Abstract
Recent evidence suggests that young rodents submitted to high fructose (FRU) diet develop metabolic, and cognitive dysfunctions. However, it remains unclear whether these detrimental effects of FRU intake can also be observed in middle-aged mice. Nine months-old C57BL/6 female mice were fed with water (Control) or 10% FRU in drinking water during 12 weeks. After that, metabolic, and neurochemical alterations were evaluated, focusing on neurotransmitters, and antioxidant defenses. Behavioral parameters related to motor activity, memory, anxiety, and depression were also evaluated. Mice consuming FRU diet displayed increased water, and caloric intake, resulting in weight gain, which was partially compensated due to decreased food pellet intake. FRU fed animals displayed increased plasma glucose, and cholesterol levels, which was not observed in overnight-fasted animals. Superoxide dismutase (SOD), and catalase (CAT) activities were markedly decreased in the prefrontal cortex of animals receiving FRU diet, while glutathione peroxidase (GPx) slightly increased. Liver (lower GPx), striatum (higher SOD and lower CAT), and hippocampus (no changes) were less impacted. No changes were observed in glutathione reductase, and thioredoxin reductase activities, two ancillary enzymes for peroxide detoxification. FRU intake did not alter serotonin, dopamine, and norepinephrine levels in the hippocampus, prefrontal cortex, and striatum. No significant alterations were observed in working, and short-term spatial memory; and in anxiety- and depressive-like behaviors in animals treated with FRU. Increased locomotor activity was observed in FRU-fed middle-aged mice, as evaluated in the open field, elevated plus-maze, Y maze, and object location tasks. Overall, these results demonstrate that high FRU consumption can disturb antioxidant defenses, and increase locomotor activity in middle-aged mice, open the opportunity for further studies to address the underlying mechanisms related to these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the dietary components that may adversely affect health, and wellness becomes an important topic, where new approaches are welcome. Fructose (FRU) is a naturally occurring monosaccharide of high palatability, and low cost; as a consequence it is largely employed as an industrial sweetener. High FRU intake contributes to brain damage, and neurogenesis impairment [1]. The Dietary Guidelines for Americans 2015–2020 [2] suggested a limit for added sugars, including FRU, to be less than 10% of ingested calories per day, which is below the current daily intake that approaches 15%. Recent studies reported that a FRU-rich diet is widely associated with dyslipidemias, behavioral impairments, dental caries, obesity, and coronary heart disease [3]. Several neurochemical changes in the rodent brain can be observed under high FRU diets, such as increased reactive gliosis, mitochondrial impairment, inflammation, and oxidative stress [4,5,6]. In adult rodents, the FRU-induced brain alterations have been associated with learning, and memory impairments, which have been addressed in several behavioral tasks [7, 8], including water maze, novel object recognition, and Y-maze.
Over the past decades, FRU has been increasingly incorporated in the Western diet, becoming a relevant component in the total caloric intake. Daily FRU consumption can be as high as 85–100 g/day [3]. In line with this, epidemic levels of metabolic dysfunctions have been related to the worldwide exponential increase in the consumption of sugary beverages, which are rich in FRU [3]. FRU consumption is widely associated with body weight gain, and increased plasma levels of triglycerides as a result of de novo lipogenesis. A recent meta-analysis found that consumption of FRU-rich beverages contributed to increased body weight gain, elevated systolic blood pressure, hyperglycemia, hyperinsulinemia, and increased serum triglycerides [9]. These findings support the “fructose hypothesis” of metabolic dysfunction, which predicts that high FRU consumption is a leading risk factor for the development, and progression of obesity, insulin resistance, dyslipidemia, fatty liver, and cardiovascular diseases [9].
The impairment in antioxidant defenses, and oxidative damage are pointed out among the potential deleterious effects of FRU intake. A number of FRU supplementation studies were performed in rats, mostly showing decreased superoxide dismutase (SOD), and catalase (CAT) activities. Rats fed with a 60% FRU diet for 2 weeks presented decreased plasma SOD, and CAT activities [10]. Similarly, rats receiving a FRU solution (10%) for two weeks presented lower hepatic SOD, and CAT activities [11]. A three-week FRU-rich diet (10%) induced a decrease in plasma CAT, and CuZnSOD relative gene, and protein expression, as well as a decrease in the CAT, and glutathione peroxidase (GPx) activities [12]. However, FRU (20%) solid diet for 10 weeks failed to induce alterations in hepatic SOD, and CAT activities in rats [13]. FRU-treated rats did not present changes in CAT, but heart SOD activity was decreased [14]. Considering the differences in these previous studies concerning methodologies (e.g., treatments, parameters, and tissues analyzed), and the conflicting results described, further studies are required.
The literature addressing the impact of FRU intake on the mood, and locomotor behavior, particularly in middle-aged, and aging rodents, is scarce. Additionally, disturbances on the brain antioxidant system following FRU diet have not been frequently investigated. Therefore, here we investigated the effects of chronic (12 weeks) FRU consumption on a set of antioxidant defenses, and nuclear factor erythroid 2-related factor 2 (Nrf2) targets, and glyoxalases in the liver and brain of middle-aged female mice. Food, and water intake, blood glucose, cholesterol, and triglycerides, and brain monoamine levels were assessed in different brain areas. Behavioral parameters related to memory, anxiety, depression, and locomotor activity were also investigated.
Materials and Methods
Animals
Female C57BL/6 mice (20–30 g) were obtained from our own breeding colony, at the Federal University of Santa Catarina (UFSC), Florianópolis, Brazil. Mice were housed in groups of 10 animals per cage (42 × 34 × 17 cm), under controlled light conditions (12 h light cycle starting at 7:00 a.m.), and temperature (22 ± 2 °C), with free access to standard chow, and water. All experimental procedures were performed in accordance with the National Institute of Health Guide for the Care, and Use of Laboratory Animals, and approved by the local ethics committee (CEUA PP0735). All efforts were made to minimize the number of animals used, and their suffering.
Experimental Design
Mice were randomly distributed in the control group (CTL), receiving tap water; and the FRU group, receiving 10% FRU in tap water. Treatment was maintained for 12 weeks, starting at 9 months of age, and the behavioral, and biochemical parameters were addressed at the age of 12 months. The consumption of liquid, and food, as well as the body mass, were registered weekly. Behavioral tasks were carried out in a sound-isolated room during the last week of the treatment, between 10:00 and 15:00 h. The experiments were performed in three independent cohorts of mice that were submitted to behavioral tests, as follows: (i) open field, Y-maze, and tail suspension test; (ii) light/dark box, marble burying test, and elevated plus maze; (iii) object location task, splash test, and rotarod. In order to provide a less aversive environment, a red lamp (15 lx) was used during behavioral tasks, except in the light/dark box test in which a white fluorescent light (60 lux) was used. Mice were anesthetized with isoflurane followed by cardiac puncture 48 h after the end of the behavioral battery. Then, mice were euthanized by cervical dislocation, and the liver, prefrontal cortex, striatum, and hippocampus were dissected on ice, and stored at − 80 °C for biochemical analysis.
Biochemical Analysis
The measurements of plasma levels of glucose, total cholesterol, and triglycerides, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were performed by enzymatic methods following manufacturer instructions (Labtest Diagnostica, Lagoa Santa, MG, Brazil, and Biotécnica, Varginha, Brazil).
The levels of norepinephrine, dopamine, and serotonin were determined by HPLC with fluorometric detection [15].
Lipid hydroperoxides present in the liver were quantified by the ferrous oxidation-xylenol orange (FOX) method, as previously described [16].
For the measurement of enzymatic activities, routine methods were used. Samples were homogenized in 20 mM Hepes, pH 7.4, and centrifuged at 20,000×g for 30 min, at 4 °C, and the supernatants were used for further analyzes. The protein was measured according to the method of Bradford [17], using bovine serum albumin as the standard. SOD activity was measured by the inhibition of NADH oxidation [18]. This method is based on the superoxide-dependent oxidation of NADH, which is formed in the presence of 2-mercaptoethanol, EDTA, and manganese. CAT activity was measured by the rate of H2O2 consumption at 240 nm [19]. GPx activity was measured indirectly by NADPH consumption. Glutathione reductase (GR) was added to the media to maintain peroxide consumption proportional to the reduction of the GPx product, GSSG [20]. Fast GSSG reduction was achieved by excess GR in the media, thus, making NADPH consumption proportional to GPx activity [21]. The thioredoxin reductase (TrxR) activity was measured by DTNB reduction in the presence of NADPH [22]. Glyoxalase 1 (Glo1) activity was measured by the formation of S-D-lactoylglutathione by reading the absorbance at 240 nm [23].
Western blot was performed after sodium dodecyl sulfate–polyacrylamide gel electrophoresis, as described initially by Laemmli [24], proteins were electrotransferred to a PVDF membrane, incubated with appropriated antibodies, and revealed by ECL.
Behavioral Tasks
Spontaneous locomotor activity, and exploratory behavior of mice, were evaluated for 5 min in a standard 40 × 40 cm open field arena. The number, and rate (%) of spontaneous alternation on the Y-maze were used to evaluate the working memory, and locomotor activity of the mice [25]. The short-term (90 min) spatial memory of mice was assessed using the object location task, that was carried out using a protocol previously described [26]. The light-dark box test was used to evaluate anxiety-related behaviors, based on the innate aversion of rodents to brightly illuminated areas, as well as assessing respective locomotor activity [27]. The marble burying test addressed repetitive and stereotyped behavior, likely associated with anxiety phenotype [28]. Anxiety-like, and exploratory behaviors were also evaluated using the elevated plus-maze apparatus [29]. The tail suspension test is a predictive test for the screening of new antidepressant drugs, as well as the analysis of depressive-like behaviors in the experimental models of depression [30]. The splash test evaluated self-grooming behavior, defined as cleaning of the fur by licking or scratching, after vaporization of 10% sucrose solution onto the mouse’s dorsal coat [31]. The pole test was used to evaluate motor coordination, and bradykinesia of mice [32]. The rotarod test was carried out to evaluate the motor coordination, and balance of mice [33].
A more detailed methodology description is presented as supplementary material.
Statistical Analysis
Differences between rates of liquid, food, and caloric intake were analyzed by linear regression. Body weight gain was analyzed by a two-way analysis of variance (ANOVA) with repeated measures, followed by Sidak’s multiple comparison tests. Student’s t test was used for single comparisons between groups (e.g. FRU versus CTL groups). Student’s t test was also used in the object location task by comparing the exploratory time (%) on the two identical objects against the random chance (50%) of exploring both object equally. The accepted level of statistical significance was p < 0.05.
Results
Metabolic Profile
The cumulative liquid intake was 85% higher in the FRU group, as compared to the control group (F(1,20) = 38.0; p < 0.001) (Fig. 1a). FRU-treated middle aged mice decreased their cumulative solid food intake by about 30% (F(1,20) = 485; p < 0.0001) (Fig. 1b), whereas their caloric intake increased by 7.3% (F(1,20) = 19.2; p < 0.001) (Fig. 1c), resulting in a significant increase in body weight of about 5% (F(1,364) = 42.0); p < 0.0001), as compared to the CTL group (Fig. 1d).
Effects of FRU consumption on cumulative liquid, food, and caloric intake, as well as the body weight gain in middle age female mice. Female C57BL/6 mice (9 months old) received water (CTL) or 10% FRU in drinking water for 12 weeks, thus, at the end of treatment, animals were 12-months old. a Liquid. Data was obtained in two experiments. b Food intake and c caloric intake. Data was obtained from the group average. d Body weight were monitored weekly. Data are presented as mean ± SEM (n = 13–17 animals per group). Significant differences are indicated by *p < 0.05, as compared to the control group
No changes were observed in the plasmatic levels of hepatic enzymes ALT (CTL: 26.96 ± 2.26; FRU: 25.86 ± 2.11), and AST (CTL: 50.39 ± 5.32; FRU: 46.86 ± 6.11). These results indicate that no major liver damage was induced by FRU intake.
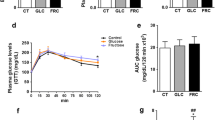
The FRU diet did not alter the blood levels of glucose (Fig. 2a) or triglycerides (Fig. 2b) in 24 h food-deprived mice, but increased plasmatic total cholesterol (Fig. 2c). In another set of experiments, during the fed state, FRU consumption increased the levels of blood glucose (D), and cholesterol (F), but not triglycerides (E). Interestingly, liver tissue of FRU fed mice presented a marked accumulation of triglycerides (Fig. 2h), and cholesterol (Fig. 2i). When normalized to lipid content, liver lipid peroxides were not altered by the FRU diet (Fig. 2g).
Effects of FRU consumption on glucose, triglycerides, total cholesterol, and lipid hydroperoxides (Lipid-OOH) levels of middle age female mice. C57BL/6 female mice were fed with 10% FRU in drinking water for 12 weeks in the plasma levels of food-deprived mice: a glucose, b triglycerides, and c total cholesterol; or fed mice: d glucose, e triglycerides, and f total cholesterol. After treatment the levels of g lipid hydroperoxides (Lipid-OOH) normalized to hepatic lipid content, h triglycerides, and i cholesterol were assayed in the liver. Data are presented as mean ± SEM (n = 13–17 animals per group for blood and n = 06 for liver). Significant differences are indicated by *p < 0.05 or **p < 0.01, as compared to control group (CTL)
Biochemical Alterations in the Liver and Brain of Fructose Treated Mice
We next investigated the effect of chronic consumption of FRU on the activity of several protective enzymes in the brain tissues, including the prefrontal cortex, hippocampus, and striatum, as well as in the liver. Regarding the enzymes GR, and TrxR1, no significant differences were observed after FRU treatment (Fig. 3). The methylglyoxal (MGO) metabolizing enzyme Glo1 was significantly increased in the liver of FRU-treated mice, but no significant changes in this parameter were observed on the analyzed brain tissues (Fig. 3).
Effects of chronic FRU consumption on the enzymatic activity of GR, TrxR1, and Glo1 in the liver and brain of middle age female mice. Female mice received water (CTL) or 10% FRU for 12 weeks before enzymatic analyzes were performed in the liver (a, e, i), prefrontal cortex (b, f, j), striatum (c, g, k) and hippocampus (d, h, l). Data are presented as mean ± SEM (n = 04–8). Significant differences are indicated by *p < 0.05 as compared to the control group
Changes in SOD activity were not observed in the liver (Fig. 4a) or hippocampus (Fig. 4d), but a significant decrease in the SOD activity was observed in the prefrontal cortex (Fig. 4b), and, inversely, we found a significant increase in the striatum (Fig. 4c). CAT activity was markedly decreased in the prefrontal cortex (Fig. 4f), and striatum (Fig. 4g), with no changes in the liver (Fig. 4e), and hippocampus (Fig. 4h). GPx activity showed a significant decrease in the liver (Fig. 4i), and in an opposite way, it was found increased in the prefrontal cortex (Fig. 4j). No significant differences were found in GPx activity in the striatum (Fig. 4k), and hippocampus (Fig. 4l).
Effects of chronic FRU consumption on the activity of SOD, CAT, and GPx in the liver and the brain of middle age female mice. Female mice received water (CTL) or 10% FRU for 3 months before enzymatic analyses were performed. Data are presented as mean ± SEM (n = 04–8). Significant differences are indicated by *p < 0.05 or **p < 0.01, as compared to control group
Cerebral cortex, and liver presented more intense alterations in previous analyzes (Figs. 3 and 4), for this reason these tissues were further tested for alterations in the protein levels. We investigated the effect of chronic FRU consumption on the protein levels of Nrf2 target genes (GCL and NQO1), thiol reductases (GR and TrxR), and MGO metabolizing enzymes (Glo1 and Glo2) in the prefrontal cortex (Fig. 5a, c), and liver (Fig. 5b, d). In the liver, we observed an increase in TrxR1, and a decrease in the GCL proteins. No alterations were observed in hepatic NQO1, Glo1, Glo2, and neither in GR activity in liver, and prefrontal cortex. There was a threefold increase in Glo2 levels in the prefrontal cortex (Fig. 5b), without changes in the other proteins analyzed (NQO1, GCL, Glo1, GR, and TrxR1).
Effects of chronic FRU consumption relative protein levels of NQO1, GCL, Glo1, Glo2, TrxR1, and GR in the prefrontal cortex and liver of middle age female mice. Representative images of blots performed with prefrontal cortex (a) and liver samples (b), and their corresponding quantifications (c and d). Data are presented as mean ± SEM (n = 04–7). Significant differences are indicated by *p < 0.05, or **p < 0.01, as compared to control group
Effects of Chronic Fructose Consumption on Brain Monoamine Levels
Monoamines analysis in the prefrontal cortex, hippocampus, and striatum indicated that 10% FRU consumption during 12 weeks did not alter the levels of norepinephrine, dopamine, and serotonin in the studied brain structures of middle age female mice (Table 1).
Effects of Chronic Fructose Consumption on Locomotor Activity
In the open field, the FRU-treated mice presented a significant increase in the total distance traveled (Fig. 6a), without affecting anxiety-related parameters, including the number of entries (Fig. 6b), and time spent in the central “aversive” area (Fig. 6c) of the open field arena.
Effects of FRU consumption on spontaneous locomotor and exploratory behavior of middle age female mice in the open field. a Total distance traveled, b number of entrances in the center, and c time in the center of the apparatus. Analysis was performed in 12-month-old C57BL/6 female mice that received water (CTL) or 10% FRU (FRU) in drinking water during 12 weeks. Data are presented as mean ± SEM (n = 13–17 animals per group). Significant differences are indicated by **p < 0.01, as compared to control group
Effects of Chronic Fructose Consumption on Learning and Memory
The effects of FRU consumption on working memory (Y-maze), and spatial short-term memory (object location task) of mice were also investigated. The FRU group displayed a significant increase in the total number of alternations in the Y-maze (Fig. 7a), and in the percentage of correct sequences (Fig. 7b).
Effects of FRU consumption on cognitive function and locomotor activity of middle age female mice addressed in the Y-maze alternation and the object location tasks. Before the tests, female mice received water (CTL) or 10% FRU (FRU) in drinking water during 12 weeks. Total number of entries (a) and percentage of correct alternations (b) in the Y-maze task. In the object relocation task, the exploratory time spent on object “A” and “B” in the training session (c) and the location index of the relocated object in the test session (d) are presented. Data are presented as mean ± SEM (n = 13–17 animals per group). Significant differences are indicated by *p < 0.05, as compared to the untreated control group. In the training session, animals receiving FRU diet presented higher overall exploratory time, as compared to the control group (##p < 0.01). In the object location task, the location index was higher the 50% (&p < 0.05), which is indicative of memory retention
In the test session of the object location task, performed 90 min after training (Fig. 7d), both the FRU, and CTL groups displayed increased time in the relocated object. This result indicates that chronic FRU consumption did not alter short-term spatial memory in middle age female mice. In the training session, both groups presented similar exploratory time of objects “A”, and “B”, indicating no biases due to a previous preference. Interestingly, the two-way ANOVA revealed a significant effect for treatment on the exploration time (Fig. 7c) on objects A, and B (F(1,56) = 4.032; p < 0.05). This indicates that FRU consumption increased the overall exploratory activity in the object relocation task, as indicated by increased exploratory time in the training session, thus, confirming the previous hyperactivity phenotype of mice, as detected on the open field.
Effects of Chronic Fructose Consumption on Anxiety-Like Behavior
The time spent in the dark compartment (Fig. 8a), and the number of crossings (Fig. 8b) of mice in light/dark box was not altered by FRU consumption. These results suggest that FRU consumption does not alter the anxiety-like behavior of mice in this task.
Effects of FRU consumption on the anxiety-like behaviors of middle age female mice evaluated in the light/dark box, marble burying test, and elevated plus-maze. a Time spent in the dark compartment of the light/dark box, b total number of crossings between light and dark compartments, c number of marbles buried in the marble burying test, d time in the open arm, the total number of entries in the e open arms and f closed arms of the elevated plus-maze. Before the tests, female mice received water (CTL) or 10% FRU (FRU) in drinking water during 12 weeks. Values are presented as mean ± SEM (n = 13–17). Statistical differences are indicated by *p < 0.05 compared to control group
FRU consumption increased the fraction of buried marble balls (Fig. 8c). Control animals buried about 82% of marble balls, while the FRU group buried 93%, which was statistically higher, as compared to the control group (p < 0.05).
The time spent in the open arms (Fig. 8d) of the elevated plus-maze apparatus was not altered by FRU consumption. However, there was a significant increase in the number of entries in the open arms (Fig. 8e), and closed arms (Fig. 8f), indicating that mice submitted to FRU diet displayed increased locomotor activity in this test.
Effects of Chronic Fructose Consumption on Motor Performance
The rotarod test evaluates general motor coordination, and balance of rodents [34], while the pole test is used to investigate the presence of bradykinesia, and more refined motor alterations [32]. The time to fall in the rotarod was not altered by FRU diet (Fig. 9a). In the pole test, both the turning time (Fig. 9b), and the descend time (Fig. 9c), were not altered by the FRU diet. These results indicate that chronic FRU consumption did not alter motor coordination, and balance, as indicated by the rotarod, and pole tests.
Effects of FRU consumption on the motor function of middle age female mice in the rotarod and pole tests. a Latency to fall in the rotarod test, b average time to turn and c descend time in the pole test performed by middle age male mice that received water (CTL) or 10% FRU (FRU) in drinking water during 12 weeks. Data are presented as mean ± SEM (n = 13–17)
Effects of Chronic Fructose Consumption on Anhedonic- and Depressive-Like Behaviors
The anhedonic-like behavior was evaluated in the splash test. It was observed that the latency to grooming (Fig. 10a), and total grooming time (Fig. 10b) were not altered by the FRU diet. The depressive-like behavior assessed by the tail suspension test showed that the immobility time was not altered (Fig. 10c). These results indicate that chronic consumption of FRU did not induce anhedonic- or depressive-like behaviors in middle age female mice.
Effects of FRU consumption on anhedonic- and depressive-like behaviors in middle age female mice. Female mice received water (CTL) or 10% FRU in drinking water for 12 weeks. Graphics show the latency to grooming (a) and total time of grooming (b) in the splash test and the immobility time in the tail suspension test (c). Data are presented as mean ± SEM (n = 13–17)
Discussion
In the present study, calories obtained in solid food were partially replaced by FRU in drinking water, resulting in a significant increase in energy intake. This increased caloric intake induced a net body weight gain in the first weeks, which was maintained along the entire period of the experimental protocol. Noteworthy, middle aged female mice displayed a marked hyperactivity-like behavior without further alterations in motor coordination, or emotional, and cognitive parameters. Previous studies demonstrated that C57BL/6 mice presented no body weight gain when FRU was provided as solid food [35], but when FRU was offered in drinking water, increased body weight, elevated circulating triglycerides [36], and hepatic steatosis [37], were observed. The current findings are in agreement with these previous results, indicating a similar response in 12-months-old female mice.
We investigated whether MGO degrading enzymes Glo1, and Glo2 would be altered as a consequence of chronic FRU treatment, since FRU intake would increase glycolytic flux, and, as a consequence, it is expected to increase MGO formation [38]. The increased Glo1 activity, and a tendency to increased protein, indicates that hepatic MGO production was increased by chronic FRU consumption, since Glo1 is the rate limiting enzyme in MGO metabolism. Interestingly, Glo2 protein was not altered in the liver, but it was markedly increased in the prefrontal cortex. Despite this detoxifying enzyme seems to be less important than Glo1 in regulating MGO degradation, there are studies indicating Glo2 actively protects cells, and ischemic animals [39, 40]. Glo2 was also considered a pro-survival factor dependent on the p53 signaling, where its induction was able to prevent DNA damage-induced apoptosis, and to inhibit MGO-induced cell death, contributing to normal development of tissues [41,42,43]. The regeneration of GSH catalyzed by Glo2 is also important to maintain an appropriate cellular redox state [39, 44]. Therefore, it is plausible to think that chronic consumption of FRU would lead to an activation of p53 signaling in the brain, leading to increased Glo2 protein, which needs to be confirmed.
Decreased SOD, and CAT activities in the prefrontal cortex would limit the protection against reactive oxygen species, thus, increasing the chance of oxidative damage. The increase in GPx activity in the prefrontal cortex can be understood as a compensatory response due to decreased SOD, and CAT activities, which remains to be confirmed. As stated above, decreased SOD, and CAT seems to be a common finding in FRU-treated rats [10,11,12], and mice [45], corroborating our results. Despite the variability on the literature data regarding the schedules of FRU treatment, time points, and tissues analyzed, most results have indicated that increased FRU intake decreases SOD, and CAT activities, reinforcing the need for further confirmation, especially in the brain, where it would be accompanied by behavioral alterations.
Given the finding that FRU uptake can decrease antioxidant protection by limiting Nrf2 activity in the brain [45], we investigated two Nrf2-target genes GCL, and NQO1. The results provided some evidence of a FRU-induced decrease in Nrf2 activity, as indicated by decreased GCL protein in the liver. Decreased Nrf2 activity can also lead to decreased aldo-keto reductases expression, enzymes capable of MGO detoxification. A possible increase in MGO burden, can increase the potential for damage due to decreased detoxification by glutathione, and glyoxalase systems [46].
Taken into account these biochemical, and metabolic alterations, as well as previous data reporting FRU-induced learning, and memory impairments in young rodents [9], we addressed putative behavioral impairments in mice, as induced by 12-weeks diet with 10% FRU in drinking water. The mechanisms, and related behaviors of long-term memory storage were not investigated in the present study. However, the effects of FRU consumption were evaluated on short-term, and working memory expressions. Previous studies showed that FRU diet reduced synaptic plasticity, hippocampal redox balance, and neurogenesis. These effects were linked to short-term spatial learning, and episodic memory impairments in mice [7, 8]. However, this study was unable to provide evidence for spatial, and working memory deficits after chronic FRU consumption in middle age mice, as evaluated in the object location, and Y-maze tasks. Moreover, no significant changes were observed at rotarod, and pole tests, two tests widely employed to evaluate murine motor function. In accordance with these findings, a previous study also demonstrated that the latency to fall from the rotarod did not differ between the FRU-treated, and control groups [47].
In the present study, we found that high FRU intake did not alter behavioral responses related to anxiety, anhedonia, and depression. For instance, mice from both FRU, and CTL groups displayed similar performance in the elevated plus-maze, tail suspension and splash tests. In accordance with these findings, it was previously demonstrated that rats fed a 10% FRU diet for five weeks did not alter the time spent in the central area of the open field, suggesting no differences in the anxiety-related behavior of these animals [48]. In addition, rats that ingested 11% FRU solution for 30 days also did not present significant differences in the anxiety-like behavior in the zero maze test, as compared to the respective control group [9]. Conversely, it was previously demonstrated that young rats consuming 65% FRU in the chow for 8 weeks exhibited decreased time in the center of the open field, and spent more time in the dark compartment of the light/dark box test, reflecting an anxiogenic profile [49]. It is important to emphasize that these studies used models of metabolic syndrome [9, 48, 49], that are distinct from the current FRU consumption protocol. The 10% FRU intake for 3 months is not expected to induce metabolic syndrome in the animals, since no concomitant fat or higher FRU concentrations were used. In addition, the fact that dopamine, serotonin, and norepinephrine levels, were not altered in different brain structures, is somewhat in line with the absence of gross behavioral alterations on cognitive function, and mood-related behaviors.
There are discrepant clinical findings around the concept stating that increased intake of added sugars may have a role in hyperactivity disorders. For example, it was reported that hyperactive children who ingested more sucrose showed greater hyperactivity indexes [50], while evidence that sugar (sucrose) intake did not alter the symptoms of attention-deficit/hyperactivity disorder [51]. Seven-years-old children, who consumed a diet rich in “junk food” with high sugar content, were more likely to exhibit hyperactivity, as compared to children who had a healthier diet [52]. Herein, we provided the first evidence that high FRU intake increased the distance traveled in the open field test, the total number of entries in the elevated plus maze arms, the number of alternations in the Y maze, and the overall exploratory time in the object location task. Therefore, following chronic 10% FRU diet, all these behavioral parameters observed in different paradigms indicate an association to a hyperlocomotion profile in middle-aged mice. In line with the present results, pregnant rats exposed to 30% sucrose solution resulted in offspring with increased locomotor activity [53]. Interestingly, FRU supplementation in solid food induced a significant increase in the number of crossings in the open field [49], and in the metabolic rate of C57BL/6 mice, as measured by the circadian increase in oxygen consumption, an effect that lasted for at least 9 weeks [35]. This higher energy expenditure suggests that animals were more active, in agreement to the hyperactivity-like phenotype found in the present work.
It is interesting to note the relationship between SOD and CAT activities, and locomotor behavior. Common carotid occlusion increased SOD activity, which was associated with decreased ambulation, and self-care behavior in rats [54]. Vitamin A induced oxidative damage in the adult rat hippocampus, which was accompanied by decreased locomotor activity in the open field [55]. This decreased ambulatory activity was paralleled by increased SOD, and lower CAT activity in the hippocampus. The overall picture suggests an association between decreased locomotor activity, and increased SOD, and CAT activities, while the converse statement is also supported. Decreased CAT, and SOD activities are related to increased ambulatory activity, implying that antioxidant defenses would be a relevant, and underrepresented topic, awaiting to be further explored. However, the available data also indicate other points that need further investigation, primarily due to differences in species, strains, tissues analyzed, and treatment regimens.
Conclusions
Collectively, our results demonstrate that 12-months-old female C57BL/6 mice submitted to a 10% FRU diet for 12 weeks displayed a marked hyperactivity-like behavior, without further alterations in motor coordination and balance, emotional, and cognitive parameters. Based on the current findings, and previous literature data, it seems plausible to speculate that locomotor activity in rodents is particularly sensitive to changes in SOD, and CAT activities in the brain. Nonetheless, further studies are necessary to confirm, and to determine the molecular mechanisms underlying the hyperlocomotion phenotype induced by FRU intake.
References
Yamazaki M, Yamada H, Munetsuna E et al (2018) Excess maternal fructose consumption impairs hippocampal function in offspring via epigenetic modification of BDNF promoter. FASEB J Off Publ Fed Am Soc Exp Biol 32:2549–2562. https://doi.org/10.1096/fj.201700783RR
USDA (2015) (US Department of Agriculture, US Department of Health and Human Services. In: Dietary Guidelines for Americans, 2015–2020, 8th edn. USDA, Washington
Mortera RR, Bains Y, Gugliucci A (2019) Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front Biosci Landmark Ed 24:186–211
Dziadek K, Kopeć A, Piątkowska E, Leszczyńska T (2019) High-fructose diet-induced metabolic disorders were counteracted by the intake of fruit and leaves of sweet cherry in wistar rats. Nutrients. 11:2638. https://doi.org/10.3390/nu11112638
Sathiya Priya C, Vidhya R, Kalpana K, Anuradha CV (2019) Indirubin-3’-monoxime prevents aberrant activation of GSK-3β/NF-κB and alleviates high fat-high fructose induced Aβ-aggregation, gliosis and apoptosis in mice brain. Int Immunopharmacol 70:396–407. https://doi.org/10.1016/j.intimp.2019.02.053
Yang N, Gonzalez-Vicente A, Garvin JL (2020) Angiotensin II-induced superoxide and decreased glutathione in proximal tubules: effect of dietary fructose. Am J Physiol Renal Physiol 318:F183–F192. https://doi.org/10.1152/ajprenal.00462.2019
Cisternas P, Salazar P, Serrano FG et al (2015) Fructose consumption reduces hippocampal synaptic plasticity underlying cognitive performance. Biochim Biophys Acta 1852:2379–2390. https://doi.org/10.1016/j.bbadis.2015.08.016
Rivera DS, Lindsay CB, Codocedo JF et al (2018) Long-term, fructose-induced metabolic syndrome-like condition is associated with higher metabolism, reduced synaptic plasticity and cognitive impairment in Octodon degus. Mol Neurobiol 55:9169–9187. https://doi.org/10.1007/s12035-018-0969-0
Tappy L (2018) Fructose-containing caloric sweeteners as a cause of obesity and metabolic disorders. J Exp Biol 221:164202. https://doi.org/10.1242/jeb.164202
Abdelrahman AM, Al Suleimani YM, Ashique M et al (2018) Effect of infliximab and tocilizumab on fructose-induced hyperinsulinemia and hypertension in rats. Biomed Pharmacother 105:182–186. https://doi.org/10.1016/j.biopha.2018.05.118
Prado VC, Quines CB, Rosa SG et al (2019) Oxidative stress and metabolic parameters are differently affected by fructose when rats were kept sedentary or underwent swimming exercise. Can J Physiol Pharmacol 97:721–728. https://doi.org/10.1139/cjpp-2018-0620
Francini F, Castro MC, Schinella G et al (2010) Changes induced by a fructose-rich diet on hepatic metabolism and the antioxidant system. Life Sci 86:965–971. https://doi.org/10.1016/j.lfs.2010.05.005
Dornas WC, de Lima WG, dos Santos RC et al (2013) High dietary salt decreases antioxidant defenses in the liver of fructose-fed insulin-resistant rats. J Nutr Biochem 24:2016–2022. https://doi.org/10.1016/j.jnutbio.2013.06.006
Calabró V, Piotrkowski B, Fischerman L et al (2016) Modifications in nitric oxide and superoxide anion metabolism induced by fructose overload in rat heart are prevented by (–)-epicatechin. Food Funct 7:1876–1883. https://doi.org/10.1039/C6FO00048G
De Benedetto GE, Fico D, Pennetta A et al (2014) A rapid and simple method for the determination of 3,4-dihydroxyphenylacetic acid, norepinephrine, dopamine, and serotonin in mouse brain homogenate by HPLC with fluorimetric detection. J Pharm Biomed Anal 98:266–270. https://doi.org/10.1016/j.jpba.2014.05.039
Nourooz-Zadeh J, Tajaddini-Sarmadi J, Wolff SP (1994) Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol orange assay in conjunction with triphenylphosphine. Anal Biochem 220:403–409. https://doi.org/10.1006/abio.1994.1357
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Paoletti F, Mocali A (1990) Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol 186:209–220. https://doi.org/10.1016/0076-6879(90)86110-h
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Wendel A (1981) Glutathione peroxidase. Methods Enzymol 77:325–333. https://doi.org/10.1016/s0076-6879(81)77046-0
Carlberg I, Mannervik B (1975) Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J Biol Chem 250:5475–5480
Holmgren A, Björnstedt M (1995) Thioredoxin and thioredoxin reductase. Methods Enzymol 252:199–208. https://doi.org/10.1016/0076-6879(95)52023-6
Thornalley PJ (1993) The glyoxalase system in health and disease. Mol Aspects Med 14:287–371. https://doi.org/10.1016/0098-2997(93)90002-u
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. https://doi.org/10.1038/227680a0
Hughes RN (2004) The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev 28:497–505. https://doi.org/10.1016/j.neubiorev.2004.06.006
Assini FL, Duzzioni M, Takahashi RN (2009) Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation. Behav Brain Res 204:206–211. https://doi.org/10.1016/j.bbr.2009.06.005
Bourin M, Hascoët M (2003) The mouse light/dark box test. Eur J Pharmacol 463:55–65. https://doi.org/10.1016/s0014-2999(03)01274-3
Kedia S, Chattarji S (2014) Marble burying as a test of the delayed anxiogenic effects of acute immobilisation stress in mice. J Neurosci Methods 233:150–154. https://doi.org/10.1016/j.jneumeth.2014.06.012
Lister RG (1987) The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92:180–185. https://doi.org/10.1007/BF00177912
Cryan JF, Mombereau C, Vassout A (2005) The tail suspension test as a model for assessing antidepressant activity: review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev 29:571–625. https://doi.org/10.1016/j.neubiorev.2005.03.009
Yalcin I, Aksu F, Belzung C (2005) Effects of desipramine and tramadol in a chronic mild stress model in mice are altered by yohimbine but not by pindolol. Eur J Pharmacol 514:165–174. https://doi.org/10.1016/j.ejphar.2005.03.029
Fleming SM, Salcedo J, Fernagut P-O et al (2004) Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci Off J Soc Neurosci 24:9434–9440. https://doi.org/10.1523/JNEUROSCI.3080-04.2004
Rial D, Castro AA, Machado N et al (2014) Behavioral phenotyping of Parkin-deficient mice: looking for early preclinical features of Parkinson’s disease. PLoS ONE 9:e114216. https://doi.org/10.1371/journal.pone.0114216
Curzon P, Zhang M, Radek RJ, Fox GB (2009) The behavioral assessment of sensorimotor processes in the mouse: acoustic startle, sensory gating, locomotor activity, rotarod, and beam walking. In: Buccafusco JJ (ed) Methods Behav Anal Neurosci, 2nd edn. CRC Press/Taylor & Francis, Boca Raton (FL)
Tillman EJ, Morgan DA, Rahmouni K, Swoap SJ (2014) Three months of high-fructose feeding fails to induce excessive weight gain or leptin resistance in mice. PLoS ONE 9:e0107206. https://doi.org/10.1371/journal.pone.0107206
Bocarsly ME, Powell ES, Avena NM, Hoebel BG (2010) High-fructose corn syrup causes characteristics of obesity in rats: Increased body weight, body fat and triglyceride levels. Pharmacol Biochem Behav 97:101–106. https://doi.org/10.1016/j.pbb.2010.02.012
Ushio M, Nishio Y, Sekine O et al (2013) Ezetimibe prevents hepatic steatosis induced by a high-fat but not a high-fructose diet. Am J Physiol 305:E293–E304. https://doi.org/10.1152/ajpendo.00442.2012
Hipkiss AR (2017) On the relationship between energy metabolism, proteostasis, aging and Parkinson’s disease: possible causative role of methylglyoxal and alleviative potential of carnosine. Aging Dis 8:334–345. https://doi.org/10.14336/AD.2016.1030
Shin MJ, Kim DW, Lee YP et al (2014) Tat-glyoxalase protein inhibits against ischemic neuronal cell damage and ameliorates ischemic injury. Free Radic Biol Med 67:195–210. https://doi.org/10.1016/j.freeradbiomed.2013.10.815
Antognelli C, Ferri I, Bellezza G et al (2017) Glyoxalase 2 drives tumorigenesis in human prostate cells in a mechanism involving androgen receptor and p53-p21 axis. Mol Carcinog 56:2112–2126. https://doi.org/10.1002/mc.22668
Kang L-L, Zhang D-M, Jiao R-Q et al (2019) Pterostilbene attenuates fructose-induced myocardial fibrosis by inhibiting ROS-driven Pitx2c/miR-15b pathway. Oxid Med Cell Longev 2019:1243215. https://doi.org/10.1155/2019/1243215
Rabbani N, Xue M, Thornalley PJ (2014) Activity, regulation, copy number and function in the glyoxalase system. Biochem Soc Trans 42:419–424. https://doi.org/10.1042/BST20140008
Xu Y, Chen X (2006) Glyoxalase II, a detoxifying enzyme of glycolysis byproduct methylglyoxal and a target of p63 and p73, is a pro-survival factor of the p53 family. J Biol Chem 281:26702–26713. https://doi.org/10.1074/jbc.M604758200
Honek JF (2015) Glyoxalase biochemistry. Biomol Concepts 6:401–414. https://doi.org/10.1515/bmc-2015-0025
Mastrocola R, Nigro D, Cento AS et al (2016) High-fructose intake as risk factor for neurodegeneration: key role for carboxy methyllysine accumulation in mice hippocampal neurons. Neurobiol Dis 89:65–75. https://doi.org/10.1016/j.nbd.2016.02.005
Rabbani N, Thornalley PJ (2015) Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem Biophys Res Commun 458:221–226. https://doi.org/10.1016/j.bbrc.2015.01.140
Rendeiro C, Masnik AM, Mun JG et al (2015) Fructose decreases physical activity and increases body fat without affecting hippocampal neurogenesis and learning relative to an isocaloric glucose diet. Sci Rep 5:e09589. https://doi.org/10.1038/srep09589
Orlandi L, Fonseca WF, Enes-Marques S et al (2015) Sickness behavior is accentuated in rats with metabolic disorders induced by a fructose diet. J Neuroimmunol 289:75–83. https://doi.org/10.1016/j.jneuroim.2015.10.014
Reddy BR, Maitra S, Jhelum P et al (2016) Sirtuin 1 and 7 mediate resveratrol-induced recovery from hyper-anxiety in high-fructose-fed prediabetic rats. J Biosci 41:407–417. https://doi.org/10.1007/s12038-016-9627-8
Prinz RJ, Roberts WA, Hantman E (1980) Dietary correlates of hyperactive behavior in children. J Consult Clin Psychol 48:760–769. https://doi.org/10.1037//0022-006x.48.6.760
Wolraich ML, Wilson DB, White JW (1995) The effect of sugar on behavior or cognition in children. A meta-analysis. JAMA 274:1617–1621. https://doi.org/10.1001/jama.1995.03530200053037
Wiles NJ, Northstone K, Emmett P, Lewis G (2009) “Junk food” diet and childhood behavioural problems: results from the ALSPAC cohort. Eur J Clin Nutr 63:491–498. https://doi.org/10.1038/sj.ejcn.1602967
Choi CS, Kim P, Park JH et al (2015) High sucrose consumption during pregnancy induced ADHD-like behavioral phenotypes in mice offspring. J Nutr Biochem 26:1520–1526. https://doi.org/10.1016/j.jnutbio.2015.07.018
Yanpallewar SU, Hota D, Rai S et al (2004) Nimodipine attenuates biochemical, behavioral and histopathological alterations induced by acute transient and long-term bilateral common carotid occlusion in rats. Pharmacol Res 49:143–150. https://doi.org/10.1016/j.phrs.2003.08.005
de Oliveira MR, Silvestrin RB, Mello e Souza T, Moreira JCF (2007) Oxidative stress in the hippocampus, anxiety-like behavior and decreased locomotory and exploratory activity of adult rats: effects of sub acute vitamin A supplementation at therapeutic doses. NeuroToxicology 28:1191–1199. https://doi.org/10.1016/j.neuro.2007.07.008
Funding
This work was supported by the Brazilian funding agency CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) to ALD (#462,333/2014-0, #306,204/2014-2, #307,057/2018-1). ALD, EAN, and RDP are CNPq research fellows. BS, AES, GRLA, and JCS students received scholarships from CNPq, and LFS received a Post-Doc Scholarship from FAPESP (Fundação de Amparo a Pesquisa do Estado de São Paulo, Brazil). MPC received a post-doctoral scholarship, and HSB a PhD scholarship from CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical Approval
All experimental procedures were performed in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and approved by the local ethics committee at the Federal University of Santa Catarina (CEUA PP0735).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
dos Santos, B., Schmitz, A.E., de Almeida, G.R.L. et al. Fructose Intake Impairs Cortical Antioxidant Defenses Allied to Hyperlocomotion in Middle-Aged C57BL/6 Female Mice. Neurochem Res 45, 2868–2883 (2020). https://doi.org/10.1007/s11064-020-03135-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03135-7