Abstract
Middle age is an early stage of the aging process, during which the consumption of diets rich in saturated fats and/or simple sugars might influence brain function, but only few data are available on this issue. We therefore investigated the impact of a diet rich in saturated fat and fructose (HFF) on mitochondrial physiology in hippocampus and frontal cortex of middle-aged rats (1 year old), by including a group of adult rats (90 days) as a “negative control,” lacking the putative effect of aging. Middle-aged rats were fed HFF or control diet for 4 weeks. Mitochondrial function was analyzed by high-resolution respirometry and by assessing the amount of respiratory complexes. Markers of oxidative balance, as well as the protein content of uncoupling protein 2 (UCP2), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), and peroxisome proliferator-activated receptor alpha (PPARα), were also assessed. A decrease in the activity of complex I was detected in both brain areas of middle-aged rats. In hippocampus, mitochondrial respiratory capacity and complex IV content decreased with age and increased with HFF diet. Higher protein oxidative damage, decreased antioxidant defenses, and increased UCP2 and PGC-1α content were found in hippocampus of middle-aged rats. HFF feeding induced a significant reduction in the amount of UCP2, PGC-1α, and PPARα, together with higher protein oxidative damage, in both brain areas. Overall, our results point to middle age as a condition of early brain aging for mitochondrial function, with hippocampus being an area more susceptible to metabolic impairment than frontal cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been estimated that the worldwide aged population (65+ years) will increase more than threefold by 2050 [1]. Therefore, the different susceptibility to environmental challenges at various life stages is an area of growing concern in the risk assessment of human health. Animal models have demonstrated age-specific responses to environmental stressors [2,3,4], thus suggesting that environment may affect the aged differently from young adults [5, 6]. A common lifestyle factor, which may interact with aging to impair brain function, is the consumption of high-fat and/or high-sugar diet [7,8,9,10,11,12,13,14,15].
Middle age (i.e., the period around the mid of the lifespan, corresponding to about 40 years in humans) is an early stage of the aging process, during which gradual physical changes and some chronic illness may occur. Such changes would affect the outcomes at older ages. Although many studies have emphasized the impact of diets rich in saturated fats or simple sugars on aging, only few data are available concerning the physiological responses at middle age [11, 12]. In particular, rats maintained on high-fat/high-fructose diet exhibited impaired spatial learning ability, reduced hippocampal dendritic spine density, and hippocampal decreased levels of a highly expressed brain neurotrophin, namely brain-derived neurotrophic factor [11]. In addition, increased memory errors and microglial activation, an index of inflammation, were observed in the hippocampus of middle-aged rats fed a diet rich in saturated fats and cholesterol [12].
One of the possible mechanisms contributing to age- and/or diet-associated brain impairment is mitochondrial dysfunction and the consequent oxidative stress [16], since these organelles are responsible for the production of ATP through oxidative phosphorylation and serve as generators of reactive oxygen species (ROS). When enzymatic and nonenzymatic antioxidant systems are overwhelmed by elevated levels of ROS, oxidative damage can occur to DNA, lipids (cell and organelle membranes), and proteins (receptors, transcription factors, and enzymes). Although this issue is central to highlight the molecular events leading to diet-induced brain impairment, to our knowledge, comprehensive investigations on the effect of a high-fat/high-fructose diet on brain respiratory activities, oxidative balance, and expression of proteins strictly linked to mitochondria at middle age are lacking.
In the light of the above considerations, this study was aimed to investigate the effect of age and/or dietary treatment on brain mitochondrial physiology. To this end, we carried out experiments in adult (3 months, equivalent to a young human adult aged 20 years), middle-aged (1 year, equivalent to a middle-aged human aged 40 years) [17], and middle-aged rats that were fed a typical “Western diet”, rich in saturated fat and fructose. This diet mimics the dietary patterns of modern society, which prefers processed foods rich in refined carbohydrates, animal fats, and edible oils [18]. On these three groups of rats, we have measured mitochondrial respiration, markers of oxidative balance, and expression of respiratory complexes I–V, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), peroxisome proliferator-activated receptor alpha (PPARα), and uncoupling protein 2 (UCP2), in two critical brain regions, such as hippocampus and frontal cortex. To investigate mitochondrial respiratory function, we used high-resolution respirometry (HRR). This novel approach enables the analysis of dysfunction in electron transport, ADP phosphorylation, and leak respiration across the inner mitochondrial membrane taking into account the entire spectrum of respiratory control, compensatory mechanisms, and cellular architecture affecting mitochondrial function. In fact, the measurements are carried out on whole tissue homogenates, where mitochondria retain their proper cellular context [19]. In addition, isolation of mitochondria drastically influences organelle function and may remove greater than 60% of the total mitochondrial population compared to brain tissue homogenates [20, 21].
Methods
Experimental Design
Male Sprague-Dawley rats were purchased from Charles River (Calco, Como, Italy) and used for the experiments. All rats were caged singly in a temperature-controlled room (23 ± 1 °C) with a 12-h light/dark cycle (06.30–18.30). Treatment, housing, and euthanasia of animals met the guidelines set by the Italian Health Ministry. All experimental procedures involving animals were approved by “Comitato Etico-Scientifico per la Sperimentazione Animale” of the University of Naples Federico II.
For the experiments, we used adult (90 days of age) and middle-aged rats (11 months old). All the rats were allowed to adapt to our animal house for 14 days before the beginning of treatment. During this adaptation period, all the rats were fed the control diet (shown in Table 1). After the adaptation period, dietary treatment started and adult rats (n = 8), as well as a group of middle-aged rats (n = 8), were fed the control diet, while another group of middle-aged rats (n = 8) received a high-fat/high-fructose diet (HFF) (see Table 1) for 4 weeks.
During the dietary treatment, body weight, food, and water intake were monitored daily. At the end of the experimental period, rats were euthanized by decapitation. The brains were quickly removed, transferred to a metal plate, on ice, carefully washed with phosphate buffer (137 mM NaCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, pH 7.4) to wipe off surface blood, and hippocampus and frontal cortex were dissected. The frontal cortex was dissected from a slice about 2.5–4.5 mm anterior to bregma, while hippocampus from a slice about 3.5–5.5 mm posterior to bregma, taking into account published stereotaxic atlas resources [22]. Little pieces of both tissues were used freshly to measure mitochondrial oxygen consumption, while the remaining samples were snap frozen in liquid nitrogen and stored at − 80 °C for subsequent analyses.
Mitochondrial Analyses in Rat Hippocampus and Frontal Cortex
Brain samples were homogenized (1:1000, w/v) in Mir05 medium containing 110 mM sucrose, 60 mM K-lactobionate, 20 mM Hepes, 20 mM taurine, 10 mM KH2PO4, 6 mM MgCl2, 0.5 mM EGTA, and 0.1% fatty acid free BSA, pH 7.0.
Homogenates (2 mg) were transferred into calibrated Oxygraph-2k (O2k, OROBOROS INSTRUMENTS, Innsbruck, Austria) 2-ml chambers. Oxygen polarography was performed at 37 ± 0.001 °C (electronic Peltier regulation), and oxygen concentration (μM) and oxygen flux (pmol O2 s−1 ml−1) were real-time recorded and corrected automatically for instrumental background by DatLab software (OROBOROS INSTRUMENTS, Innsbruck, Austria).
After addition of the homogenates, the O2 flux was allowed to stabilize. A substrate, uncoupler, inhibitor titration (SUIT) protocol was applied to assess qualitative and quantitative mitochondrial changes [23]. After stabilization, leak respiration supported primarily by electron flow through complex I (CI) of the respiratory chain (CIL) was evaluated by adding the substrates malate (0.5 mM), pyruvate (5 mM), and glutamate (10 mM). Electron transfer was coupled to phosphorylation by the addition of 2.5 mM ADP, assessing phosphorylating respiration with electron transfer supported by complex I (CIP). Succinate at 10 mM was added to the chamber to induce maximal phosphorylating respiration with parallel electron input from complexes I and II (CI&IIP). Oligomycin at 2.5 mM was added to assess leak respiration when substrates and ADP were provided, but ATP synthase is inhibited (CI&IIL). Maximum capacity of the electron transport chain (CI&IIE) was obtained by addition of the uncoupler carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 0.5 mM). Rotenone (0.5 μM) was added to inhibit CI; hence, the maximal capacity supported by CII alone was determined (CIIE). Residual oxygen consumption was established by addition of the inhibitor antimycin A (2.5 mM) and the resulting value was subtracted from the fluxes in each run, to correct for non-mitochondrial respiration. All samples were run in duplicates and the mean was used for analysis. Representative traces of oxygen flux in the hippocampus are shown in Supplementary Fig. 1.
Procedures to test mitochondrial integrity were routinely carried out at the beginning of each measurement, by evaluating the stimulating effect of 10 mM exogenous cytochrome c on mitochondrial respiration in the presence of complex I-linked substrates and ADP.
Calculation of Intrinsic Mitochondrial Function
HRR allows the determination of numerous respiratory states within the same sample, from which respiratory control ratios/factors can be calculated [19, 24, 25]. Maximum capacity of the electron transport chain (CI&IIE) was used to normalize other respiratory states, which were measured within the capacity of the electron transfer system. Thus, flux control ratios can be calculated for leak respiration with electron provision from complex I (FCRL) and complex I and II (Eqs. 5 and 6), phosphorylating respiration with electron provision from complex I (FCRP), and complexes I and II (FCRPI&II) (Eqs. 7 and 8):
In addition, flux control factors (FCF) can be calculated as follows:
Coupling efficiency of oxidative phosphorylation = 1 − CI&IIL/CI&IIP
Excess capacity of the electron transport chain = 1 − CI&IIP / CI&IIE
Citrate synthase (CS) activity was measured using homogenates from hippocampus and frontal cortex according to Srere [26] by following the appearance of the free SH group of the released CoASH at 412 nm by using 5,5′-dithiobis-(2-nitrobenzoate).
Oxidative Status in Rat Hippocampus and Frontal Cortex
Lipid peroxidation was determined according to Fernandes et al. [27], by measuring thiobarbituric reactive substances (TBARS), using the thiobarbituric acid assay. Aliquots of hippocampal or frontal cortex homogenates prepared in KCl medium were added to 0.3 ml of ice-cold 40% trichloroacetic acid. Then, 1 ml of 0.67% of aqueous thiobarbituric acid containing 0.01% of 2,6-di-tert-butyl-p-cresol was added. The mixtures were heated at 90 °C for 15 min, then cooled in ice for 10 min, and centrifuged at 850×g for 10 min. The supernatant fractions were collected, and lipid peroxidation was estimated spectrophotometrically at 530 nm. The amount of TBARS formed was calculated using a molar extinction coefficient of 1.56 × 105/M/cm and expressed as nmol TBARS per g tissue.
Nitrotyrosine (N-Tyr) titration was carried out by ELISA in hippocampal and frontal cortex homogenates as previously described [28]. Samples were diluted (1:500, 1:1500, 1:3000, and 1:6000) with coating buffer, and aliquots (50 μl) were then incubated in the wells of a microtiter plate (overnight, 4 °C). After four washes by T-TBS (130 mM NaCl, 20 mM Tris-HCl, 0.05% Tween 20, pH 7.4) and four washes by high-salt TBS (500 mM NaCl, 20 mM Tris-HCl at pH 7.4), the wells were blocked with TBS containing 0.5% BSA (1 h, 37 °C).
After washing, the wells were incubated (2 h, 37 °C) with 50 μl of rabbit anti-N-Tyr (Covalab, distributed by VinciBiochem, Vinci, Italy; 1:600 dilution in T-TBS containing 0.25% BSA) followed by 60 μl of goat anti-rabbit horseradish peroxidase-conjugated IgG (GAR-HRP, Immunoreagents, distributed by Microtech, Naples, Italy; 1:3500 dilution; 1 h, 37 °C). Peroxidase-catalyzed color development from o-phenylenediamine was measured at 492 nm. Data were reported as OD per mg of proteins.
Catalase activity was measured in homogenates from frontal cortex or hippocampus in 50 mM phosphate buffer, pH 7.0 containing 10 mM H2O2, and 0.25% Triton X-100. Determinations were carried out spectrophotometrically (240 nm) at 25 °C, by monitoring the decrease in absorbance due to the decomposition of H2O2. The rate of H2O2 loss from solution was linear when the natural log of the absorbance was plotted against time, in accord with the usual first-order kinetics exhibited by catalase [29]. Linear regression analysis was carried out to calculate the first order reaction rate constant (k) and the values were then referred to unit of tissue weight.
Superoxide dismutase activity (SOD) was measured in homogenates from frontal cortex or hippocampus in a medium containing 0.1 mM EDTA, 50 mM KH2PO4 pH 7.8, 20 mM cytochrome c, 0.1 mM xanthine, and 0.01 units of xanthine oxidase. Determinations were carried out spectrophotometrically (550 nm) at 25 °C, by monitoring the decrease in the reduction rate of cytochrome c by superoxide radicals, generated by the xanthine-xanthine oxidase system. One unit of SOD activity is defined as the concentration of enzyme that inhibits cytochrome c reduction by 50% in the presence of xanthine + xanthine oxidase [30].
Western Blotting
Proteins were extracted from hippocampus or frontal cortex by homogenizing frozen tissues (− 80 °C) in seven volumes (w/v) of cold RIPA buffer (150 mM NaCl, 50 mM Tris-HCl, 0.5% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, pH 8) containing Tissue Protease Inhibitor Cocktail (Sigma-Aldrich, 1:500, v/v) and Tissue Phosphatase inhibitor cocktail (Sigma-Aldrich, 1:100, v/v). Homogenates were then centrifuged (14,000×g, 45 min, 4 °C) and protein concentration of supernatants was measured.
Supernatants were then used to evaluate the content of several mitochondrial proteins, namely complexes I–V and UCP2, as well as PPARα and PGC-1α. Respiratory complexes were detected by incubation (overnight, 4 °C) with anti-OXPHOS (Abcam, Cambridge, UK; 1:400 dilution in T-TBS containing 3% BSA) followed by goat anti-mouse horseradish peroxidase-conjugated IgG (GAM-HRP; Sigma-Aldrich; 1:120,000 dilution for detection of complex I, 1:170,000 for complexes II and III, 1:60,000 for complex IV, 1:200,000 for complex V, in T-TBS containing 1% non-fat milk; 1 h, 37 °C). Different dilutions of secondary antibody were used to optimize band intensity and accurate quantification of each complex. Representative western blot of respiratory complexes I–V detected using a single secondary antibody dilution is reported in Supplementary Fig. 2.
UCP2 was detected by incubation (overnight, 4 °C) with anti-UCP2 IgG (Merck, Darmstadt, Germany; 1:600 dilution in T-TBS containing 3% BSA) followed by GAR-HRP IgG (Immunoreagents, distributed by Microtech, Naples, Italy; 1:80,000 dilution in T-TBS containing 1% non-fat milk; 1 h, 37 °C).
PGC-1α was revealed by incubation (overnight, 4 °C) with anti-human PGC-1α IgG (Merck, Darmstadt, Germany; 1:1000 dilution in T-TBS containing 0.25% non-fat milk), followed by GAR-HRP IgG (1: 90,000 dilution in T-TBS containing 0.25% non-fat milk; 1 h, 37 °C).
PPARα was detected by incubation (overnight, 4 °C) with anti-PPARα IgG (Thermo Fisher, IL, USA; 1:1000 dilution in T-TBS containing 3% BSA) followed by GAR-HRP IgG (Sigma-Aldrich; 1:80,000 dilution in T-TBS containing 1% non-fat milk; 1 h, 37 °C).
For loading control, the membranes were stripped [31] and then incubated (overnight, 4 °C) with mouse anti-β-actin IgG (Sigma-Aldrich; 1000 dilution in T-TBS containing 0.25% non-fat milk) followed by GAM-HRP IgG (GAM-HRP; Sigma-Aldrich; 1:10,000 dilution in 0.25% non-fat milk; 1 h, 37 °C).
All the above immunocomplexes were detected by the ECL detection system, using the Excellent Chemiluminescent detection Kit (ElabScience, Microtech, Naples, Italy). Quantitative densitometry of the bands was carried out by analyzing chemidoc images or digital images of X-ray films exposed to immunostained membranes, and the quantification of the signal was performed by Un-Scan-It gel software (Silk Scientific, UT, USA).
Statistical Analysis
Data were expressed as mean values ± SEM. The program GraphPad Prism 6 (GraphPad Software, San Diego, CA) was used to perform one-way ANOVA, followed by the Bonferroni’s post hoc test. P < 0.05 was considered significant.
Results
Animal Data
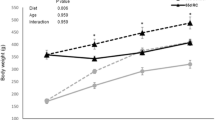
Middle-aged rats, when subjected to a 4-week period of HFF feeding, gained significantly more weight compared to their age-matched controls (Table 2). Cumulative food intake significantly decreased in middle-aged HFF rats, but this difference disappeared when the intake was expressed as metabolizable energy, thus indicating an isocaloric feeding between the two groups of middle-aged rats.
Electron Transport Chain Pathway
In order to have an overall picture of the changes in mitochondrial activity induced by aging or HFF diet, the integrated pathway of the electron transport chain was assessed using HRR to determine the FCR in the hippocampus and frontal cortex homogenates from adult, middle-aged, and middle-aged HFF diet rats using a SUIT protocol.
As for mitochondrial oxygen consumption rates, in hippocampus, we found a significant age-induced decrease in ADP-supported respiration with complex I-linked substrates, while no variation was evident with complex I- and II-linked substrates, both in the presence of ADP and oligomycin. FCCP-stimulated respiration with complex I- and II-linked substrates was significantly lower in middle-aged rats and significantly higher after HFF feeding. On the other hand, FCCP-stimulated respiration with complex II-linked respiration was higher in middle-aged rats (Fig. 1a). In the frontal cortex, a significant age-related decrease in respiratory rates was found, except that in the presence of oligomycin or rotenone. No effect of HFF feeding was evident (Fig. 1b).
Respiratory activity in hippocampus and frontal cortex. Non-normalized respiration after addition of malate + pyruvate + glutamate (PMG), ADP, succinate (S), oligomycin (O), FCCP, rotenone (R), and antimycin A (A) in the hippocampus (a) and frontal cortex (b) from adult, middle-aged, and middle-aged HFF rats. Data are reported as oxygen flux (pmol/(s*ml). Values are the means ± SEM of eight different rats. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA followed by Bonferroni’s post-test). FCCP carbonyl cyanide p-trifluoromethoxyphenylhydrazone
In the hippocampus, the FCRLI (CIL/CI&IIE) and FCRLI&II (CI&IIL/CI&IIE) significantly increased in middle-aged rats, and consequently, the coupling efficiency (1 − CI&IIL/CI&IIP) was significantly lower, while no diet-induced variation was evident (Fig. 2a–c). The FCRPI (CIP/CI&IIETS) significantly decreased with age (Fig. 2d), while FCRPI&II (CI&IIP/CI&IIE) exhibited an opposite trend, i.e., significantly increased with age, with no diet-induced variation (Fig. 2e). Finally, the excess capacity of the electron transport chain (1 − CI&IIP/CI&IIE) drastically decreased with age and significantly increased in middle-aged rats after dietary treatment (Fig. 2f).
Integrated pathway of the electron transport chain in hippocampus. Leak respiration with electron provision from complex I (CIL)/(CI&IIE) (a) and complexes I and II (CI&IIL/CI&IIE) (b), coupling efficiency of oxidative phosphorylation (1 − CI&IIL/CI&IIP) (c), phosphorylating respiration with electron provision from complex I (CIP/CI&IIE) (d), and complexes I and II (CI&IIP/CI&IIE) (e), apparent excess capacity of the electron transport chain (1 − CI&IIP/CI&IIE) (f) in the hippocampus from adult, middle-aged, and middle-aged HFF rats. Values are the means ± SEM of eight different rats. *P < 0.05, **P < 0.01, ****P < 0.0001 (one-way ANOVA followed by Bonferroni’s post-test). HFF high-fat-high-fructose; CIL leak respiration with complex I substrate; CI&IIL leak respiration with complex I and II substrates; CIP phosphorylating respiration with complex I substrate; CI&IIP phosphorylating respiration with complex I and II substrates; CI&IIE maximum capacity of the electron transport chain with complex I and II substrates
In the frontal cortex, the FCRLI (CIL/CI&IIE) significantly decreased with age (Fig. 3a), while the FCRLI&II (CI&IIL/CI&IIE) significantly increased in middle-aged rats (Fig. 3b), and consequently, the coupling efficiency (1 − CI&IIL/CI&IIP) was significantly lower, while no diet-induced variation was evident (Fig. 3c). The FCRPI (CIP/CI&IIE) significantly decreased with age, (Fig. 3d), while FCRPI&II (CI&IIP/CI&IIE) did not show any significant change (Fig. 3e). Finally, the excess capacity of the electron transport chain (1 − CI&IIP/CI&IIET) did not show any significant variation with age or dietary treatment (Fig. 3f).
Integrated pathway of the electron transport chain in frontal cortex. Leak respiration with electron provision from complex I (CIL)/(CI&IIE) (a) and complexes I and II (CI&IIL/CI&IIE) (b), coupling efficiency of oxidative phosphorylation (1 − CI&IIL/CI&IIP) (c), phosphorylating respiration with electron provision from complex I (CIP/CI&IIE) (d), and complexes I and II (CI&IIP/CI&IIE) (e), apparent excess capacity of the electron transport chain (1 − CI&IIP/CI&IIE) (f) in the frontal cortex from adult, middle-aged, and middle-aged HFF rats. Values are the means ± SEM of eight different rats. *P < 0.05, **P < 0.01, ****P < 0.0001 (one-way ANOVA followed by Bonferroni’s post-test). HFF high-fat-high-fructose; CIL leak respiration with complex I substrate; CI&IIL leak respiration with complex I and II substrates; CIP phosphorylating respiration with complex I substrate; CI&IIP phosphorylating respiration with complex I and II substrates; CI&IIE maximum capacity of the electron transport chain with complex I and II substrates
Citrate synthase (CS) activity is a commonly used functional marker for the content of intact mitochondria representing a single-enzyme marker of the mitochondrial matrix [32]. No significant effect of age or dietary treatment was found in hippocampus and frontal cortex, thus suggesting no variation in mitochondrial mass in both areas due to age or diet (CS activity (μmol CoA min−1 × mg tissue), hippocampus adult = 21.6 ± 3.2, middle-aged = 19.1 ± 1.2, middle-aged HFF = 20.0 ± 2.3; cortex adult = 22.0 ± 1.9, middle-aged = 21.1 ± 2.2, middle-aged HFF = 23.0 ± 3.7).
Since the alteration of mitochondrial function could be the consequence of an altered amount of proteins constituting respiratory complexes, we analyzed the abundance of complexes I–V. The analysis of the various components of the mitochondrial respiratory chain in the hippocampus revealed a significant age-dependent increase in complexes I, II, and III, a reduction in complex IV, and no significant variation in complex V (Fig. 4). In addition, the amounts of the complexes I and II were found significantly reduced, while the level of complex IV was increased, in the hippocampus of middle-aged rats following the HFF diet, with no significant alteration of the complex III or V (Fig. 4). These results point to a differential regulation of the respiratory complexes by age or HFF diet.
Mitochondrial complexes’ amount in hippocampus. The amount of mitochondrial complexes I, II, III, IV, and V was assessed by western blot on protein extracts from hippocampus of adult, middle-aged, and middle-aged HFF rats. Representative western blot is shown. Quantitative densitometry was carried out, band intensities were calculated, and protein concentrations were expressed relative to β-actin level. Data are reported as means ± SEM of eight different rats. *P < 0.05, **P < 0.01 (one-way ANOVA followed by Bonferroni’s post-test)
No significant age- or diet-dependent changes in the amount of mitochondrial complexes I–V were detected in the frontal cortex samples (data not shown).
Markers of Oxidative Status
Taking into account the strong link between mitochondrial function and ROS production, we investigated whether a 4-week treatment affects brain oxidative status. To this aim, we evaluated lipid peroxidation as marker of oxidative damage to lipids, and N-Tyr, the footprint of protein oxidative damage induced by peroxynitrite [33] as marker of oxidative damage to proteins, in both hippocampus and cortex. Further, the activity of two antioxidant enzymes, namely SOD and catalase, known markers of the antioxidant defense system, was measured.
In hippocampus, enhanced oxidative damage to proteins, namely an increase in N-Tyr, was found with age (Fig. 5b), while lipid peroxidation was not affected (Fig. 5a). In addition, an age-related decrease in antioxidant enzymes SOD (Fig. 5c) and catalase (Fig. 5d) was found. These results are in line with the well-known increase in oxidative stress associated with aging [34].
Oxidative balance in hippocampus. Lipid peroxidation (a), N-tyrosine (N-Tyr) (b), antioxidant enzyme superoxide dismutase (c), and antioxidant enzyme catalase (d) in hippocampus from adult, middle-aged, and middle-aged HFF rats. Values are the means ± SEM of eight different rats. *P < 0.05, **P < 0.01, ****P < 0.0001 (one-way ANOVA followed by Bonferroni’s post-test)
Oxidative damage to proteins and lipid peroxidation significantly increased with HFF diet (Fig. 5a, b), with no variation in antioxidant enzymes SOD (Fig. 5c) and catalase (Fig. 5d). These results suggest that the increased diet-induced oxidative damage is due to an increase in ROS production.
In the frontal cortex, lipid peroxidation did not show any significant change (Fig. 6a), while both aging and diet were associated with an increase of N-Tyr level (Fig. 6b). In addition, an age-related decrease in antioxidant enzyme SOD was found, with no effect of dietary treatment (Fig. 6c). No significant changes with aging or HFF dietary treatment were detected in catalase activity (Fig. 6d). These results led us to suppose that cerebral cortex is more resistant than hippocampus to lipid peroxidation.
Oxidative balance in frontal cortex. Lipid peroxidation (a), N-tyrosine (N-Tyr) (b), antioxidant enzyme superoxide dismutase (c), and antioxidant enzyme catalase (d) in frontal cortex from adult, middle-aged, and middle-aged HFF rats. Values are the means ± SEM of eight different rats. *P < 0.05, **P < 0.01 (one-way ANOVA followed by Bonferroni’s post-test)
UCP2, PGC-1α, and PPARα Protein Expression
As UCP2 is crucial for reducing the production of ROS and consequent oxidative stress [35], thus playing a neuroprotective effect in brain [36, 37], we evaluated whether aging or diet affects the level of this mitochondrial protein. An age-related increase of UCP2 was observed both in hippocampus (Fig. 7a) and frontal cortex (Fig. 8a), while the amount of the protein significantly decreased with HFF diet in both tissues (Figs. 7a and 8a).
UCP2, PGC-1α, and PPARα levels in hippocampus. UCP2 (a), PGC-1α (b), and PPARα (c) levels were assessed by western blot on protein extracts from hippocampus of adult, middle-aged, and middle-aged HFF rats. Representative western blots are shown (bottom). Quantitative densitometry was carried out, band intensities were calculated, and protein concentrations were expressed relative to β-actin level (top). Data are reported as means ± SEM of eight different rats. *P < 0.05, **P < 0.01 (one-way ANOVA followed by Bonferroni’s post-test)
UCP2, PGC-1α, and PPARα levels in frontal cortex. UCP2 (a), PGC-1α (b), and PPARα (c) levels were assessed by western blot on protein extracts from frontal cortex of adult, middle-aged, and middle-aged HFF rats. Representative western blots are shown (bottom). Quantitative densitometry was carried out, band intensities were calculated, and protein concentrations were expressed relative to β-actin level (top). Data are reported as means ± SEM of eight different rats. *P < 0.05, **P < 0.01, ***P < 0.001 (one-way ANOVA followed by Bonferroni’s post-test)
The protein levels of PGC-1α and PPARα, a coactivating target of PGC-1α that plays a central role in the regulation of cellular energy metabolism, and in the protection against oxidative stress, were also assessed. The amount of PGC-1α in both hippocampus (Fig. 7b) and frontal cortex (Fig. 8b) significantly increased with aging, while decreased with dietary treatment (Figs. 7b and 8b). PPARα protein content significantly decreased with dietary treatment in hippocampus of middle-aged rats, while no age-dependent variations were found (Fig. 7c). The level of this marker was not affected by either age or diet in brain frontal cortex (Fig. 8c). The decrease in UCP2, PGC-1α, and PPARα in hippocampus, in agreement with the results from the analysis of both oxidative stress markers and antioxidant enzymes, shows that the HFF diet has a detrimental effect on the maintenance of redox homeostasis.
Discussion
The aim of the present work was to provide insight into potential brain mitochondrial dysfunction following aging or Western diet consumption in hippocampus and frontal cortex, two critical brain regions crucial for learning and memory, particularly susceptible to modifications by aging or dietary factors [38,39,40].
In the attempt to highlight putative early modifications of mitochondrial function, rats were fed HFF diet for 4 weeks, the minimal period needed to induce brain bioenergetic changes, since the life cycle of a rodent mitochondrion is estimated to be of 3 weeks [41].
To better clarify the mechanism underlying changes in mitochondrial function, which could arise from altered activity and/or amount of respiratory complexes, we analyzed mitochondrial oxidative function through HRR together with the abundance of the respiratory complexes I–V. The obtained results showed drastic age-induced changes in mitochondrial physiology, with a significant decrease in phosphorylation capacity linked to complex I, as well as in the mitochondrial coupling efficiency in both brain regions. In hippocampus, an age-related decrease in excess capacity of the electron transport chain, which is partly reversed by HFF diet, was also evident. All these changes point to a severe age-dependent derangement in mitochondrial capacity to face with ATP needs in a tissue with high metabolic activity, while no additive effect of the HFF diet is evident.
As for hippocampus, a significant decrease in maximal respiratory capacity was evidenced when respiration rates were measured in the presence of complex I- and II-linked substrates and FCCP, thus ruling out the regulation exerted by ATP synthase complex. Taking into account the data from the amount of the different respiratory complexes, it can be concluded that the age-related decreased maximal mitochondrial capacity is driven by the decrease in complex IV amount, while the age-related increase in complexes I, II, and III seems to be ineffective in regulating mitochondrial function. This result suggests that complex IV exerts a high control on mitochondrial respiration when both complex I- and II-linked substrates are oxidized without the control of ATP synthase, in agreement with previous findings [42]. The decrease of maximal mitochondrial respiratory capacity was lower after HFF feeding, and this effect could be again ascribed to the HFF-induced increase in the amount of complex IV.
In the presence of ADP, mitochondrial respiration driven by complex I- and II-linked substrates is also limited by ATP synthase and ATP/ADP carrier, as shown by the lower values obtained in comparison with those obtained with FCCP. In this condition, when only complex I-linked substrates are being oxidized, an age-dependent decrease was evident, although less marked compared to results obtained with FCCP. Since the amount of complex I was higher in middle-aged rats, it can be suggested that this increase is compensatory to a loss of function, probably through the oxidative inactivation of this protein. With complex I- and II-linked substrates, no difference was found, in agreement with the age-induced increase in the amount of complex II.
Leak-related respiration (i.e., measured in the presence of oligomycin) did not show any significant variation. However, the contribution of leak to total respiration significantly increased with age, due to the decrease in maximal capacity, thus evidencing a decrease in coupling efficiency with age.
In the frontal cortex, no variation was found in the amount of the various respiratory complexes. Thus, the age- and diet-induced changes in respiratory activity are likely due to protein oxidative damage that was found in the cortex. The absence of variations in the presence of rotenone are indicative of a normal functioning of the electron transport chain from complex II onwards, thus pointing to a functional impairment of complex I with age in the cortex.
The age-induced decrease in the activity of complex I in hippocampus and frontal cortex is of relevance, since the brain is the tissue with the highest ATP production rate with complex I substrates [43]. Accordingly, chronic reductions in complex I function have been implicated in exacerbating chronic inflammation and cellular damage in brain disease [44, 45]. Our results also point to the higher metabolic vulnerability of the hippocampus, since its excess respiratory capacity becomes obliterated with age, such that mitochondrial activity cannot meet tissue needs for ATP [46].
On the other hand, the increase of complexes I, II, and III with aging might represent a compensatory-like mechanism to counteract loss of mitochondrial activity, which would lead to a dysfunction in ATP synthesis. Accordingly, increased gene expression of mitochondrial encoded subunits of the respiratory complexes has been found in the hippocampus to counteract the reduction in mitochondrial respiration in middle-aged mice, while this compensatory mechanism has not been found to extend into senescence [47]. To date, few studies have been performed to compare the effects of aging on the abundance of respiratory complexes in rats, and a decrease of complexes’ amount and activity were reported [48,49,50,51]. However, it should be pointed out that the above results were obtained using rats of different age [48, 49] or by extracting the five complexes of the oxidative phosphorylation system in the form of active supercomplexes [50,51,52].
Several studies reported alterations in the activity of respiratory chain enzymes in animals fed high-fat diet, but inconsistent results were obtained, as these enzyme activities have been shown to decline, remain unchanged, or even increase [53, 54]. Indeed, bioenergetics consequences of high-fat diet strongly depend on fat source, treatment duration, rodent species or strain, or initial age [54].
It is interesting to note that in middle-aged rats, the dietary regimen consisting of high-fat and high-fructose intake does not exert a strong effect on mitochondrial functioning in the hippocampus, while we have previously found that in adult rats, only 2 weeks of a fructose-rich diet was able to induce a decrease in mitochondrial function [14, 15]. It is therefore possible that, contrary to what is generally believed, dietary stimuli are more detrimental for mitochondria during adulthood than at middle age.
The aging process, as well as obesity onset, are long far known to be associated with the accumulation of ROS. Any stress factor, able to increase ROS production and to overwhelm the cellular antioxidant systems, compromises the bioenergetic function of mitochondria, by impairing the tricarboxylic acid cycle and the respiratory chain with further enhanced production of ROS. This triggers a vicious circle that progressively aggravates oxidative stress and mitochondrial dysfunction [45, 55]. Our data actually show that aging is associated with an increased brain oxidative stress, characterized by increased oxidative damage, decreased antioxidant defenses, and increased UCP2 content. Again, the hippocampus exhibits a higher propensity to metabolic insults, with a higher oxidative damage to proteins concomitant to a decreased catalase activity, compared to frontal cortex. The oxidative stress of these two important brain regions is even more pronounced following HFF diet, especially in the hippocampus, where oxidative damage not only to proteins but also to lipids is detected. The HFF diet-induced increase in oxidative damage is concomitant to a significant reduction in the amount of UCP2 in both regions, in agreement with the fact that this protein protects neurons by reducing the production of free radicals [56]. Taken as a whole, our results on oxidative status indicate that in middle-aged rats, the HFF diet elicits a condition of oxidative stress, which is widely linked to brain dysfunction, even in the absence of marked mitochondrial changes.
To better characterize the changes in redox status induced in brain by aging or high-fat/high-sugar diet, we also analyzed the protein levels of PGC-1α and PPARα, which is a coactivating target of PGC-1α. Indeed, PGC-1α is involved in the regulation of genes that protect neuronal cells from oxidative stress, such as mitochondrial superoxide dismutase [57]. In addition, it has been suggested that PPARα plays a role in controlling the synaptic plasticity in hippocampal neurons in central nervous system [58, 59]. In middle-aged rats, we found an increase in PGC-1α that, however, does not activate key mechanisms to preserve the health and functionality of this area. It cannot be excluded that posttranslational mechanisms may take place. On the other hand, in agreement with previous reports on fat-induced PGC-1α decrease in brain [60, 61], the HFF diet induces an impairment of these defensive mechanisms, since PGC-1α (in hippocampus and frontal cortex) and PPARα (in hippocampus) levels significantly decreased.
Overall, our results point to middle age as a condition of early brain aging for mitochondrial function, with hippocampus being an area more susceptible to metabolic impairment than frontal cortex. An unexpected result is the marginal effect of HFF diet on brain mitochondrial function in middle-aged rats, notwithstanding the condition of oxidative stress. It therefore seems that middle-aged rats, although exhibiting signs of initial brain aging compared to adults, are more resistant to dietary stimuli. Further research devoted at investigating the response to high-fat/high-fructose diet of adult rats compared to middle-aged ones will help to substantiate the above hypothesis. In addition, it cannot be excluded that the Western diet here used, which is able to initiate brain oxidative stress in middle-aged rats, if administered to older age rats and/or for longer time periods, could also induce mitochondrial impairment. Finally, since mitochondrial function and oxidative stress can strongly affect brain insulin signaling as well as synaptic transmission, further studies will be important to highlight whether concomitant changes in these additional parameters take place in middle-aged rats following the HFF diet.
References
National Institute on Aging/World Health Organization (2011) Global health and aging. NIH Publication no:11–7737
Elder AC, Finkelstein J, Johnston C, Gelein R, Oberdorster G (2000) Induction of adaptation to inhaled lipopolysaccharide in young and old rats and mice. Inhal Toxicol 12:225e243
Kodavanti PR, Royland JE, Richards JE, Besas J, Macphail RC (2011) Toluene effects on oxidative stress in brain regions of young-adult, middle-age, and senescent Brown Norway rats. Toxicol Appl Pharmacol 256:386e398
MacPhail RC, Farmer JD, Jarema KA (2012) Toluene effects on the motor activity of adolescent, young-adult, middle-age and senescent male Brown Norway rats. Neurotoxicol 33:111e118
Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J (2005) Effects of air pollution on heart rate variability: the VA normative aging study. Environ Health Perspect 113:304e309
Royland JE, Kodavanti PR, Schmid JE, MacPhail RC (2012) Toluene effects on gene expression in the hippocampus of young adult, middle-age, and senescent Brown Norway rats. Toxicol Sci 126:193e212
Bruce-Keller AJ, White CL, Gupta S, Knight AG, Pistell PJ, Ingram DK, Morrison CD, Keller JN (2010) NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med 49:22–30
Morrison CD, Pistell PJ, Ingram DK, Johnson WD, Liu Y, Fernandez-Kim SO, White CL, Purpera MN et al (2010) High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem 114:1581–1589
Spencer SJ, D'Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM (2017) High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol Aging 58:88–101
Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, Dasuri K, Keller NJ (2010) Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. J Neurochem 114:344–361
Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP (2008) Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18:1085–1088
Granholm AC, Bimonte-Nelson H, Moore AB, Nelson ME, Freeman LR, Sambamurti K (2008) Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis 14:133–145
Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm AC (2014) Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr Neurosci 7:241–251
Cigliano L, Spagnuolo MS, Crescenzo R, Cancelliere R, Iannotta L, Mazzoli A, Liverini G, Iossa S (2018) Short-term fructose feeding induces inflammation and oxidative stress in the hippocampus of young and adult rats. Mol Neurobiol 55(4):2869–2883
Spagnuolo MS, Bergamo P, Crescenzo R, Iannotta L, Treppiccione L, Iossa S, Cigliano L (2018) Brain Nrf2 pathway, autophagy, and synaptic function proteins are modulated by a short-term fructose feeding in young and adult rats. Nutr Neurosci 24:1–12. https://doi.org/10.1080/1028415X.2018.1501532
Papa L, Rockwell P (2008) Persistent mitochondrial dysfunction and oxidative stress hinder neuronal cell recovery from reversible proteasome inhibition. Apoptosis 13:588–599
Collier TJ, Coleman PD (1991) Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol Aging 12:685–693
Odermatt A (2011) The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Ren Physiol 301:F919–F931
Pesta D, Gnaiger E (2012) High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 810:25–58
Picard M, Taivassalo T, Ritchie D, Wright KJ, Thomas MM, Romestaing C, Hepple RT (2011) Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One 6(3):e18317
Picard M, Taivassalo T, Gouspillou G, Hepple RT (2011) Mitochondria: isolation, structure and function. J Physiol 589:4413–4421
Papp EA, Leergaarda TB, Calabrese E, Johnson GA, Bjaalie JG (2014) Waxholm Space atlas of the Sprague Dawley rat brain. NeuroImage 97:374–386
Burtscher J, Zangrandi L, Schwarzer C, Gnaiger E (2015) Differences in mitochondrial function in homogenated samples from healthy and epileptic specific brain tissues revealed by high-resolution respirometry. Mitochondrion 25:104–112
Gnaiger E (2009) Capacity of oxidative phosphorylation in human skeletal muscle: new perspectives of mitochondrial physiology. Int J Biochem Cell Biol 41:1837–1845
Gnaiger E (2014) Mitochondrial pathways and respiratory control. An introduction to OXPHOS analysis. OROBOROS MiPNet Publications (4th ed): ISBN 978-973-9502399-9502398-9502390
Srere PA (1969) Citrate synthase. Methods Enzymol 13:3–11
Fernandes MA, Custódio JB, Santos MS, Moreno AJ, Vicente JA (2006) Tetrandrine concentrations not affecting oxidative phosphorylation protect rat liver mitochondria from oxidative stress. Mitochondrion 6:176–185
Spagnuolo MS, Mollica MP, Maresca B, Cavaliere G, Cefaliello C, Trinchese G, Scudiero R, Crispino M et al (2015) High fat diet and inflammation—modulation of haptoglobin level in rat brain. Front Cell Neurosci 9:479. https://doi.org/10.3389/fncel.2015.00479
Maehly AC, Chance B (1954) The assay of catalases and peroxidases. Methods Biochem Anal 1:357–424
Flohè L, Otting F (1974) Superoxide dismutase assay. Methods Enzymol 105:93–104
Spagnuolo MS, Maresca B, Mollica MP, Cavaliere G, Cefaliello C, Trinchese G, Esposito MG, Scudiero R et al (2014) Haptoglobin increases with age in rat hippocampus and modulates apolipoprotein E mediated cholesterol trafficking in neuroblastoma cell lines. Front Cell Neurosci 8:212
Holloszy JO, Oscai LB, Don IJ, Molé PA (1970) Mitochondrial citric acid cycle and related enzymes: adaptive response to exercise. Biochem Biophys Res Commun 40(6):1368–1373
Halliwell B, Gutteridge JMC (2000) Free radicals, other reactive species and disease. In: Halliwell B, Gutteridge JMC (eds) Free radicals in biology and medicine. Oxford University Press, Oxford, pp. 617–783
Garaschuk O, Semchyshyn HM, Lushchak VI (2018) Healthy brain aging: interplay between reactive species, inflammation and energy supply. Ageing Res Rev 43:26–45
Krauss S, Zhang CY, Lowell BB (2005) The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol 6:248–261
Mattiasson G, Shamloo M, Gido G, Mathi K, Tomasevic G, Yi S, Warden CH, Castilho RF et al (2003) Uncoupling protein-2 prevents neuronal death and diminishes brain dysfunction after stroke and brain trauma. Nat Med 9:1062–1068
Barnstable CJ, Reddy R, Li H, Horvath TL (2016) Mitochondrial uncoupling protein 2 (UCP2) regulates retinal ganglion cell number and survival. J Mol Neurosci 58:461–469
Preston AR, Eichenbaum H (2013) Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23(17):R764–R773
Monti JM, Baym CL, Cohen NJ (2014) Identifying and characterizing the effects of nutrition on hippocampal memory. Adv Nutr 5(3):337S–343S
Beilharz JE, Maniam J, Morris MJ (2015) Diet-induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients 7(8):6719–6738
Rajwade MS, Katyare SS, Fatterpaker P, Sreenivasan A (1975) Regulation of mitochondrial protein turnover by thyroid hormone(s). Biochem J 152:379–387
Davey GP, Clark JB (1996) Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem 66(4):1617–1624
Cocco T, Pacelli C, Sgobbo P, Villani G (2009) Control of OXPHOS efficiency by complex I in brain mitochondria. Neurobiol Aging 30:622–629
Navarro A, Boveris A (2009) Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J Bioenerg Biomembr 41:517–521
Grimm A, Eckert A (2017) Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem 143(4):418–431
Braidy N, Poljak A, Grant R, Jayasena T, Mansour H, Chan-Ling T, Guillemin GJ, Smythe G et al (2014) Mapping NAD+ metabolism in the brain of ageing Wistar rats: potential targets for influencing brain senescence. Biogerontol 15:177–198
Manczak M, Jung Y, Park BS, Partovi D, Reddy PH (2005) Time-course of mitochondrial gene expressions in mice brains: implications for mitochondrial dysfunction, oxidative damage, and cytochrome c in aging. J Neurochem 92:494–504
Navarro A, Bandez MJ, Lopez-Cepero JM, Gomez C, Boveris A (2011) High doses of vitamin E improve mitochondrial dysfunction in rat hippocampus and frontal cortex upon aging. Am J Phys Regul Integr Comp Phys 300:R827–R834
Thomsen K, Yokota T, Hasan-Olive MM, Sherazi N, Fakouri NB, Desler C, Regnell CE, Larsen S et al (2018) Initial brain aging: heterogeneity of mitochondrial size is associated with decline in complex I-linked respiration in cortex and hippocampus. Neurobiol Aging 61:215–224
Dencher NA, Frenzel M, Reifschneider NH, Sugawa M, Krause F (2007) Proteome alterations in rat mitochondria caused by aging. Ann N Y Acad Sci 1100:291–298
Frenzel M, Rommelspacher H, Sugawa MD, Dencher NA (2010) Ageing alters the supramolecular architecture of OxPhos complexes in rat brain cortex. Exp Gerontol 45:563–572
Dudkina NV, Kouril R, Peters K, Braun HP, Boekema EJ (2010) Structure and function of mitochondrial supercomplexes. Biochim Biophys Acta 1797:664–670
Franko A, von Kleist-Retzow JC, Neschen S, Wu M, Schommers P, Böse M, Kunze A, Hartmann U et al (2014) Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J Hepatol 60:816–823
Kakimoto PA, Kowaltowski AJ (2016) Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol 8:216–225
Wang CH, Wu SB, Wu YT, Wei YH (2013) Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp Biol Med (Maywood) 238:450–460
Lu M, Sun XL, Qiao C, Liu Y, Ding JH, Hu G (2014) Uncoupling protein 2 deficiency aggravates astrocytic endoplasmic reticulum stress and nod-like receptor protein 3 inflammasome activation. Neurobiol Aging 35:421–430
St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K et al (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127(2):397–408
Roy A, Jana M, Corbett GT, Ramaswamy S, Kordower JH, Gonzalez FJ, Pahan K (2013) Regulation of cyclic AMP response element binding and hippocampal plasticity-related genes by peroxisome proliferator-activated receptor alpha. Cell Rep 4:724–737
Roy A, Pahan K (2015) PPARα signaling in the hippocampus: crosstalk between fat and memory. J NeuroImmune Pharmacol 10(1):30–34
Sajan M, Hansen B, Ivey R, Sajan J, Ari C, Song S, Braun U, Leitges M et al (2016) Brain insulin signaling is increased in insulin-resistant states and decreases in FOXOs and PGC-1α and increases in Aβ1-40/42 and phospho-tau may abet Alzheimer development. Diabetes 65(7):1892–1903
Morselli E, Frank AP, Palmer BF, Rodriguez-Navas C, Criollo A, Clegg DJ (2016) A sexually dimorphic hypothalamic response to chronic high-fat diet consumption. Int J Obes 40:206–209
Acknowledgements
The authors wish to thank Dr. Emilia de Santis for skillful management of animal house.
Funding
This work was supported by a grant from University of Naples Federico II - Ricerca Dip 2017 and by a FIRB - Futuro in Ricerca grant (RBFR12QW4I_004) from the Italian Ministry of Education, University and Research (MIUR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Treatment, housing, and euthanasia of animals met the guidelines set by the Italian Health Ministry. All experimental procedures involving animals were approved by “Comitato Etico-Scientifico per la Sperimentazione Animale” of the University of Naples Federico II.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Figure 9
Representative traces of oxygen flux in the hippocampus of middle-aged rats. (PNG 1.71 mb)
Supplementary Figure 10
Representative western blot of respiratory complexes I-V carried out on protein extracts from hippocampus of adult (1) middle-aged (2) and middle-aged HFF (3) rats. (PNG 12.9 mb)
Rights and permissions
About this article
Cite this article
Crescenzo, R., Spagnuolo, M.S., Cancelliere, R. et al. Effect of Initial Aging and High-Fat/High-Fructose Diet on Mitochondrial Bioenergetics and Oxidative Status in Rat Brain. Mol Neurobiol 56, 7651–7663 (2019). https://doi.org/10.1007/s12035-019-1617-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-019-1617-z