory tests. One study found an interaction effect with binge drinking (n.b. without alcohol-related addiction diagnosis; Mahmood et al. 2010), in which greater alcohol hangover symptoms predicted worse verbal learning and memory scores for NC, but not for CU.
Of the 12 EO studies examining memory and learning, seven found memory and learning deficits.
Visual Spatial
Only four studies (two EO studies) examined differences in visual spatial functioning between CU and NC. Here, two studies found differences (CU one better/one worse). From this scarce evidence only subtle impairments in visual spatial functioning can be concluded. The EO studies found inconsistent results after sustained abstinence (Schweinsburg et al. 2010, Jacobus et al. 2014), while Medina et al. (2007a) found better visual spatial functioning in CU than in NC after four weeks of abstinence.
Functional Imaging
Seventeen studies examined neurocognitive alterations in CU compared to NC with functional imaging technology, and 16 studies found neurocognitive alterations. Quantifiable data (n = 11 studies) resulted in a medium ES of r = .479 (CI 95 % = [.409; .554]). There is clear evidence for differences in activation patterns between CU and NC even after a washout period of more than 14 days. It seems that differences in activation patterns are demonstrated in prefrontal, temporal and occipital as well as cerebellar regions. Bolla et al. (2005) reported greater activation in the left cerebellum and less activation in the right lateral orbitofrontal cortex and the right dorsolateral prefrontal cortex for CU. In the study of Pujol et al. (2014), CU showed increased functional connectivity in the core of the default and insula networks and in selective enhancement of functional anticorrelation between both. Schweinsburg et al. (2010) demonstrated an increased response in the right precentral gyrus clusters for CU. Also, this group reported significantly more activity during spatial working memory (SWM) relative to vigilance in adolescent CU in the right parietal cluster, but less activity during the use of SWM relative to vigilance than NC in right dorsolateral prefrontal cortex (Schweinsburg et al. 2008b). Sevy et al. (2008) reported decreased glucose metabolism in CU compared to controls after more than 12 weeks of abstinence. Herning et al. (2008) found that chronic CU was associated with reduced EEG power in alpha and beta bands at posterior sites, alterations that were associated with changes in cerebral perfusion and/or thyroid function. Herning et al. (2005) reported that the pulsatility index, a measure of cerebrovascular resistance, and systolic blood flow velocity were still significantly increased in heavy CU after a month of monitored abstinence. In a visual attention study, active CU with positive urine toxicology screens evidenced greater reductions in right prefrontal fMRI response than abstinent users (Chang et al. 2006), a finding that suggests a change in neural recruitment throughout the course of abstinence. Sneider et al. (2008) also examined changes over abstinence duration and found that while cerebral blood flow levels begin to normalize with continued abstinence from cannabis after 28 days, specifically in frontal areas, other temporal and cerebellar brain regions show slower blood flow decreases. Only one study found no functional imaging differences between CU and NC: Jacobus et al. (2012) identified no relationships between cerebral blood flow and executive functioning, nonverbal memory, structural verbal memory, or visuospatial processing.
Eleven EO studies examined neurocognitive alterations in CU compared to NC with functional imaging technology, of which 10 studies found neurocognitive alterations.
Structural Imaging
Ten studies examined structural imaging differences between CU and NC, of which nine found differences. Quantifiable data (n = 6 studies) gave a small mean ES of r = .287 (CI 95 % = [.198; .371]). Structural differences are found in cortical areas such as the limbic system, the orbitofrontal region (decision making) or the hippocampus (memories) and in subcortical areas that are involved in emotion processing such as the amygdala as well as in the nucleus accumbens, a region that is central to the reward system. These differences are indicated by volume differences (esp. white matter volume). In a positron emission tomography (PET) imaging study, CB1 receptor density returned to normal levels after a time of abstinence (Hirvonen et al. 2012). Medina et al. (2007b) found smaller white matter volume in CU, and other than in controls, white matter volume was negatively associated with depressive symptoms for CU. Ashtari et al. (2011) found that heavy CU had significantly smaller left and right hippocampus volumes as compared to their matched controls, but no significant changes in the left and right amygdala volume. Matochik et al. (2005) found lower gray matter density in the right parahippocampal gyrus and greater density bilaterally near the precentral gyrus and the right thalamus in CU. In the same group, lower white matter density was found in the left parietal lobe and higher density around the parahippocampal and fusiform gyri compared to controls. Longer duration of marijuana use was significantly correlated with higher white matter tissue density in the left precentral gyrus. Three studies reported interaction effects: Female CU demonstrated comparatively larger PFC volumes while male CU had smaller volumes compared with same-gender controls (Medina et al. 2009). Another study from Medina et al. (2010) found that CU exhibited a larger inferior posterior (lobules VIII-X) vermis volume, and that larger vermis volumes were associated with poorer executive functioning.
Six EO studies examined structural imaging differences between CU and NC, and all six studies found differences.
Additional Meta-Analytic Information
Neurocognitive Performance
The overall data basis is N = 1.428. A total of 31 studies reported neurocognitive outcome effects, while concerning five studies (about 16 %) supplied no applicable data to calculate ES. In Pillay et al. (2008) (motor function), 12 effects were reported. As these proved to be homogeneous after Q-testing, only one pooled effect size (ES) has been used for this study. As shown in Table 4, mean overall ES across all studies is r mean = .305 with CI 95 % = [.254; .358], which amounts to a significant medium sized effect. However, Q-testing (p = .000) reveals that there could be population effects which allow only for limited generalizability. ES in motor function is significantly higher than in the remaining domains (p ≤ .005), but Cohen’s q ≤ .217 gives only small ES. In terms of I 2, heterogeneity is typically low to medium even in highly significant Q-measures. In sum, in quantifiable studies, CU appear to be adversely affected in neurocognitive performances compared to NC. For assessing possible publication bias, a funnel plot based on 75 observed effects is given in Fig. 1. Upon inspection, it is slightly asymmetric, but this is because zero and small ES findings have been published and this does not speak for a publication bias, unless the tendency for underpowered studies counts as “bias”.
Functional and structural imaging
The overall data basis is N = 732. Seventeen studies (11 EO studies) examined alterations in neural activity in CU compared to NC with functional imaging technology. Of these 17 studies, 16 studies (10 EO studies) found functional alterations with a medium effect size of r = .479 (CI 95 % = [.409; .554]), while only one study did not. Ten studies (6 EO studies) examined structural imaging differences between CU and NC. Of these 10 (6) studies, 9 (5) found differences with a mean effect of r = .287 (CI 95 % = [.198; .371]), while only one (0) did not. Three studies reported interaction effects, one with life-time alcohol use and two with executive functioning. ES in functional imaging is significantly higher than in structural imaging (p ≤ .006), but again Cohen’s q = .226 gives only a small ES. In sum, 27 studies reported functional and structural imaging outcome effects, 10 (37 %) supplied no applicable data. As shown in Table 5, mean overall ES across all studies is r mean = .446 with CI 95 % = [.383; .505], which amounts to a significant, almost large-sized effect. Thus, findings in studies with meta-analytic information suggest that CU are adversely affected in measures of functional and structural imaging compared to NC. But again, Q-testing (p = .000) reveals that there is limited generalizability, which is confirmed by the I 2-statistic that yields a low to medium heterogeneity. Possible publication bias is negligible as assessed by the funnel plot in Fig. 2, based on 54 observed effects. The plot is slightly asymmetric, but again this is because zero and small ES findings have been published, thus not speaking for a publication bias. Again, a small-samples-size “bias” appears, which comes as no surprise since there is considerable overlap between studies from Figs. 1 and 2.
Early versus later onset
Concerning neurocognitive performance, EO studies (age of onset < 18 years) reveal a low heterogeneity (I 2 = 39.4 %) and an almost medium ES of r mean = .250 with CI 95 % = [.173; .324], whereas studies employing subjects with a later age of onset show medium heterogeneity (I 2 = 66.0 %) and a medium ES of r mean = .359 with CI 95 % = [.280; .432]. These ES differ with q = .120, i.e. a small ES according to Cohen’s convention. Hence, in neurocognitive performance, neurocognitive impairment over all domains in CU with early age of onset seems less pronounced than in CU with later onset, compared to NC. In the area of functional and structural imaging, we found medium heterogeneity (I 2 = 61.5 %) and a large ES of r mean = .617 with CI 95 % = [.487; .767] in EO studies, but in studies employing subjects with later onset we found no heterogeneity (I 2 = 0 % by definition; Q = 12.50 with p = .641) and a medium ES of r mean = .328 with CI 95 % = [.246; .415]. These ES differ with q = .380 to medium ES. This finding is opposed to the finding in the area of neurocognitive performance because, here, differences in functional and structural imaging according ES in CU with early age of onset appear more pronounced than in CU with later onset, compared to NC.
Discussion
In this review, we sought to collect and report current knowledge concerning (1) the long-term effects of cannabis use on neurocognitive functioning after a prolonged abstinence period of at least 14 days, (2) the conclusiveness of findings, especially in studies providing data for meta-analysis, and (3) the question if EO of cannabis use exacerbates long-term effects of cannabis use.
Regarding (1) we conclude that findings concerning neurocognitive impairments remain heterogeneous. Most studies found some attention or concentration deficits in CU. Also, there is evidence that chronic CU might experience sustained deficits in the area of information encoding, storage and retrieval. Findings are mixed regarding impairments in inhibition, impulsivity and decision making for CU, but there is a trend toward worse performance. Four out of five studies found evidence that motor function remains impaired even after a time of abstinence, while the data basis concerning impairments in visual spatial functioning (four studies) is inconsistent. Functional imaging demonstrates clear differences in activation patterns between CU and controls. Structural differences are found in cortical areas, like the orbitofrontal and temporal region, or the hippocampus and in subcortical areas, such as amygdala and nucleus accumbens. On the whole, neurocognitive findings remain quite mixed even after our attempt to account for additional factors such as abstinence period, study quality, method, and onset of CU. This heterogeneity is more evident in some domains than in others. For example, while 16 out of 17 functional imaging/EEG studies find different brain activation patterns, blood flow or other alterations in CU, only five out of ten structural imaging studies report significant differences between CU and NC (three more report differences in interaction with confounders). This may be due to the fact that, while volumetric effects will be especially observed in heavy users, functional effects might generally be easier to detect. The heterogeneity of findings is discussed further below. In conclusion, we detected neurocognitive differences between CU in comparison to NC – mostly appearing as impairments – that lasted for more than two weeks of abstinence. Also, across all domains, functional and structural differences between CU and NC were found that persisted longer than 14 days after terminating THC consumption. Drawing associations between both domains (neuropsychological testing and imaging) is out of our scope, however this promising avenue should be pursued by further research.
Regarding (2) we conclude that, in most domains, substantial medium or large detrimental effects of cannabis use can be expected as per 20 similarly well-designed clinical trials which supply quantifiable data. ES and homogeneity measures suggest that in CU compared to NC, impairment in neurocognitive performances can be expected on a level of medium- to large-sized effects. We detected medium-sized heterogeneity/variance between populations, employed measures, and domains. About half of the studies are underpowered (Median N = 32). T-testing, for instance, requires a sample total of N ≥ 40 to detect medium ES. This means that several small effects may have been overlooked and that, consequentially, the true detrimental effects of cannabis are probably still underestimated in this review due to shortcomings in respective reports.
Regarding (3), our results indicate that – in terms of ES – individuals with earlier EO of cannabis use differed more heavily from individuals with later age of onset in structural and functional imaging (q = .380), but comparatively less in neuropsychological tests (q = .120). These findings are more in accordance with a hypothesis that cannabis consumption is of serious impact in adolescence due to age-related vulnerability (Lisdahl et al. 2014; Volkow et al. 2014). We will return to the role of EO later.
All in all, the heterogeneity of findings warrants interpretation. Are some studies overrating the long-term effects of cannabis use – or were these effects not detected in some studies? In the following, we explore these possibilities further.
Why Sustained Effects of Cannabis use may be Overrated
There are two main arguments that speak for a cautious stance toward the effects we found:
First, there is the time factor: how long does abstinence need to persist before effects can truly be viewed as long-term? Even though our review already places a large emphasis on abstinence, it is unclear whether this suffices. Possibly, longer washout periods are required (Schreiner and Dunn 2012; Pope et al. 1995) due, for instance, to prolonged intoxication within fatty tissue (Iversen 2003). Animal studies showed reversible downregulation of brain CB1 receptors after chronic exposure to cannabis (Gonzalez et al. 2005; Sim-Selley 2003). Using positron emission tomography imaging, Hirvonen et al. (2012) detected reversible and regionally selective downregulation of brain CB1 receptors in human subjects who chronically smoked cannabis. The downregulation correlated with years of cannabis smoking and was selective to cortical brain regions. After 4 weeks (26 ± 5 days) of continuously monitored abstinence from cannabis, CB1 receptor density returned to normal levels. This was the first direct demonstration of cortical CB1 receptor downregulation as a neuroadaptation that may promote cannabis dependence in the human brain. Therefore, reversibility may be possible after an abstinence period even longer than 14 or 28 days (as suggested by Schreiner and Dunn 2012; Grant et al. 2003), though there might be a dose-related aspect to reversibility (Verdejo-Garcia et al. 2007). In sum, study effects might still be viewed as temporary deficits due to a residue of cannabinoids in the brain, even if they cannot be attributed to acute withdrawal effects from stopping cannabis use, and not as effects of cumulative THC exposure on brain functioning.
Second, there is the question of cause and effect: some evidence suggests that preexisting brain abnormalities predate and predict the onset of substance use (e.g., Cheetham et al. 2012). The cross sectional nature of the studies at hand makes it impossible to judge whether chronic cannabis use impairs cognitive performance, or whether worse cognitive performance is a premorbid condition for problems with cannabis use. The same goes for the association of cannabis use with poorer educational attainment (Stiby et al. 2015). Possibly, both conditions are caused by common factors rooted in cognitive deficiencies (e.g., Gonzalez 2007). But not only cognitive deficits may influence an individual’s development of dependency symptoms. The “pre-existing conditions”, meaning state of health before first episode of THC could be influenced by a widespread conglomerate of factors: certain premorbid personality characteristics such as neuroticism, risk-taking or sensation-seeking as well as deficits in inhibitory control and affect regulation may also precede regular cannabis use (e.g., Crane et al. 2013; Gonzalez 2007; Jacobus et al. 2009a) and then be falsely attributed to cannabis consumption in neurocognitive tests. For example, could a correlation between craving and amygdala volume in abstinent cannabis users be mediated by frustration tolerance or inhibition control? And: should it be explained by focal damage specific to the amygdala through THC or are there more widespread alterations to brain networks involved like the reward system or emotional regulation (Padula et al. 2015)? Only few studies did control for family history of substance use disorders, early school dropouts, uncertain family structures or instable family situations as well as different sexual or cultural health awareness. Only incompletely did studies exclude subjects with Axis I comorbid psychiatric disorders. Thus, more evidence from longitudinal studies is needed to determine whether brain and cognitive abnormalities may have predated the onset of drug use, evidence gained by thoroughly controlling for a variety of potential confounders, e.g., pre-existing or current mood disorder, prodromal psychosis, other drug use, etc.
Why Sustained Effects of Cannabis use may Have Gone Undetected
There are three essential arguments that speak for taking the effects we found seriously:
First and foremost, there is the issue of deficient methodology. The studies at hand utilized advanced electrophysiological or imaging technology, too often employing very small sample sizes in a laboratory setting. It is very likely that more small to medium-sized effects would have been detected if more of the studies in this review had been supplied with adequate sample sizes. Also, there are barriers in comparability grounded in different definitions of terms such as “regular use” or “heavy use”. Because it is impossible to administer cannabis to volunteers for many years in a laboratory, any study of long-term cannabis effects must rely on naturalistic studies of “users” in the field that are hard to compare (Pope et al. 1995). The attempt to exclude confounders such as premorbid cognitive reserves, psychiatric functioning, other substance use, or head injuries, neuropsychological deficits such as attention deficit hyperactivity disorder, conduct disorder, antisocial behavior and family history of schizophrenia render the remaining sample even more artificial with regard to clinical relevance. Other confounders such as anxiety (Jacobus et al. 2012), alcohol hangover symptoms (Mahmood et al. 2010), concomitant alcohol abuse (Jacobus et al. 2014) or nicotine withdrawal (Jacobsen et al. 2007) may have gone undetected in many studies. In addition, different neuropsychological tests are influenced in complex ways by attention and motivation of subjects, are diverse and hard to compare and have their limits regarding everyday life relevance. Or, as in the case of memory and learning, the localization of this domain within the brain is difficult, since it is broadly distributed within different regions of the brain such as the hippocampus, temporal lobe, and hippocampal formation (Garcia-Lazaro et al. 2012), and is highly dense in CB1 receptors. Functional differences compared to non-users were detected in adult and adolescent CU when activated and at rest status. Former CU showed different activity patterns compared to NC in functional imaging studies, however, these are unspecific and inconsistent in many cases. Structural magnetic resonance cannot provide information on the microstructure of the brain. Changes in neuronal numbers (for example, atrophy) or neuronal disorders (e.g., inflammation), or structural changes in synaptic density may possibly affect signal strength, which cannot be detected by structural magnetic resonance imaging methods (Matochik et al. 2005).
Second, there is the possibility of compensatory mechanisms. The human brain seems to be capable of a certain degree of functional reorganization between different brain regions. For instance, Chang et al. (2006) demonstrated an altered pattern of brain activation during visual attention in chronic CU and greater activation in a reserve brain network in active CU suggesting neuroadaptation in the attention network due to chronic marijuana exposure. The recruitment of additional regions, such as the prefrontal cortex and hippocampus differentiates users from controls during cognitive performance (Block et al. 2002; Eldreth et al. 2004; Gruber and Yurgelun-Todd 2005; Jager et al. 2007). This may indicate that increased neurocognitive resources are required to maintain memory and executive processes in this group. Moderately greater task-related activation in these areas may reflect impaired efficiency of processing following cannabis use, such that more activation is required to maintain normal performance. This is broadly consistent with the cognitive efficiency hypothesis (Vernon 1983) proposing that more direct connections between task-critical brain regions may correspond to decreases in task-related neural activity and improvements in performance (Rypma and D’Esposito 2000). Within imaging, activation patterns were not always clearly distributed. In understanding why some studies found evidence and other not, a functional compensation (Rajah and D’Esposito 2005) could be an answer: CU recruit more neural tissue in areas with high CB1 receptor density such as the frontal lobe, hippocampal/temporal regions, basal ganglia, and cingulate cortices to adequately perform the tasks. Findings suggest a change in neural recruitment throughout the course of abstinence. This could relate to “residual drug effects or withdrawal symptoms during early abstinence, less need for neural compensation, or a change in neurocognitive strategy as the brain adapts to different stages of sobriety” (Schweinsburg et al. 2008b, p. 8). Some polymorphisms from the endocannabinoid system have been repeatedly associated with drug addiction (Lopez-Moreno et al. 2012) but their exact influence on neurocognitive impairments is not well understood. However, even though the brain seems to be capable of activating brain regions in CU that are not usually engaged in NC to achieve the cognitive demand, the real impact of such alterations in daily users’ lives and its possibility to induce psychiatric disorders are still controversial (Martin-Santos et al. 2010).
Third, inter-substance interactions may have acted as confounders for (non-)effects. Prior research suggests differential neurocognitive effects of cannabis that depend on whether the individual also uses other substances (Lundqvist 2005; van Holst and Schilt 2011). There is marked inter-individual variability in the patterns of substance use (e.g., duration, frequency, dosage, type) and, with the exception of a few studies, most researchers were not able to definitively isolate the effects of a single specific substance due to a history of polysubstance use (Yucel et al. 2007). Some studies (e.g., Ashtari et al. 2011) did not control for the amount of alcohol or other drug intake. In other studies, interaction effects with binge drinking were found (Jacobus et al. 2009b; Mahmood et al. 2010). An under-investigated issue is whether concurrent use of different substances (e.g., cannabis and alcohol or methamphetamine) may potentiate the long-term adverse effects of each drug (Winward et al. 2014; Cuzen et al. 2015; Nguyen-Louie et al. 2015). For example, Jacobus et al. (2014) demonstrated significantly worse results in CU with concomitant alcohol use in the domains of complex attention, memory, processing speed, and visuospatial functioning compared to controls with limited substance use histories. However, the study was limited by a minimum abstinence of one day before testing and the question of different inter-substance effects. While synergistic or additive effects are plausible, there is also speculation that use of some substances may mask or protect against the neurocognitive sequelae of other substances (Jacobus et al. 2009b; Mahmood et al. 2010; Medina et al. 2007b; Schweinsburg et al. 2011). This even goes for inter-substance effects within cannabis itself: some evidence indicates that cannabidiol (CBD) might moderate the adverse effects of cannabis on mental health (Schoeler and Bhattacharyya 2013). As reported by Hermann (2011), cannabis and CBD can exert opposing effects that could be interpreted as a protecting factor. Next to neuroadaptive processes, this might explain the better performance by CU in some studies, for instance in the “visual spatial” domain (Chang et al. 2006; Medina et al. 2007a). To date, it is unclear whether increased cannabis content has been accompanied by changes in levels of CBD.
The Role of Early Onset
Impairments in EO users―compared to users with later onset of cannabis use―were less pronounced in neuropsychological tests than in the area of functional and structural imaging (for example, Jacobus et al. 2014). One possible explanation relates to the fact that imaging may be more powerful than test performance. As Pope et al. (2001a, b, p. 509) state, “individuals may display grossly normal performance on crude paper and pencil tests, yet show obvious abnormalities on more technically sophisticated tests, such as electrophysiological or imaging assessments”. Thus, while structural or functional imaging technology may detect a small but statistically significant difference between EO and later onset users on a given measure, the behavioral consequences of this difference may be subtle and undetectable in self-report and neuropsychological test. This, however, does not explain our findings as well as the idea that persons who begin consumption at early age may display stronger compensatory mechanisms that are made visible by functional and structural imaging. Their brain may have learned to balance deficits better through longer experience, but it uses more neurocognitive resources in performing the same tasks than the brain of users with later onset. Also, the neuropsychological tests chosen may have failed to tap important domains: even if an individual does not underperform on a variety of quantitative measures, he or she might display “subtle impairments of judgment or social inappropriateness that are readily detectable to a human observer” (Pope et al. 2001a, b, p. 509).
Perhaps our criterion of “onset under age 18” led to excluding studies with very similar subjects compared with the “EO” subjects (i.e., not much older when starting consumption). We may have missed some “EO” studies in cases where the information provided did not suffice to determine the exact age of onset. Besides this, age of onset is usually based on anamnestic data. The duration of time between consumption onset and testing is different for each individual. Both points in time are heterogeneous between studies, since neither age of onset nor an optimal age for testing are clearly defined. Hence, we can merely search for tentative associations between the beginning of consumption and trends regarding performance or activation. Above and beyond this, it is likely that there is another effect with far more impact than the age of substance use onset, and that is the intensity of use over time. Several findings indicate that there may be a threshold level, i.e. a dose-effect relationship that separates moderate and recreational CU from high-risk, heavy CU (Crean et al. 2011). In fact, all of the studies in our review explicitly referring to “heavy cannabis users” (Herning et al. 2005; Mahmood et al. 2010; Matochik et al. 2005; Pillay et al. 2008; Pujol et al. 2014) detected effects of CU in different domains. In contrast, results from a longitudinal cohort study by Pardini et al. (2015) suggest that adolescents who engage only in low to moderate marijuana use may experience an increase in observable attention and academic problems. However, these problems appeared to be minimal and were eliminated following sustained abstinence. It remains likely that residual impairments are linked to the duration and quantity of cannabis use, maybe more so than to the sole variable “age of onset“that – at least in the studies at hand – does not consistently separate moderate from heavy users. Block and Ghoneim (1993) compared intellectual functioning before the onset of drug use and in adult CU and NC by testing mathematical skills and verbal expression. They found that impairments depended on the frequency of chronic cannabis use. Cheetham et al. (2012) found smaller orbitofrontal cortex volumes at age 12 years that predicted initiation of cannabis use by age 16 years. The volumes of other regions (amygdala, hippocampus and anterior cingulate cortex) did not predict later cannabis use. In a longitudinal study, Fried et al. (2005) assessed cognitive functioning in 9- to 12-year-olds before the initiation of cannabis use, and again when youths were ages 17 to 21. After controlling for baseline performance and demographics, they found that current heavy cannabis use showed deficits in immediate and delayed memory, processing speed and overall IQ (cave: abstinence duration of 24 h). In general, prospective longitudinal studies have provided evidence for additional cognitive and brain abnormalities following the onset of CU that are above and beyond premorbid differences in personality, cognition and brain structure (Maurage et al. 2009; White and Batty 2012; Hicks et al. 2012; Meier et al. 2012; Jacobus et al. 2014; Nguyen-Louie et al. 2015). In the Dunedin study conducted in New Zealand, Meier et al. (2012) collected neuropsychological data of individuals at age 13 and again at age 38, after a pattern of persistent cannabis use had developed (although the status of abstinence was not asserted by testing). Persistent cannabis use was associated with neurocognitive decline broadly across domains of functioning, even after controlling for confounders such as education level. Impairment was concentrated among adolescent-onset CU, with more persistent use associated with greater decline. Further, cessation of cannabis use did not fully restore neurocognitive functioning among adolescent-onset CU.
Limitations
Regarding evidence level, studies did not exhibit much variance, so this criterion did not yield particularly meaningful differences for interpretation (studies of 2+ versus 2++ design quality according to SIGN). We tested if ES were biased depending on study quality. In neurocognitive function measures, ES was r = .343 (CI 95 % [.283; .400]) in 2+ studies, and r = .202 (CI 95 % [.082; .318]) in 2++ studies. Though studies of better design quality reveal lower ES than those of lower quality here, the difference is not significant (Z = 0.557; p = .289). In functional and structural imaging measures, ES was r = .449 (CI 95 % [.376; .517]) in 2+ studies, and r = .434 (CI 95 % [.287; .562]) in 2++ studies. Again, the difference between studies of different design quality is not significant (Z = 0.048; p = .481).
Seventeen of the included 38 empirical studies and three of the above-mentioned reviews were conducted by the same laboratory in San Diego/California (SDCA; research group around S.F. Tapert) which might hint at some bias in publications. However, in neurocognitive performance, both SDCA studies (I 2 = 49.7 %) and others (I 2 = 59.3 %) are of medium heterogeneity and their ES (SDCA r mean = .216; others r mean = .368) differ only with a small effect (q = .156; Cohen 1988). Thus, we assume the same underlying population effect could have been measured. In functional and structural imaging studies, SDCA studies (r mean = .673) and others (r mean = .392) do differ with a medium ES (q = .402), and SDCA studies reveal a high heterogeneity (I 2 = 72.0 %) whereas the others reveal zero heterogeneity (I 2 = −4.8 % ≈ 0 % by definition). But, inspecting Table 3, we assume that heterogeneity in SDCA imaging studies mainly reflects the variety of measures employed by this research group rather than population effects. In sum, we consider any possible bias contributed by the SDCA studies negligible.
Other factors further limit the interpretation of our results. For one thing, the methodological heterogeneity described above as well as differences in subjects, particularly with regards to amount and duration of cannabis use, make comparisons between studies difficult. For instance, drug amount used prior to abstinence was operationalized as “joints per day” or duration of time was “6 times per week”. The variability in the respondent’s definition of a ‘joint’ was as large as was the variability in “length of use” that was defined as “the time from initial use to time of current abstinence period” in some studies and as “beginning of time with heavy consumption” in others. Though these are common kinds of limitations in literature reviews, they must be mentioned here. Also the definition and measurement of our outcome parameters is difficult. For example what exactly are executive functions? One concept of executive functions could be that they are a heterogeneous psychological construct summarizing cognitive processes such as planning, task flexibility, impulsive control or problem solving as well as decision making and execution. It is not hard to understand the difficulty of subsuming all these different aspects in order to make one clear statement. Next, the inclusion criterion of a two week abstinence may have led to exclusion of subjects with poorer executive functions who were not able to refrain from the drug in this period of time. The characteristics of a drug-using lifestyle such as higher risk-taking make head injuries during intoxication hard to control. This plays a role in measuring neurocognitive performance. The cross-sectional nature of the studies at hand limits the interpretation of cause and effect as well as the domains included here, something that must be remarked after decades of cannabis research. Of specific relevance here are questions of co-occurring psychiatric disorders, including mood, anxiety, personality and especially psychotic disorders. Finally, gender as a variable of possible influence has not yet been adequately addressed. Research on the pharmacology of alcohol shows that men and women are affected differently. Several of the reported studies use all-male samples, and frequently, males are over-represented.
Where to go from Here
There is no easy explanation for complex reality. Like others before, this systematic review encountered shortcomings in preceding research and revealed certain desiderata. It is our goal to foster the ongoing scientific discussion on potential risks and neurocognitive long-term effects in CU. In this sense, this review contributes to the body of information towards a responsible social and health policy on cannabis and its use. Results are heterogenic and until now not fully understood. In our review we found no clear and general associations between neuropsychology, neurocognition and neuromorphology. However, our findings add a neurocognitive aspect to the growing body of evidence documenting the long-term psychosocial problems associated with phases of regular cannabis consumption (Wadsworth et al. 2006; Richer and Bergeron 2009; van Ours and Williams 2009; Best et al. 2005; Flory et al. 2004). They also point out barriers to a deeper understanding of neurocognitive effects caused by cannabis use that are yet to be overcome. We need studies with larger sample sizes that possess a larger generalizability and enable researchers to identify small(er) effects, also because a statistically small ES can be of major clinical relevance. And as Hall (2015) stated “there is a need for larger, better-controlled neuroimaging studies that use standardized tasks and measures” (p. 24). Also, we need better classification of terms and more accurate assessment methods with regard to predictors, confounders, and outcomes. Twin studies like Lyons et al. (2004) would help in the area of comparability and untangling cause and effect such as the abovementioned issue of preexisting brain abnormalities. Finally, we need more longitudinal-design studies. Recently, there have been first efforts to gain longitudinal data (Squeglia et al. 2014; Nguyen-Louie et al. 2014; Pardini et al. 2015). An ideal study would obtain neuropsychological testing results from a large general population, and then obtain neuropsychological results many years later, after some of the individuals had become CU and combining functional as well as structural information of the human brain. By taking into account a broader array of variables, longitudinal studies can put neurocognitive research back into proportion by uncovering the brain’s role in shaping young and heavy cannabis consumer’s trajectories toward adverse psychosocial outcomes over time.
Notes
2++ = “high quality systematic reviews of case control or cohort studies, high quality case control or cohort studies with a very low risk of confounding or bias and a high probability that the relationship is causal”
2+ = “well conducted case control or cohort studies with a low risk of confounding or bias and a moderate probability that the relationship is causal” (Baker et al. 2010, p. 359)
By convention, effects of r = .10 to .29 count as small, effects of r = .30 to 0.49 as medium and effects of r = 0.50 or more as large (Cohen 1988).
References
Agurell, S., Halldin, M., Lindgren, J. E., Ohlsson, A., Widman, M., Gillespie, H., & Hollister, L. (1986). Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacological Reviews, 38(1), 21–43.
Ashtari, M., Avants, B., Cyckowski, L., Cervellione, K. L., Roofeh, D., Cook, P., et al. (2011). Medial temporal structures and memory functions in adolescents with heavy cannabis use. Journal of Psychiatric Research, 45(8), 1055–1066. doi:10.1016/j.jpsychires.2011.01.004.
Ashtari, M., Cervellione, K., Cottone, J., Ardekani, B. A., Sevy, S., & Kumra, S. (2009). Diffusion abnormalities in adolescents and young adults with a history of heavy cannabis use. Journal of Psychiatric Research, 43(3), 189–204. doi:10.1016/j.jpsychires.2008.12.002.
Ashton, C. H. (1999). Adverse effects of cannabis and cannabinoids. British Journal of Anaesthesia, 83(4), 637–649.
Ashton, C. H. (2001). Pharmacology and effects of cannabis: a brief review. British Journal of Psychiatry, 178, 101–106.
Baker, A., Young, K., Potter, J., & Madan, I. (2010). A review of grading systems for evidence-based guidelines produced by medical specialties. Clinical Medicine, 10(4), 358–363.
Barnes, T. R., Mutsatsa, S. H., Hutton, S. B., Watt, H. C., & Joyce, E. M. (2006). Comorbid substance use and age at onset of schizophrenia. British Journal of Psychiatry, 188, 237–242. doi:10.1192/bjp.bp.104.007237.
Batalla, A., Bhattacharyya, S., Yucel, M., Fusar-Poli, P., Crippa, J. A., Nogue, S., et al. (2013). Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS One, 8(2), e55821. doi:10.1371/journal.pone.0055821.
Belue, R. C., Howlett, A. C., Westlake, T. M., & Hutchings, D. E. (1995). The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicology and Teratology, 17(1), 25–30.
Best, D., Gross, S., Manning, V., Gossop, M., Witton, J., & Strang, J. (2005). Cannabis use in adolescents: the impact of risk and protective factors and social functioning. Drug and Alcohol Review, 24(6), 483–488. doi:10.1080/09595230500292920.
Block, R. I., & Ghoneim, M. M. (1993). Effects of chronic marijuana use on human cognition. Psychopharmacology, 110(1–2), 219–228.
Block, R. I., O’Leary, D. S., Hichwa, R. D., Augustinack, J. C., Boles Ponto, L. L., Ghoneim, M. M., et al. (2002). Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacology Biochemistry and Behavior, 72(1–2), 237–250.
Bolla, K. I., Brown, K., Eldreth, D., Tate, K., & Cadet, J. L. (2002). Dose-related neurocognitive effects of marijuana use. Neurology, 59(9), 1337–1343.
Bolla, K. I., Eldreth, D. A., Matochik, J. A., & Cadet, J. L. (2005). Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage, 26(2), 480–492. doi:10.1016/j.neuroimage.2005.02.012.
Bosker, W. M., Karschner, E. L., Lee, D., Goodwin, R. S., Hirvonen, J., Innis, R. B., et al. (2013). Psychomotor function in chronic daily Cannabis smokers during sustained abstinence. PloS One, 8(1), e53127. doi:10.1371/journal.pone.0053127.
Brook, J. S., Stimmel, M. A., Zhang, C., & Brook, D. W. (2008). The association between earlier marijuana use and subsequent academic achievement and health problems: a longitudinal study. The American Journal on Addictions, 17(2), 155–160. doi:10.1080/10550490701860930.
Budney, A. J., Hughes, J. R., Moore, B. A., & Vandrey, R. (2004). Review of the validity and significance of cannabis withdrawal syndrome. The American Journal of Psychiatry, 161(11), 1967–1977. doi:10.1176/appi.ajp.161.11.1967.
Budney, A. J., Roffman, R., Stephens, R. S., & Walker, D. (2007). Marijuana dependence and its treatment. Addiction Science & Clinical Practice, 4(1), 4–16.
Chang, L., & Chronicle, E. P. (2007). Functional imaging studies in cannabis users. The Neuroscientist, 13(5), 422–432. doi:10.1177/1073858406296601.
Chang, L., Yakupov, R., Cloak, C., & Ernst, T. (2006). Marijuana use is associated with a reorganized visual-attention network and cerebellar hypoactivation. Brain, 129(Pt 5), 1096–1112. doi:10.1093/brain/awl064.
Cheetham, A., Allen, N. B., Whittle, S., Simmons, J. G., Yucel, M., & Lubman, D. I. (2012). Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biological Psychiatry, 71(8), 684–692. doi:10.1016/j.biopsych.2011.10.029.
Clark, L., Roiser, J. P., Robbins, T. W., & Sahakian, B. J. (2009). Disrupted ‘reflection’ impulsivity in cannabis users but not current or former ecstasy users. Journal of Psychopharmacology, 23(1), 14–22. doi:10.1177/0269881108089587.
Cohen, J. (1988). Statistical power analysis for the behavioral sciences (2nd ed.). Hillsdale: Lawrence Erlbaum Associates.
Crane, N. A., Schuster, R. M., Fusar-Poli, P., & Gonzalez, R. (2013). Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychology Review, 23(2), 117–137. doi:10.1007/s11065-012-9222-1.
Crean, R. D., Crane, N. A., & Mason, B. J. (2011). An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine, 5(1), 1–8. doi:10.1097/ADM.0b013e31820c23fa.
Cuzen, N. L., Koopowitz, S., Ferrett, H. L., Stein, D. J., & Yurgelun-Todd, D. (2015). Methamphetamine and cannabis abuse in adolescence: a quasi-experimental study on specific and long-term neurocognitive effects. BMJ, 5(1), e005833. doi:10.1136/bmjopen-2014-005833.
Danovitch, I., & Gorelick, D. A. (2012). State of the art treatments for cannabis dependence. The Psychiatric Clinics of North America, 35(2), 309–326. doi:10.1016/j.psc.2012.03.003.
De Bellis, M. D., Wang, L., Bergman, S. R., Yaxley, R. H., Hooper, S. R., & Huettel, S. A. (2013). Neural mechanisms of risky decision-making and reward response in adolescent onset cannabis use disorder. Drug and Alcohol Dependence, 133(1), 134–145. doi:10.1016/j.drugalcdep.2013.05.020.
Devane, W. A., Dysarz, F. A., 3rd, Johnson, M. R., Melvin, L. S., & Howlett, A. C. (1988). Determination and characterization of a cannabinoid receptor in rat brain. Molecular Pharmacology, 34(5), 605–613.
Di Forti, M., Morrison, P. D., Butt, A., & Murray, R. M. (2007). Cannabis use and psychiatric and cogitive disorders: the chicken or the egg? Current Opinion in Psychiatry, 20(3), 228–234. doi:10.1097/YCO.0b013e3280fa838e.
Earleywine, M. (2002). Understanding marijuana: a new look at the scientific evidence Oxford University Press.
Egerton, A., Allison, C., Brett, R. R., & Pratt, J. A. (2006). Cannabinoids and prefrontal cortical function: insights from preclinical studies. Neuroscience and Biobehavioral Reviews, 30(5), 680–695. doi:10.1016/j.neubiorev.2005.12.002.
Eldreth, D. A., Matochik, J. A., Cadet, J. L., & Bolla, K. I. (2004). Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage, 23(3), 914–920. doi:10.1016/j.neuroimage.2004.07.032.
Fernandez-Serrano, M. J., Perez-Garcia, M., & Verdejo-Garcia, A. (2010). What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neuroscience and Biobehavioral Reviews, 35(3), 377–406. doi:10.1016/j.neubiorev.2010.04.008.
Flory, K., Lynam, D., Milich, R., Leukefeld, C., & Clayton, R. (2004). Early adolescent through young adult alcohol and marijuana use trajectories: early predictors, young adult outcomes, and predictive utility. Development and Psychopathology, 16(1), 193–213.
Freedland, C. S., Whitlow, C. T., Miller, M. D., & Porrino, L. J. (2002). Dose-dependent effects of Delta9-tetrahydrocannabinol on rates of local cerebral glucose utilization in rat. Synapse, 45(2), 134–142. doi:10.1002/syn.10089.
Fried, P. A., Watkinson, B., & Gray, R. (2005). Neurocognitive consequences of marihuana--a comparison with pre-drug performance. Neurotoxicology and Teratology, 27(2), 231–239. doi:10.1016/j.ntt.2004.11.003.
Garcia-Lazaro, H. G., Ramirez-Carmona, R., Lara-Romero, R., & Roldan-Valadez, E. (2012). Neuroanatomy of episodic and semantic memory in humans: a brief review of neuroimaging studies. Neurology India, 60(6), 613–617. doi:10.4103/0028-3886.105196.
Gogtay, N., Giedd, J. N., Lusk, L., Hayashi, K. M., Greenstein, D., Vaituzis, A. C., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–8179. doi:10.1073/pnas.0402680101.
Gonzalez, R. (2007). Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychology Review, 17(3), 347–361. doi:10.1007/s11065-007-9036-8.
Gonzalez, S., Cebeira, M., & Fernandez-Ruiz, J. (2005). Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacology Biochemistry and Behavior, 81(2), 300–318. doi:10.1016/j.pbb.2005.01.028.
Grant, I., Gonzalez, R., Carey, C. L., Natarajan, L., & Wolfson, T. (2003). Non-acute (residual) neurocognitive effects of cannabis use: a meta-analytic study. Journal of the International Neuropsychological Society, 9(5), 679–689. doi:10.1017/s1355617703950016.
Gruber, S. A., Silveri, M. M., Dahlgren, M. K., & Yurgelun-Todd, D. (2011). Why so impulsive? White matter alterations are associated with impulsivity in chronic marijuana smokers. Experimental and Clinical Psychopharmacology, 19(3), 231–242. doi:10.1037/a0023034.
Gruber, S. A., & Yurgelun-Todd, D. A. (2005). Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Research. Cognitive Brain Research, 23(1), 107–118. doi:10.1016/j.cogbrainres.2005.02.016.
Hall, W. (2015). What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction, 110(1), 19–35. doi:10.1111/add.12703.
Hall, W., & Degenhardt, L. (2014). The adverse health effects of chronic cannabis use. Drug Testing and Analysis, 6(1–2), 39–45. doi:10.1002/dta.1506.
Hanson, K. L., Winward, J. L., Schweinsburg, A. D., Medina, K. L., Brown, S. A., & Tapert, S. F. (2010). Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors, 35(11), 970–976. doi:10.1016/j.addbeh.2010.06.012.
Harbour, R., & Miller, J. (2001). A new system for grading recommendations in evidence based guidelines. BMJ, 323(7308), 334–336.
Herkenham, M., Lynn, A. B., Little, M. D., Johnson, M. R., Melvin, L. S., de Costa, B. R., & Rice, K. C. (1990). Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the United States of America, 87(5), 1932–1936.
Hermann, D. (2011). Wirkung von Cannabinoiden auf das Gehirn: Ein Überblick über MRI Befunde. [Cannabinoids and the brain: review of MRI studies]. Sucht, 57(3), 161–171. doi:10.1024/0939-5911.a000108.
Herning, R. I., Better, W., & Cadet, J. L. (2008). EEG of chronic marijuana users during abstinence: relationship to years of marijuana use, cerebral blood flow and thyroid function. Clinical Neurophysiology, 119(2), 321–331. doi:10.1016/j.clinph.2007.09.140.
Herning, R. I., Better, W. E., Tate, K., & Cadet, J. L. (2005). Cerebrovascular perfusion in marijuana users during a month of monitored abstinence. Neurology, 64(3), 488–493. doi:10.1212/01.wnl.0000150882.69371.dd.
Hicks, B. M., Durbin, C. E., Blonigen, D. M., Iacono, W. G., & McGue, M. (2012). Relationship between personality change and the onset and course of alcohol dependence in young adulthood. Addiction, 107(3), 540–548. doi:10.1111/j.1360-0443.2011.03617.x.
Hirvonen, J., Goodwin, R. S., Li, C. T., Terry, G. E., Zoghbi, S. S., Morse, C., et al. (2012). Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Molecular Psychiatry, 17(6), 642–649. doi:10.1038/mp.2011.82.
Hoch, E., Bonnet, U., Thomasius, R., Ganzer, F., Havemann-Reinecke, U., & Preuss, U. W. (2015). Risks associated with the non-medicinal use of cannabis. Deutsches Ärzteblatt International, 112(16), 271–278. doi:10.3238/arztebl.2015.0271.
Hooper, S. R., Woolley, D., & De Bellis, M. D. (2014). Intellectual, neurocognitive, and academic achievement in abstinent adolescents with cannabis use disorder. Psychopharmacology, 231(8), 1467–1477. doi:10.1007/s00213-014-3463-z.
Horti, A. G., & Van Laere, K. (2008). Development of radioligands for in vivo imaging of type 1 cannabinoid receptors (CB1) in human brain. Current Pharmaceutical Design, 14(31), 3363–3383.
Huedo-Medina, T. B., Sanchez-Meca, J., Marin-Martinez, F., & Botella, J. (2006). Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological Methods, 11(2), 193–206. doi:10.1037/1082-989x.11.2.193.
Iversen, L. (2003). Cannabis and the brain. Brain, 126(Pt 6), 1252–1270.
Iversen, L. (2005). Long-term effects of exposure to cannabis. Current Opinion in Pharmacology, 5(1), 69–72. doi:10.1016/j.coph.2004.08.010.
Jacobsen, L. K., Mencl, W. E., Westerveld, M., & Pugh, K. R. (2004). Impact of cannabis use on brain function in adolescents. Annals of the New York Academy of Sciences, 1021, 384–390. doi:10.1196/annals.1308.053.
Jacobsen, L. K., Pugh, K. R., Constable, R. T., Westerveld, M., & Mencl, W. E. (2007). Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry, 61(1), 31–40. doi:10.1016/j.biopsych.2006.02.014.
Jacobus, J., Bava, S., Cohen-Zion, M., Mahmood, O., & Tapert, S. F. (2009a). Functional consequences of marijuana use in adolescents. Pharmacology Biochemistry and Behavior, 92(4), 559–565. doi:10.1016/j.pbb.2009.04.001.
Jacobus, J., Goldenberg, D., Wierenga, C. E., Tolentino, N. J., Liu, T. T., & Tapert, S. F. (2012). Altered cerebral blood flow and neurocognitive correlates in adolescent cannabis users. Psychopharmacology, 222(4), 675–684. doi:10.1007/s00213-012-2674-4.
Jacobus, J., McQueeny, T., Bava, S., Schweinsburg, B. C., Frank, L. R., Yang, T. T., & Tapert, S. F. (2009b). White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology, 31(6), 349–355. doi:10.1016/j.ntt.2009.07.006.
Jacobus, J., Squeglia, L. M., Sorg, S. F., Nguyen-Louie, T. T., & Tapert, S. F. (2014). Cortical thickness and neurocognition in adolescent marijuana and alcohol users following 28 days of monitored abstinence. Journal of Studies on Alcohol, 75(5), 729–743. doi:10.15288/jsad.2014.75.729.
Jacobus, J., & Tapert, S. F. (2014). Effects of cannabis on the adolescent brain. Current Pharmaceutical Design, 20(13), 2186–2193. doi:10.2174/13816128113199990426.
Jager, G., Block, R. I., Luijten, M., & Ramsey, N. F. (2010). Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. Journal of the American Academy of Child and Adolescent Psychiatry, 49(6), 561–572. doi:10.1016/j.jaac.2010.02.001.
Jager, G., Van Hell, H. H., De Win, M. M., Kahn, R. S., Van Den Brink, W., Van Ree, J. M., & Ramsey, N. F. (2007). Effects of frequent cannabis use on hippocampal activity during an associative memory task. European Neuropsychopharmacology, 17(4), 289–297. doi:10.1016/j.euroneuro.2006.10.003.
Johns, A. (2001). Psychiatric effects of cannabis. British Journal of Psychiatry, 178, 116–122.
Khiabani, H. Z., & Mørland, J. (2007). Cannabis og cannabinoider som legemidler. Tidsskrift for den Norske Lægeforening, 127(5), 579–582.
Kouri, E. M., & Pope, H. G., Jr. (2000). Abstinence symptoms during withdrawal from chronic marijuana use. Experimental and Clinical Psychopharmacology, 8(4), 483–492.
Lenhard, W., & Lenhard, A. (2015). Berechnung von Effektstärken [Computation of Effect Sizes]. Software from Psychometrica http://www.psychometrica.de/freeware.html.
Lenroot, R. K., & Giedd, J. N. (2006). Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews, 30(6), 718–729. doi:10.1016/j.neubiorev.2006.06.001.
Lisdahl, K. M., Gilbart, E. R., Wright, N. E., & Shollenbarger, S. (2013). Dare to delay? The impacts of adolescent alcohol and marijuana use onset on cognition, brain structure, and function. Front Psychiatry, 4, 53. doi:10.3389/fpsyt.2013.00053.
Lisdahl, K. M., Wright, N. E., Kirchner-Medina, C., Maple, K. E., & Shollenbarger, S. (2014). The effects of regular cannabis use on neurocognition in adolescents and young adults. Current Addiction Reports, 1(2), 144–156. doi:10.1007/s40429-014-0019-6.
Lopez-Moreno, J. A., Echeverry-Alzate, V., & Buhler, K. M. (2012). The genetic basis of the endocannabinoid system and drug addiction in humans. Journal of Psychopharmacology, 26(1), 133–143. doi:10.1177/0269881111416689.
Lorenzetti, V., Solowij, N., Fornito, A., Lubman, D. I., & Yucel, M. (2014). The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Current Pharmaceutical Design, 20(13), 2138–2167.
Lubman, D. I., Cheetham, A., & Yucel, M. (2015). Cannabis and adolescent brain development. Pharmacology and Therapeutics, 148, 1–16. doi:10.1016/j.pharmthera.2014.11.009.
Lundqvist, T. (2005). Cognitive consequences of cannabis use: comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacology Biochemistry and Behavior, 81(2), 319–330. doi:10.1016/j.pbb.2005.02.017.
Lyons, M. J., Bar, J. L., Panizzon, M. S., Toomey, R., Eisen, S., Xian, H., & Tsuang, M. T. (2004). Neuropsychological consequences of regular marijuana use: a twin study. Psychological Medicine, 34(7), 1239–1250.
Mahmood, O. M., Jacobus, J., Bava, S., Scarlett, A., & Tapert, S. F. (2010). Learning and memory performances in adolescent users of alcohol and marijuana: interactive effects. Journal of Studies on Alcohol and Drugs, 71(6), 885–894.
Martin-Santos, R., Fagundo, A. B., Crippa, J. A., Atakan, Z., Bhattacharyya, S., Allen, P., et al. (2010). Neuroimaging in cannabis use: a systematic review of the literature. Psychological Medicine, 40(3), 383–398. doi:10.1017/s0033291709990729.
Matochik, J. A., Eldreth, D. A., Cadet, J. L., & Bolla, K. I. (2005). Altered brain tissue composition in heavy marijuana users. Drug and Alcohol Dependence, 77(1), 23–30. doi:10.1016/j.drugalcdep.2004.06.011.
Maurage, P., Pesenti, M., Philippot, P., Joassin, F., & Campanella, S. (2009). Latent deleterious effects of binge drinking over a short period of time revealed only by electrophysiological measures. Journal of Psychiatry and Neuroscience, 34(2), 111–118.
Maykut, M. O. (1985). Health consequences of acute and chronic marihuana use. Progress in Neuropsychopharmacology and Biological Psychiatry, 9(3), 209–238.
McQueeny, T., Padula, C. B., Price, J., Medina, K. L., Logan, P., & Tapert, S. F. (2011). Gender effects on amygdala morphometry in adolescent marijuana users. Behavioural Brain Research, 224(1), 128–134. doi:10.1016/j.bbr.2011.05.031.
Medina, K. L., Hanson, K. L., Schweinsburg, A. D., Cohen-Zion, M., Nagel, B. J., & Tapert, S. F. (2007a). Neuropsychological functioning in adolescent marijuana users: subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society, 13(5), 807–820. doi:10.1017/s1355617707071032.
Medina, K. L., McQueeny, T., Nagel, B. J., Hanson, K. L., Yang, T. T., & Tapert, S. F. (2009). Prefrontal cortex morphometry in abstinent adolescent marijuana users: subtle gender effects. Addiction Biology, 14(4), 457–468. doi:10.1111/j.1369-1600.2009.00166.x.
Medina, K. L., Nagel, B. J., Park, A., McQueeny, T., & Tapert, S. F. (2007b). Depressive symptoms in adolescents: associations with white matter volume and marijuana use. Journal of Child Psychology and Psychiatry, 48(6), 592–600. doi:10.1111/j.1469-7610.2007.01728.x.
Medina, K. L., Nagel, B. J., & Tapert, S. F. (2010). Abnormal cerebellar morphometry in abstinent adolescent marijuana users. Psychiatry Research, 182(2), 152–159. doi:10.1016/j.pscychresns.2009.12.004.
Meier, M. H., Caspi, A., Ambler, A., Harrington, H., Houts, R., Keefe, R. S., et al. (2012). Persistent cannabis users show neuropsychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America, 109(40), E2657–2664. doi:10.1073/pnas.1206820109.
Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine, 6(7), e1000097. doi:10.1371/journal.pmed.1000097.
Moore, D. R., Florsheim, P., & Butner, J. (2007). Interpersonal behavior, psychopathology, and relationship outcomes among adolescent mothers and their partners. Journal of Clinical Child and Adolescent Psychology, 36(4), 541–556. doi:10.1080/15374410701662709.
Nguyen-Louie, T. T., Castro, N., Matt, G. E., Squeglia, L. M., Brumback, T., & Tapert, S. F. (2015). Effects of emerging alcohol and marijuana use behaviors on adolescents’ neuropsychological functioning over four years. Journal of Studies on Alcohol and Drugs, 76(5), 738–748. doi:10.15288/jsad.2015.76.738.
Padula, C. B., McQueeny, T., Lisdahl, K. M., Price, J. S., & Tapert, S. F. (2015). Craving is associated with amygdala volumes in adolescent marijuana users during abstinence. The American Journal of Drug and Alcohol Abuse, 41(2), 127–132. doi:10.3109/00952990.2014.966198.
Padula, C. B., Schweinsburg, A. D., & Tapert, S. F. (2007). Spatial working memory performance and fMRI activation interaction in abstinent adolescent marijuana users. Psychology of Addictive Behaviors, 21(4), 478–487. doi:10.1037/0893-164x.21.4.478.
Pardini, D., White, H. R., Xiong, S., Bechtold, J., Chung, T., Loeber, R., & Hipwell, A. (2015). Unfazed or dazed and confused: does early adolescent marijuana use cause sustained impairments in attention and academic functioning? Journal of Abnormal Child Psychology, 43(7), 1203–1217. doi:10.1007/s10802-015-0012-0.
Pertwee, R. G. (2005). Pharmacological actions of cannabinoids. Handbook of Experimental Pharmacology (168):1–51.
Pillay, S. S., Rogowska, J., Kanayama, G., Gruber, S., Simpson, N., Pope, H. G., & Yurgelun-Todd, D. A. (2008). Cannabis and motor function: fMRI changes following 28 days of discontinuation. Experimental and Clinical Psychopharmacology, 16(1), 22–32. doi:10.1037/1064-1297.16.1.22.
Pontieri, F. E., Conti, G., Zocchi, A., Fieschi, C., & Orzi, F. (1999). Metabolic mapping of the effects of WIN 55212-2 intravenous administration in the rat. Neuropsychopharmacology, 21(6), 773–776. doi:10.1016/s0893-133x(99)00064-0.
Pope, H. G., Jr., Gruber, A. J., Hudson, J. I., Huestis, M. A., & Yurgelun-Todd, D. (2001a). Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry, 58(10), 909–915.
Pope, H. G., Jr., Gruber, A. J., & Yurgelun-Todd, D. (1995). The residual neuropsychological effects of cannabis: the current status of research. Drug and Alcohol Dependence, 38(1), 25–34.
Pope, H. G., Jr., Gruber, A. J., & Yurgelun-Todd, D. (2001b). Residual neuropsychologic effects of cannabis. Current Psychiatry Reports, 3(6), 507–512.
Pujol, J., Blanco-Hinojo, L., Batalla, A., Lopez-Sola, M., Harrison, B. J., Soriano-Mas, C., et al. (2014). Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. Journal of Psychiatric Research, 51, 68–78. doi:10.1016/j.jpsychires.2013.12.008.
Quickfall, J., & Crockford, D. (2006). Brain neuroimaging in cannabis use: a review. The Journal of Neuropsychiatry and Clinical Neurosciences, 18(3), 318–332. doi:10.1176/appi.neuropsych.18.3.318.
Radbruch, L., & Nauck, F. (2003). Cannabinoide--Nebenwirkungen und Komplikationen. Der Schmerz, 17(4), 274–279. doi:10.1007/s00482-003-0232-z.
Rajah, M. N., & D’Esposito, M. (2005). Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain, 128(Pt 9), 1964–1983. doi:10.1093/brain/awh608.
Ranganathan, M., & D’Souza, D. C. (2006). The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology, 188(4), 425–444. doi:10.1007/s00213-006-0508-y.
Realini, N., Rubino, T., & Parolaro, D. (2009). Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacological Research, 60(2), 132–138. doi:10.1016/j.phrs.2009.03.006.
Richer, I., & Bergeron, J. (2009). Driving under the influence of cannabis: links with dangerous driving, psychological predictors, and accident involvement. Accident Analysis and Prevention, 41(2), 299–307. doi:10.1016/j.aap.2008.12.004.
Rocchetti, M., Crescini, A., Borgwardt, S., Caverzasi, E., Politi, P., Atakan, Z., & Fusar-Poli, P. (2013). Is cannabis neurotoxic for the healthy brain? A meta-analytical review of structural brain alterations in non-psychotic users. Psychiatry and Clinical Neurosciences, 67(7), 483–492. doi:10.1111/pcn.12085.
Romero, J., Garcia, L., Fernandez-Ruiz, J. J., Cebeira, M., & Ramos, J. A. (1995). Changes in rat brain cannabinoid binding sites after acute or chronic exposure to their endogenous agonist, anandamide, or to delta 9-tetrahydrocannabinol. Pharmacology Biochemistry and Behavior, 51(4), 731–737.
Roncero, C., Egido, A., Rodriguez-Cintas, L., Perez-Pazos, J., Collazos, F., & Casas, M. (2015). Substance use among medical students: a literature review 1988–2013. Actas Españolas de Psiquiatría, 43(3), 109–121.
Rosenberg, M. F., & Anthony, J. C. (2001). Early clinical manifestations of cannabis dependence in a community sample. Drug and Alcohol Dependence, 64(2), 123–131.
Rosenthal, R., & DiMatteo, M. R. (2001). Meta-analysis: recent developments in quantitative methods for literature reviews. Annual Review of Psychology, 52, 59–82. doi:10.1146/annurev.psych.52.1.59.
Roten, A., Baker, N. L., & Gray, K. M. (2015). Cognitive performance in a placebo-controlled pharmacotherapy trial for youth with marijuana dependence. Addictive Behaviors, 45, 119–123. doi:10.1016/j.addbeh.2015.01.013.
Rubino, T., & Parolaro, D. (2008). Long lasting consequences of cannabis exposure in adolescence. Molecular and Cellular Endocrinology, 286(1–2 Suppl 1), S108–113. doi:10.1016/j.mce.2008.02.003.
Rypma, B., & D’Esposito, M. (2000). Isolating the neural mechanisms of age-related changes in human working memory. Nature Neuroscience, 3(5), 509–515. doi:10.1038/74889.
Saban, A., Flisher, A. J., Grimsrud, A., Morojele, N., London, L., Williams, D. R., & Stein, D. J. (2014). The association between substance use and common mental disorders in young adults: results from the South African Stress and Health (SASH) survey. The Pan African Medical Journal, 17(Suppl 1), 11. doi:10.11694/pamj.supp.2014.17.1.3328.
Schneider, M. (2008). Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addiction Biology, 13(2), 253–263. doi:10.1111/j.1369-1600.2008.00110.x.
Schoeler, T., & Bhattacharyya, S. (2013). The effect of cannabis use on memory function: an update. Substance Abuse and Rehabilitation, 4, 11–27. doi:10.2147/sar.s25869.
Schreiner, A. M., & Dunn, M. E. (2012). Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Experimental and Clinical Psychopharmacology, 20(5), 420–429. doi:10.1037/a0029117.
Schulte, M. H. J., Cousijn, J., den Uyl, T. E., Goudriaan, A. E., van den Brink, W., & Veltman, D. J. (2014). Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clinical Psychology Review, 34(7), 531–550. doi:10.1016/j.cpr.2014.08.002.
Schweinsburg, A. D., Brown, S. A., & Tapert, S. F. (2008a). The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Reviews, 1(1), 99–111.
Schweinsburg, A. D., Nagel, B. J., Schweinsburg, B. C., Park, A., Theilmann, R. J., & Tapert, S. F. (2008b). Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Research, 163(1), 40–51. doi:10.1016/j.pscychresns.2007.04.018.
Schweinsburg, A. D., Schweinsburg, B. C., Medina, K. L., McQueeny, T., Brown, S. A., & Tapert, S. F. (2010). The influence of recency of use on fMRI response during spatial working memory in adolescent marijuana users. Journal of Psychoactive Drugs, 42(3), 401–412. doi:10.1080/02791072.2010.10400703.
Schweinsburg, A. D., Schweinsburg, B. C., Nagel, B. J., Eyler, L. T., & Tapert, S. F. (2011). Neural correlates of verbal learning in adolescent alcohol and marijuana users. Addiction, 106(3), 564–573. doi:10.1111/j.1360-0443.2010.03197.x.
Scott, J., Scott, E. M., Hermens, D. F., Naismith, S. L., Guastella, A. J., White, D., et al. (2014). Functional impairment in adolescents and young adults with emerging mood disorders. British Journal of Psychiatry, 205(5), 362–368. doi:10.1192/bjp.bp.113.134262.
Sevy, S., Smith, G. S., Ma, Y., Dhawan, V., Chaly, T., Kingsley, P. B., et al. (2008). Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology, 197(4), 549–556. doi:10.1007/s00213-008-1075-1.
Silins, E., Horwood, L. J., Patton, G. C., Fergusson, D. M., Olsson, C. A., Hutchinson, D. M., et al. (2014). Young adult sequelae of adolescent cannabis use: an integrative analysis. The Lancet Psychiatry, 1(4), 286–293. doi:10.1016/S2215-0366(14)70307-4.
Sim-Selley, L. J. (2003). Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Critical Reviews in Neurobiology, 15(2), 91–119.
Sneider, J.T., Mashhoon, Y., & Silveri, M.M. (2013). A review of magnetic resonance spectroscopy studies in marijuana using adolescents and adults. Journal of Addiction Research & Theraphy, Suppl 4. doi: 10.4172/2155-6105.S4-010
Sneider, J. T., Pope, H. G., Jr., Silveri, M. M., Simpson, N. S., Gruber, S. A., & Yurgelun-Todd, D. A. (2008). Differences in regional blood volume during a 28-day period of abstinence in chronic cannabis smokers. European Neuropsychopharmacology, 18(8), 612–619. doi:10.1016/j.euroneuro.2008.04.016.
Solowij, N., & Battisti, R. (2008). The chronic effects of cannabis on memory in humans: a review. Current Drug Abuse Reviews, 1(1), 81–98.
Solowij, N., & Pesa, N. (2010). Anormalidades cognitivas no uso da cannabis. Revista Brasileira de Psiquiatria, 32(1), 31–40.
Sowell, E. R., Thompson, P. M., Leonard, C. M., Welcome, S. E., Kan, E., & Toga, A. W. (2004a). Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience, 24(38), 8223–8231. doi:10.1523/jneurosci.1798-04.2004.
Sowell, E. R., Thompson, P. M., & Toga, A. W. (2004b). Mapping changes in the human cortex throughout the span of life. The Neuroscientist, 10(4), 372–392. doi:10.1177/1073858404263960.
Squeglia, L. M., Jacobus, J., Nguyen-Louie, T. T., & Tapert, S. F. (2014). Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology, 28(5), 782–790. doi:10.1037/neu0000083.
Stiby, A. I., Hickman, M., Munafo, M. R., Heron, J., Yip, V. L., & Macleod, J. (2015). Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction, 110(4), 658–668. doi:10.1111/add.12827.
Tapert, S. F., Schweinsburg, A. D., Drummond, S. P., Paulus, M. P., Brown, S. A., Yang, T. T., & Frank, L. R. (2007). Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology, 194(2), 173–183. doi:10.1007/s00213-007-0823-y.
Trezza, V., Cuomo, V., & Vanderschuren, L. J. (2008). Cannabis and the developing brain: insights from behavior. European Journal of Pharmacology, 585(2–3), 441–452. doi:10.1016/j.ejphar.2008.01.058.
van Holst, R. J., & Schilt, T. (2011). Drug-related decrease in neuropsychological functions of abstinent drug users. Current Drug Abuse Reviews, 4(1), 42–56.
van Ours, J. C., & Williams, J. (2009). Why parents worry: initiation into cannabis use by youth and their educational attainment. Journal of Health Economics, 28(1), 132–142. doi:10.1016/j.jhealeco.2008.09.001.
Verdejo-Garcia, A., Benbrook, A., Funderburk, F., David, P., Cadet, J. L., & Bolla, K. I. (2007). The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcohol Dependence, 90(1), 2–11. doi:10.1016/j.drugalcdep.2007.02.004.
Verdejo-Garcia, A., Lopez-Torrecillas, F., Gimenez, C. O., & Perez-Garcia, M. (2004). Clinical implications and methodological challenges in the study of the neuropsychological correlates of cannabis, stimulant, and opioid abuse. Neuropsychology Review, 14(1), 1–41.
Vernon, P. A. (1983). Speed of information processing and general intelligence. Intelligence, 7, 53–70. doi:10.1016/0160-2896(83)90006-5.
Volkow, N. D., Baler, R. D., Compton, W. M., & Weiss, S. R. (2014). Adverse health effects of marijuana use. New England Journal of Medicine, 370(23), 2219–2227. doi:10.1056/NEJMra1402309.
Volkow, N. D., Gillespie, H., Mullani, N., Tancredi, L., Grant, C., Valentine, A., & Hollister, L. (1996). Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Research, 67(1), 29–38.
Wadsworth, E. J., Moss, S. C., Simpson, S. A., & Smith, A. P. (2006). A community based investigation of the association between cannabis use, injuries and accidents. Journal of Psychopharmacology, 20(1), 5–13. doi:10.1177/0269881105056642.
White, J., & Batty, G. D. (2012). Intelligence across childhood in relation to illegal drug use in adulthood: 1970 British Cohort Study. Journal of Epidemiology and Community Health, 66(9), 767–774. doi:10.1136/jech-2011-200252.
Whiting, P. F., Wolff, R. F., Deshpande, S., Di Nisio, M., Duffy, S., Hernandez, A. V., et al. (2015). Cannabinoids for medical use: a systematic review and meta-analysis. JAMA, 313(24), 2456–2473. doi:10.1001/jama.2015.6358.
Wiesbeck, G. A., Schuckit, M. A., Kalmijn, J. A., Tipp, J. E., Bucholz, K. K., & Smith, T. L. (1996). An evaluation of the history of a marijuana withdrawal syndrome in a large population. Addiction, 91(10), 1469–1478.
Winward, J. L., Hanson, K. L., Tapert, S. F., & Brown, S. A. (2014). Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. Journal of the International Neuropsychological Society, 20(8), 784–795. doi:10.1017/S1355617714000666.
Wrege, J., Schmidt, A., Walter, A., Smieskova, R., Bendfeldt, K., Radue, E. W., et al. (2014). Effects of cannabis on impulsivity: a systematic review of neuroimaging findings. Current Pharmaceutical Design, 20(13), 2126–2137.
Wu, L. T., Blazer, D. G., Gersing, K. R., Burchett, B., Swartz, M. S., & Mannelli, P. (2013). Comorbid substance use disorders with other Axis I and II mental disorders among treatment-seeking Asian Americans, Native Hawaiians/Pacific Islanders, and mixed-race people. Journal of Psychiatric Research, 47(12), 1940–1948. doi:10.1016/j.jpsychires.2013.08.022.
Yucel, M., Lubman, D. I., Solowij, N., & Brewer, W. J. (2007). Understanding drug addiction: a neuropsychological perspective. The Australian and New Zealand Journal of Psychiatry, 41(12), 957–968. doi:10.1080/00048670701689444.
Yusoff, N., Yuan, J., & Yang, J. (2013). A review of neuropsychological status in cannabis users. Procedia Social and Behavioral Sciences, 97, 2–11. doi:10.1016/j.sbspro.2013.10.198.
Zimmer, A., Zimmer, A. M., Hohmann, A. G., Herkenham, M., & Bonner, T. I. (1999). Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 96(10), 5780–5785. doi:10.1073/pnas.96.10.5780.
Acknowledgments
Authors thank Levente Kriston and Christiane Baldus for their critical comments on a former draft of this paper, Paula Schäffer for proof reading and Juliette Bernardini and Lilly von Osten for their committed help.
Authors’ contributions
FG, SK, SB planned the design, in-/excluded eligible literature and drafted the manuscript. PMS contributed methods, consulting and critical revision. PMS, SB, FG, SK analyzed and interpreted data. Under SB’s supervision, JB and LvO collected data, researched articles, and graded studies. FG, RT were involved in all parts of the review as general supervisors of the research group. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors declare that there is no conflict of interest.
Funding
There was no specific funding for this work. The German Center for Addiction Research in Childhood and Adolescence is a University-based non-government institute and partially funded by the City of Hamburg.
Additional information
This article was reviewed for publication under the editorship of Dr. Edith Sullivan.
Rights and permissions
About this article
Cite this article
Ganzer, F., Bröning, S., Kraft, S. et al. Weighing the Evidence: A Systematic Review on Long-Term Neurocognitive Effects of Cannabis Use in Abstinent Adolescents and Adults. Neuropsychol Rev 26, 186–222 (2016). https://doi.org/10.1007/s11065-016-9316-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11065-016-9316-2