Abstract
Dopamine (DA) is critical for motivation, reward, movement initiation, and learning. Mechanisms that control DA signaling have a profound impact on these important behaviors, and additionally play a role in DA-related neuropathologies. The presynaptic SLC6 DA transporter (DAT) limits extracellular DA levels by clearing released DA, and is potently inhibited by addictive and therapeutic psychostimulants. Decades of evidence support that the DAT is subject to acute regulation by a number of signaling pathways, and that endocytic trafficking strongly regulates DAT availability and function. DAT trafficking studies have been performed in a variety of model systems, including both in vitro and ex vivo preparations. In this review, we focus on the breadth of DAT trafficking studies, with specific attention to, and comparison of, how context may influence DAT’s response to different stimuli. In particular, this overview highlights that stimulated DAT trafficking not only differs between in vitro and ex vivo environments, but also is influenced by both sex and anatomical subregions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dopamine (DA) is a modulatory neurotransmitter that plays a central role in a variety of complex, evolutionarily conserved behaviors. Midbrain DA neurons in the substantia nigra project to the dorsal striatum (DS), where DA is required for motor control and habit formation. DAergic neurons in the ventral tegmental area (VTA) project primarily to the prefrontal cortex and ventral striatum (VS), where DA critically influences reward, motivation, anxiety, and predictive cue conditioning [1, 2]. DA neurons fire tonically with phasic bursting, and rewarding stimuli drive enhanced bursting [3]. Once released, DA’s extracellular half-life is strictly limited by presynaptic reuptake, mediated by the Na+/Cl−-dependent DA transporter (DAT). DAT is potently inhibited by addictive and therapeutic psychostimulants, such as cocaine, methylphenidate (Ritalin), and amphetamines, which are competitive antagonists (cocaine, methylphenidate) and substrates (amphetamines), and their binding to DAT is requisite to elicit rewarding behaviors [4,5,6]. Multiple DAT coding variants have been identified in patients with attention-deficit/hyperactivity disorder, ADHD [7,8,9,10], autism spectrum disorder, ASD [9, 11, 12], and Parkinson’s-like neurodegenerative disorders [13,14,15], illustrating that DAT dysfunction has a marked impact on DAergic homeostasis.
Decades of effort from multiple investigators support that DAT is not static in the plasma membrane, but is dynamically regulated by endocytic trafficking. Multiple signaling pathways modulate DAT endocytic trafficking, which ultimately impacts DAT surface expression. Given the profound impact that DAT dysfunction imparts on baseline DAergic tone and function, regulated DAT surface expression is mechanistically well poised to likewise influence DA signaling and DA-dependent behaviors. The majority of investigations into the individual mechanisms that mediate regulated DAT trafficking, and their potential impact on DAergic function, have primarily been conducted outside the context of DAergic terminals. Recent technical advances in conditional gene expression and delivery, as well as in optical and ex vivo approaches, have facilitated examining DAT regulation and trafficking in its appropriate context, and have raised the possibility that the complex circuitry inherent to DAergic terminal regions may converge to dynamically regulate DAT. Here, we review regulated DAT trafficking studies to date, with an emphasis on how context may influence DAT trafficking. It is our hope that viewing DAT trafficking studies in this light may set the stage for where future DAT regulatory studies may be aimed.
DRD2- and Gi-Mediated DAT Plasma Membrane Delivery
The D2 DA receptor subtype (DRD2) is a Gi-coupled receptor expressed widely throughout the striatum. Presynaptically, DRD2 is an autoreceptor on DAergic terminals. Post-synaptically, DRD2 is expressed in striatal glutamatergic terminals, cholinergic interneurons, and GABAergic medium spiny neurons of the indirect pathway, which project either (1) from the dorsal striatum to the globus pallidus, or (2) from the nucleus accumbens to the ventral pallidum [16]. DRD2 is a member of the DRD2-like receptor subfamily, which is comprised of DRD2, DRD3, and DRD4. Multiple lines of evidence, both from ex vivo and transfected cell line studies, support that DRD2 activation increases DAT function and plasma membrane expression. Initial studies in rat striatal synaptosomes revealed that the DRD2-like agonist, quinpirole, increased DA uptake as measured by rotating disk voltammetry [17]. Moreover, in vivo chronoamperometry demonstrated that DA clearance decreased following systemic injection with the broad-spectrum DRD antagonist, haloperidol [17]. Subsequent kinetic studies in Xenopus oocytes co-expressing DAT and DRD2 observed both increased DA uptake Vmax and [3H]WIN35,428 whole cell binding Bmax, suggesting that DRD2 activation may increase DAT activity via enhanced surface expression [18]. DRD2-mediated DAT functional upregulation was further confirmed by Liu and colleagues [19], who reported that DRD2 associates with DAT in isolated protein complexes from rat striatal lysates, and that DAT residues 1–26 were sufficient to recover DRD2 in vitro. One potential confound in studies using [3H]DA uptake to measure how DRD2 activation impacts DAT function, is that the inherent addition of DA to the assay will also activate DRD2. To eliminate this potential pitfall, Shippenberg and colleagues leveraged the fluorescent DAT substrate, 4-[4-(diethylamino)-styryl]-N-methylpyridinium iodide (ASP+), which is taken up by DAT, but does not activate DRD2 [20]. Using ASP+ uptake, these studies found that DRD2-mediated increases in DAT function required ERK1/2, but not PI3-kinase, activity [20] in HEK and N2a cells. Further, using BRET they confirmed the DRD2-DAT association, but found that DAT N-terminal residues 1–55 were not required for the DRD2-DAT association by co-immunoprecipitation. Taken together, these initial studies clearly demonstrated that DRD2 increases DAT activity, and were consistent with the hypothesis DRD2-mediated DAT upregulation was likely due to enhanced surface expression.
DRD2-mediated DAT surface delivery was first directly demonstrated by Gnegy and colleagues, using a surface biotinylation approach in ex vivo mouse striatal synaptosomes, prepared from total striatum that included both DS and VS [21]. Moreover, using both PKCβ-specific inhibitors and PKCβ−/− mice, they found that DRD2-mediated DAT surface delivery requires PKCβ [21, 22]. These landmark results have opened the door to a variety of new potential questions regarding DRD2-mediated DAT trafficking: Is DRD2-activated DAT trafficking mediated by DRD2 autoreceptors, or is there a retrograde signaling contribution via DRD2 receptors expressed throughout the striatum? Are there regional differences in DRD2-mediated DAT surface delivery? Blakely and colleagues recently reported that DRD2-dependent DAT trafficking differs between DS and VS in ex vivo slices, where the DRD2 agonist, quinpirole, significantly increased DAT surface expression in DS, but had no effect on DAT surface levels in VS [23]. The mechanisms governing these regional differences remain unknown. However, it should be noted that quinpirole can activate all D2-like receptors (i.e. DRD2, DRD3, DRD4; Ki ~ 4.8, 24, and 30 nM, respectively), as well as DRD1 (1.9 µM). Since their study used 1 µM quinpirole, there is a possibility that region-specific effects reported may reflect a net integrated signal from multiple DRDs, which would be equally interesting to discern. Alternatively, region-specific, DRD2-mediated DAT trafficking could arise from distinct DRD2 signaling, which is differentially sensitive to DA in the DS vs. VS [24].
Does DRD2-dependent DAT trafficking occur in vivo? In vivo chronoamperometric studies revealed that hypoinsulinemic rats exhibit reduced DA clearance, due to decreased insulin receptor-mediated PI3K/Akt signaling [25]. Interestingly, DAT activity in hypoinsulinemic rats was restored in a DRD2-dependent manner by treating with AMPH [26], which drives DA efflux through the DAT [27, 28]. These results strongly suggest that DRD2-mediated DAT membrane insertion occurs in vivo, in response to elevated extracellular DA.
Do other Gi-coupled GPCRs promote DAT surface expression? Studies from Shippenberg and colleagues found that kappa opiate receptor (KOR) activation increased DA uptake and DAT surface expression in cell lines and synaptosomes, and likewise found that KOR activation increased DA uptake in minced striatal preparations, using rotating disk voltammetry [29]. Given that KOR activation has aversive properties, KOR-mediated DAT trafficking is poised as a pivotal interaction point between the opiate and reward circuitry, and may have future therapeutic potential [30].
DAT PDZ Domain-Dependent Surface Expression
Multiple DAT domains have been identified that are required either (1) to maintain DAT surface expression, or (2) to promote biosynthetic (i.e. “forward”) DAT trafficking. The final carboxy terminal amino acids of DAT, “LKV”, constitute a PDZ-binding domain, and are required for DAT binding to the PDZ protein, PICK1 (protein interacting with C kinase 1) [31]. Initial studies in HEK293 cells and cultured DA neurons found that PICK1 potentiated DAT function in an LKV-dependent manner. Moreover, truncating the LKV residues from the DAT carboxy terminus substantially reduced DA uptake and DAT axonal targeting, suggesting that the PDZ domain, possibly through the PICK1 association, is required for DAT surface delivery [31]. A subsequent study by Gether and colleagues confirmed that truncating the LKV motif indeed resulted in DAT retention in the endoplasmic reticulum (ER). They further found that replacing the LKV motif with the β2-adrenergic receptor PDZ domain (SLL) sufficed to rescue DAT surface targeting, but not PICK1 binding, indicating that PDZ-dependent plasma membrane targeting may not be solely dependent upon the DAT-PICK1 interaction [32]. Moreover, using an alanine substitution mutant (DAT-AAA), our laboratory recently found that the LKV PDZ domain is required for retromer-dependent, DAT endosomal surface delivery in the rat mesencephalic cell line, AN27 [33]. However, DAT-AAA relative surface levels were comparable to wildtype DAT, indicating that the DAT LKV motif, per se, might not be required for DAT biosynthesis and forward trafficking in AN27 cells [33].
In order to address the role of the LKV motif in situ, Gether and colleagues generated a knock-in mouse expressing DAT-AAA, which had significantly reduced affinity for purified PICK1 protein [34]. The DAT-AAA mouse had a striking loss in striatal DAT protein. Furthermore, DAT-AAA was not retained in the ER in neuronal cultures made from the knock-in mouse, in agreement with their previous cell line report [32]. However, PICK1 was not required in vivo for proper DAT protein levels or axonal targeting, as demonstrated by the PICK1 knockout mouse [34]. In summary, these data indicate that (1) the DAT PDZ domain is required in vivo for DAT protein expression, but not for DAT’s overall surface: intracellular distribution, and (2) PICK1, though initially thought to be required for PDZ-dependent DAT plasma membrane targeting, is not required in vivo for DAT protein expression. Together, these reports highlight the importance of investigating DAT trafficking mechanisms in DAergic neurons, especially if there are contradictory findings among various expression systems.
Constitutive DAT Endocytosis
Constitutive DAT internalization and recycling has been reported in a variety of heterologous expression systems [33, 35,36,37,38,39,40], as well as in primary DAergic neuronal cultures [38, 41], as measured using biochemical and imaging approaches [35, 36, 38,39,40,41,42,43,44]. Constitutively internalized DAT can reportedly target to several endocytic compartments, including those positive for EEA1, rab4, rab5, and the Vps35 retromer complex component. DAT also targets, albeit to a lesser extent, to rab11- and rab7-positive loci [33, 38, 41].
Despite these findings, constitutive DAT trafficking in intact DA terminals has proven difficult to assess. In cell lines, basal DAT endocytic trafficking can be readily measured using reversible biotinylation assays [45]. However, the rapid and dramatic temperature shifts required for this approach are not optimal for acute brain slice viability, creating a sizable obstacle in measuring DAT internalization in bona fide DAergic terminals. Using cultured rat midbrain DA neurons and the fluorescent cocaine analog JHC 1-64, which selectively labels DAT [46], Gether and colleagues found that native DAT indeed constitutively internalizes [38]. Hong and Amara further confirmed this finding, and found that internalized DAT co-localizes with Rab11+ recycling endosomes in rat embryonic mesencephalic primary cultured neurons [41]. To track DAT internalization in DAergic terminals in situ, Sorkin and colleagues generated a DAT knock-in mouse, in which an HA epitope was engineered into the DAT extracellular loop 2 (HA-EL2-DAT), and used this mouse to monitor DAT internalization by tracking anti-HA antibody internalization in ex vivo striatal slices. They found only sparse intracellular HA immunoreactivity via electron microscopy [47], and therefore concluded that DAT undergoes little, if any, constitutive or regulated endocytosis in axon terminals. This result is in contrast to biochemical studies that demonstrate that various stimuli can modulate DAT surface expression in ex vivo striatal slices (elaborated below), and raises the possibility that technical obstacles may have impacted their study. For example, studies were performed in 800 µm brain slices, which are relatively thick in comparison to the standard 250–400 µm thickness typically prepared, which maximizes tissue oxygenation for ex vivo studies. Moreover, several recent reports demonstrated that large immunoglobulins cannot efficiently penetrate thick tissue slices beyond 50–100 µm [48, 49]. Similarly, our laboratory recently reported that although PRIME (PRobe Incorporation Mediated by Enzyme) labeling can efficaciously label surface DAT and track its internalization in monolayer culture, it cannot be used to successfully label DAT in 300 µm acute brain slices, presumably due to an inability of the lipoic acid ligase (LpIA) enzyme to effectively penetrate the slice [33]. Given that the HA-EL2-DAT mouse study did not present controls for either slice viability or antibody access to deep tissue loci, it is not clear whether the approach used was able to accurately measure endogenous DAT trafficking events.
Recent studies using super-resolution microscopy techniques such as PALM (photoactivated localization microscopy) and STORM (stochastic optical reconstruction microscopy) have allowed researchers to more precisely measure DAT surface dynamics in cultured DA neurons [50], however this type of high-resolution approach has not yet been employed to study basal or stimulated DAT trafficking in DA terminals. Thus, it remains unclear whether DAT undergoes constitutive internalization in the striatum.
PKC-Stimulated DAT Endocytosis
Early studies in Xenopus oocytes, COS cells, and striatal synaptosomes demonstrated that the Vmax of DA uptake rapidly decreases in response to acute protein kinase C (PKC) activation with phorbol esters [51,52,53], suggesting that DAT may be subject to either PKC-mediated catalytic inactivation, decreased surface expression, or both. Subsequent studies in heterologous expression systems demonstrated that acute PKC activation decreases DAT surface expression [54, 55], and that the shift in DAT from the cell surface to endosomal loci is mediated by increased DAT internalization combined with decreased plasma membrane delivery [35, 41, 53]. PKC-stimulated DAT surface downregulation has been demonstrated in both neuronal and non-neuronal cell culture models (for review see: [56]). We further reported that PKC activation decreases DAT surface expression in ex vivo acute (total) striatal slices, demonstrating that PKC activation impacts DAT surface expression in bona fide DAergic terminals [40]. More recently, we further explored whether there are region-specific differences in the ability of PKC to drive DAT internalization. Surprisingly, PKC activation in DS had no effect on DAT surface expression, whereas in the VS, PKC activation significantly decreased DAT surface levels in male and female mice [57]. Given that PKC activation decreases surface DAT in total striatal slices (i.e. that include both DS and VS) [40], these results suggest that any PKC-mediated effects on DAT trafficking observed in total striatum were driven solely from VS.
We recently reported that in vivo, conditional Rit2 (AKA: Rin) knockdown (Rit2-KD) in DAergic neurons decreased DAT protein levels in total striatum of male mice [58]. Given that Rit2 is required for PKC-stimulated DAT internalization in cell lines [43], we subsequently leveraged shRNA-mediated DAergic Rit2-KD to directly test whether Rit2 is required for the PKC-mediated DAT surface loss in DAergic terminals [57]. In male and female VS, Rit2 was indeed required for PKC-mediated DAT internalization. Surprisingly, following Rit2-KD, PKC activation increased DAT surface expression in male DS and had no effect on DAT surface expression in female DS. The mechanism(s) through which PKC increases DAT surface expression in the absence of Rit2 in male DS are not yet known. These results emphasize the importance of studying DAT endocytic mechanisms not only the specific context where DAT is endogenously expressed, but also in both male and female subjects, as the mechanisms are not necessarily the same, and should not be assumed to be so.
As described above, PKCβ is required for D2-dependent DAT insertion (see “DRD2- and Gi-Mediated DAT Plasma Membrane Delivery”), however it remains unknown which PKC isoform(s) are required for PKC-stimulated DAT internalization in response to phorbol ester treatment. PMA activates two diacylglycerol (DAG)-sensitive PKC isozyme subtypes: classical (DAG- and Ca2+-dependent) and novel (DAG-dependent, Ca2+-independent) PKCs [59]. Candidate PKCs can be further narrowed, as PMA-stimulated DAT internalization is blocked by the PKC inhibitor, bisindolylmaleimide (BIM I, GF 109203X, Gö 6850) [54, 60, 61], which is selective for α, βI, δ, ε, and ζ PKC isozymes. However, PKCζ is not DAG-dependent, and therefore not activated by PMA. Thus, PKC-stimulated DAT internalization likely requires either PKCα, βI, δ, or ε.
What are the physiological means that drive PKC-stimulated DAT internalization? Conventional and novel PKCs are typically activated in response to stimulating Gq-coupled GPCRs (G-protein-coupled receptors), which activate PKC and release Ca2+ from intracellular stores, in parallel, downstream of phospholipase C activation. However, it still not clear whether activating endogenously expressed, Gq-coupled GPCRs stimulates DAT internalization in intact DA terminals. Studies in transfected HEK293 and N2a cells demonstrated that activating the Gq-coupled receptor neurokinin (NK)-1 with its endogenous ligand, substance P, reduced DAT surface expression in a PKC-dependent manner [62], providing a possible candidate for endogenous PKC-dependent DAT endocytosis. However, substance P-dependent DAT internalization has not yet been reported in DAergic terminals. The Gq-coupled, Group I metabotropic glutamate receptor 5 (mGluR5) has also been implicated in DAT functional downregulation. DHPG, a Group I selective mGluR agonist, decreased DAT function in rat striatal synaptosomes, which was blocked by the mGluR5-specific antagonist, MPEP, as well as the PKC inhibitor, Ro-31–8220 [63]. However, Ro-21-8220 was used at a relatively high concentration that can also inhibit other kinases (e.g. GSK3β, MAPKAP-K1β), raising the possibility that mGluR5-mediated DAT downregulation may be mediated via signaling pathways other than PKC. Fast-scan cyclic voltammetry studies from Alvarez and colleagues recently found that activating the muscarinic receptor M5, a Gq-coupled GPCR selectively expressed in DA neurons [64, 65], significantly decreased DA clearance rates in VS [66]. Given that mGluR5, and possibly M5, receptors are expressed on other cell types throughout the striatum, such as cholinergic interneurons and medium spiny neurons, it is unclear whether or not mGluR5 and M5-mediated DAT downregulation occur cell autonomously. Thus, whether activating a Gq-coupled GPCR expressed on DA terminals can stimulate PKC-dependent DAT internalization, and whether this mechanism is subject to regional differences, remains to be tested.
Amphetamine-Stimulated DAT Endocytosis
Amphetamine (AMPH) is an addictive psychostimulant that increases extracellular DA concentrations via multiple actions at the DA terminal. AMPH is a competitive substrate for DAT, thus increases DA by blocking reuptake through DAT [67]. AMPH also depletes vesicular DA content and induces DA efflux through DAT, further enhancing DA levels at the synapse [27, 67,68,69,70,71]. Moreover, AMPH exposure induces DAT internalization from the plasma membrane, thus decreasing surface DAT availability [41, 61, 72,73,74,75,76,77,78].
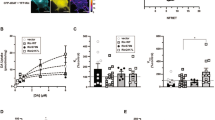
AMPH-stimulated DAT surface loss was originally characterized in HEK293 cells treated with AMPH [72]. This result was later replicated in synaptosomes made from whole rat striatum [75] and in primary DA neuronal cultures [77]. AMPH-induced DAT internalization was further demonstrated in ex vivo mouse midbrain slices, and was shown to be dependent on Rho GTPase activity downstream of the trace amine-associated receptor (TAAR) 1 [77, 78]. However, it is still unknown whether AMPH stimulates DAT internalization in bona fide DA terminals. Using the HA-αEL2-DAT and electron microscopy techniques, Block and colleagues found that i.p. AMPH injection did not subsequently affect DAT surface distribution in axon terminals or DA cell bodies, however it is unclear whether the DAT labeling method employed was sufficient to detect drug-induced changes (see “Constitutive DAT Endocytosis”) [47]. To directly test this possibility, we performed ex vivo striatal slice biotinylation (as previously described [40, 44, 58], and found that AMPH treatment (10 µM, 30 min, 37 °C) induced significant DAT surface loss, both in DS and VS (Fig. 1). These results confirm that AMPH drives DAT surface loss in DAergic terminals. However, whether the mechanisms required for AMPH-stimulated endocytosis in DAergic terminals are similar to those in somatodendritic regions remains to be tested.
AMPH stimulates DAT internalization in mouse dorsal and ventral striatum. Ex vivo striatal slice biotinylation. Acute striatal slices were prepared and treated ± 10 µM AMPH, 30 min, 37 °C. Surface proteins were biotinylated and isolated by streptavidin pulldown, and DAT was detected by immunoblot using rat αDAT (Millpore MAB369), as previously described [40, 58]; Wu, 2015 #35}. aStriatal slice subdissection. Slices including VS were identified by presence of the anterior commissure (AC). Prior to lysis, slices were subdissected to enrich for dorsal and ventral striatum, by cutting from the lateral ventricle (LV) to the olfactory tract (OT). bTop representative blots of surface and total (input) DAT following the indicated treatment(s). Bottom average DAT surface levels, expressed as %total DAT input. *Significantly less than vehicle-treated control, p < 0.05, one-tailed Student’s t test, n = 6–9
Receptor Tyrosine Kinase-Mediated DAT Trafficking
DAT surface expression is also regulated by receptor tyrosine kinases (RTKs). Broad-spectrum tyrosine kinase inhibitors, such as genistein, tyrphostin 23, and tyrphostin 25, significantly decreased DAT function in DS synaptosomes and Xenopus oocytes [79, 80]. Additional studies indicate that direct RTK activation modulates DAT surface expression [81,82,83,84]. Insulin-like growth factor receptor (IGFR-1) activation increased DAT function and surface expression in transfected cell lines, and was dependent on PI3-kinase and Akt activity, as defined with PI3-kinase and Akt inhibitors [81, 82]. Moreover, hypoinsulinemia induced either by streptozotocin treatment or high fat diet significantly reduced DA clearance rates, DA reuptake, and DAT surface expression compared to controls, as measured in rat striatal synaptosomes [25, 85, 86], consistent with the results obtained in cell lines.
Glial cell line-derived neurotrophic factor (GDNF) also regulates DAT surface expression through receptor Ret activation and downstream signaling [84]. GDNF+/− mice exhibited increased DA uptake in the VS, but not DS, as measured via in vivo chronoamperometry, and reduced striatal DA tissue content in both VS and DS [87]. Furthermore, a similar, regional-specific increase in DA levels and DAT function was observed in synaptosomes prepared from Ret+/− DS and VS [84]. GDNF/Ret-dependent negative regulation of DAT surface expression was demonstrated to require Vav2, a guanine exchange factor (GEF) that activates Rho and Rac GTPases [84]. In striatal synaptosomes prepared from Vav2−/− mice, DAT exhibited enhanced DA uptake and surface expression specifically in the VS, but not DS. Moreover, GDNF-dependent Ret activation increased Vav2 phosphorylation, and Ret co-expression increased the DAT-Vav2 interaction, suggesting that Ret RTK signaling may negatively regulate DAT surface expression through Vav2 activation [84].
In summary, regulated DAT trafficking occurs in response to multiple cellular signaling pathways, and is poised to significantly impact DAergic signaling, as well as DA-dependent behaviors and neuropathologies. The recent increase in DAT trafficking studies, carried out both in vivo and in ex vivo preparations, will undoubtedly glean mechanisms that impact DAT surface presentation, as well as how converging signaling pathways within DAergic terminal regions are integrated to impact DAT surface availability and function.
References
Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5:483–494
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565–598
Keiflin R, Janak PH (2015) Dopamine prediction errors in reward learning and addiction: from theory to neural circuitry. Neuron 88:247–263
Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH (2006) Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA 103:9333–9338
Thomsen M, Han DD, Gu HH, Caine SB (2009) Lack of cocaine self-administration in mice expressing a cocaine-insensitive dopamine transporter. J Pharmacol Exp Ther 331:204–211
Tilley MR, O’Neill B, Han DD, Gu HH (2009) Cocaine does not produce reward in absence of dopamine transporter inhibition. NeuroReport 20:9–12
Bowton E, Saunders C, Reddy IA, Campbell NG, Hamilton PJ, Henry LK, Coon H, Sakrikar D, Veenstra-VanderWeele JM, Blakely RD, Sutcliffe J, Matthies HJ, Erreger K, Galli A (2014) SLC6A3 coding variant Ala559Val found in two autism probands alters dopamine transporter function and trafficking. Transl Psychiatry 4:e464
Mazei-Robison MS, Bowton E, Holy M, Schmudermaier M, Freissmuth M, Sitte HH, Galli A, Blakely RD (2008) Anomalous dopamine release associated with a human dopamine transporter coding variant. J Neurosci 28:7040–7046
Mergy MA, Gowrishankar R, Gresch PJ, Gantz SC, Williams J, Davis GL, Wheeler CA, Stanwood GD, Hahn MK, Blakely RD (2014) The rare DAT coding variant Val559 perturbs DA neuron function, changes behavior, and alters in vivo responses to psychostimulants. Proc Natl Acad Sci USA 111:E4779–4788
Mazei-Robison MS, Couch RS, Shelton RC, Stein MA, Blakely RD (2005) Sequence variation in the human dopamine transporter gene in children with attention deficit hyperactivity disorder. Neuropharmacology 49:724–736
DiCarlo GE, Aguilar JI, Matthies HJ, Harrison FE, Bundschuh KE, West A, Hashemi P, Herborg F, Rickhag M, Chen H, Gether U, Wallace MT, Galli A (2019) Autism-linked dopamine transporter mutation alters striatal dopamine neurotransmission and dopamine-dependent behaviors. J Clin Investig 129:3407–3419
Hamilton PJ, Campbell NG, Sharma S, Erreger K, Herborg Hansen F, Saunders C, Belovich AN, Consortium NAAS, Sahai MA, Cook EH, Gether U, McHaourab HS, Matthies HJ, Sutcliffe JS, Galli A (2013) De novo mutation in the dopamine transporter gene associates dopamine dysfunction with autism spectrum disorder. Mol Psychiatry 18:1315–1323
Hansen FH, Skjorringe T, Yasmeen S, Arends NV, Sahai MA, Erreger K, Andreassen TF, Holy M, Hamilton PJ, Neergheen V, Karlsborg M, Newman AH, Pope S, Heales SJ, Friberg L, Law I, Pinborg LH, Sitte HH, Loland C, Shi L, Weinstein H, Galli A, Hjermind LE, Moller LB, Gether U (2014) Missense dopamine transporter mutations associate with adult Parkinsonism and ADHD. J Clin Investig 124:3107–3120
Kurian MA, Li Y, Zhen J, Meyer E, Hai N, Christen HJ, Hoffmann GF, Jardine P, von Moers A, Mordekar SR, O’Callaghan F, Wassmer E, Wraige E, Dietrich C, Lewis T, Hyland K, Heales S Jr, Sanger T, Gissen P, Assmann BE, Reith ME, Maher ER (2011) Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol 10:54–62
Kurian MA, Zhen J, Cheng SY, Li Y, Mordekar SR, Jardine P, Morgan NV, Meyer E, Tee L, Pasha S, Wassmer E, Heales SJ, Gissen P, Reith ME, Maher ER (2009) Homozygous loss-of-function mutations in the gene encoding the dopamine transporter are associated with infantile Parkinsonism-dystonia. J Clin Investig 119:1595–1603
Gerfen CR, Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34:441–466
Meiergerd SM, Patterson TA, Schenk JO (1993) D2 receptors may modulate the function of the striatal transporter for dopamine: kinetic evidence from studies in vitro and in vivo. J Neurochem 61:764–767
Mayfield RD, Zahniser NR (2001) Dopamine D2 receptor regulation of the dopamine transporter expressed in Xenopus laevis oocytes is voltage-independent. Mol Pharmacol 59:113–121
Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, Liu F (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 26:2127–2136
Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, Ramamoorthy S, Javitch JA, Zapata A, Shippenberg TS (2007) D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol 71:1222–1232
Chen R, Daining CP, Sun H, Fraser R, Stokes SL, Leitges M, Gnegy ME (2013) Protein kinase Cbeta is a modulator of the dopamine D2 autoreceptor-activated trafficking of the dopamine transporter. J Neurochem 125:663–672
Luderman KD, Chen R, Ferris MJ, Jones SR, Gnegy ME (2015) Protein kinase C beta regulates the D(2)-like dopamine autoreceptor. Neuropharmacology 89:335–341
Gowrishankar R, Gresch PJ, Davis GL, Katamish RM, Riele JR, Stewart AM, Vaughan RA, Hahn MK, Blakely RD (2018) Region-specific regulation of presynaptic dopamine homeostasis by D2 autoreceptors shapes the in vivo impact of the neuropsychiatric disease-associated DAT variant Val559. J Neurosci 38:5302–5312
Marcott PF, Gong S, Donthamsetti P, Grinnell SG, Nelson MN, Newman AH, Birnbaumer L, Martemyanov KA, Javitch JA, Ford CP (2018) Regional heterogeneity of D2-receptor signaling in the dorsal striatum and nucleus accumbens. Neuron 98:575–587.e574
Owens WA, Sevak RJ, Galici R, Chang X, Javors MA, Galli A, France CP, Daws LC (2005) Deficits in dopamine clearance and locomotion in hypoinsulinemic rats unmask novel modulation of dopamine transporters by amphetamine. J Neurochem 94:1402–1410
Owens WA, Williams JM, Saunders C, Avison MJ, Galli A, Daws LC (2012) Rescue of dopamine transporter function in hypoinsulinemic rats by a D2 receptor-ERK-dependent mechanism. J Neurosci 32:2637–2647
Sitte HH, Huck S, Reither H, Boehm S, Singer EA, Pifl C (1998) Carrier-mediated release, transport rates, and charge transfer induced by amphetamine, tyramine, and dopamine in mammalian cells transfected with the human dopamine transporter. J Neurochem 71:1289–1297
Sulzer D, Sonders MS, Poulsen NW, Galli A (2005) Mechanisms of neurotransmitter release by amphetamines: a review. Prog Neurobiol 75:406–433
Kivell B, Uzelac Z, Sundaramurthy S, Rajamanickam J, Ewald A, Chefer V, Jaligam V, Bolan E, Simonson B, Annamalai B, Mannangatti P, Prisinzano TE, Gomes I, Devi LA, Jayanthi LD, Sitte HH, Ramamoorthy S, Shippenberg TS (2014) Salvinorin A regulates dopamine transporter function via a kappa opioid receptor and ERK1/2-dependent mechanism. Neuropharmacology 86:228–240
Kivell BM, Ewald AW, Prisinzano TE (2014) Salvinorin A analogs and other kappa-opioid receptor compounds as treatments for cocaine abuse. Adv Pharmacol 69:481–511
Torres GE, Yao W-D, Mohn AR, Quan H, Kim K-M, Levey AI, Staudinger J, Caron MG (2001) Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 30:121–134
Bjerggaard C, Fog JU, Hastrup H, Madsen K, Loland CJ, Javitch JA, Gether U (2004) Surface targeting of the dopamine transporter involves discrete epitopes in the distal C terminus but does not require canonical PDZ domain interactions. J Neurosci 24:7024–7036
Wu S, Fagan RR, Uttamapinant C, Lifshitz LM, Fogarty KE, Ting AY, Melikian HE (2017) The dopamine transporter recycles via a retromer-dependent postendocytic mechanism: tracking studies using a novel fluorophore-coupling approach. J Neurosci 37:9438–9452
Rickhag M, Hansen FH, Sorensen G, Strandfelt KN, Andresen B, Gotfryd K, Madsen KL, Vestergaard-Klewe I, Ammendrup-Johnsen I, Eriksen J, Newman AH, Fuchtbauer EM, Gomeza J, Woldbye DP, Wortwein G, Gether U (2013) A C-terminal PDZ domain-binding sequence is required for striatal distribution of the dopamine transporter. Nat Commun 4:1580
Loder MK, Melikian HE (2003) The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem 278:22168–22174
Holton KL, Loder MK, Melikian HE (2005) Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci 8:881–888
Furman CA, Lo CB, Stokes S, Esteban JA, Gnegy ME (2009) Rab 11 regulates constitutive dopamine transporter trafficking and function in N2A neuroblastoma cells. Neurosci Lett 463:78–81
Eriksen J, Bjorn-Yoshimoto WE, Jorgensen TN, Newman AH, Gether U (2010) Postendocytic sorting of constitutively internalized dopamine transporter in cell lines and dopaminergic neurons. J Biol Chem 285:27289–27301
Sakrikar D, Mazei-Robison MS, Mergy MA, Richtand NW, Han Q, Hamilton PJ, Bowton E, Galli A, Veenstra-Vanderweele J, Gill M, Blakely RD (2012) Attention deficit/hyperactivity disorder-derived coding variation in the dopamine transporter disrupts microdomain targeting and trafficking regulation. J Neurosci 32:5385–5397
Gabriel LR, Wu S, Kearney P, Bellve KD, Standley C, Fogarty KE, Melikian HE (2013) Dopamine transporter endocytic trafficking in striatal dopaminergic neurons: differential dependence on dynamin and the actin cytoskeleton. J Neurosci 33:17836–17846
Hong WC, Amara SG (2013) Differential targeting of the dopamine transporter to recycling or degradative pathways during amphetamine- or PKC-regulated endocytosis in dopamine neurons. FASEB J 27:2995–3007
Sorkina T, Hoover BR, Zahniser NR, Sorkin A (2005) Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic 6:157–170
Navaroli DM, Stevens ZH, Uzelac Z, Gabriel L, King MJ, Lifshitz LM, Sitte HH, Melikian HE (2011) The plasma membrane-associated GTPase Rin interacts with the dopamine transporter and is required for protein kinase C-regulated dopamine transporter trafficking. J Neurosci 31:13758–13770
Wu S, Bellve KD, Fogarty KE, Melikian HE (2015) Ack1 is a dopamine transporter endocytic brake that rescues a trafficking-dysregulated ADHD coding variant. Proc Natl Acad Sci USA 112:15480–15485
Gabriel L, Stevens Z, Melikian H (2009) Measuring plasma membrane protein endocytic rates by reversible biotinylation. J Vis Exp. https://doi.org/10.3791/1669
Eriksen J, Rasmussen SG, Rasmussen TN, Vaegter CB, Cha JH, Zou MF, Newman AH, Gether U (2009) Visualization of dopamine transporter trafficking in live neurons by use of fluorescent cocaine analogs. J Neurosci 29:6794–6808
Block ER, Nuttle J, Balcita-Pedicino JJ, Caltagarone J, Watkins SC, Sesack SR, Sorkin A (2015) Brain region-specific trafficking of the dopamine transporter. J Neurosci 35:12845–12858
Wakayama S, Kiyonaka S, Arai I, Kakegawa W, Matsuda S, Ibata K, Nemoto YL, Kusumi A, Yuzaki M, Hamachi I (2017) Chemical labelling for visualizing native AMPA receptors in live neurons. Nat Commun 8:14850
Biermann B, Sokoll S, Klueva J, Missler M, Wiegert JS, Sibarita JB, Heine M (2014) Imaging of molecular surface dynamics in brain slices using single-particle tracking. Nat Commun 5:3024
Rahbek-Clemmensen T, Lycas MD, Erlendsson S, Eriksen J, Apuschkin M, Vilhardt F, Jorgensen TN, Hansen FH, Gether U (2017) Super-resolution microscopy reveals functional organization of dopamine transporters into cholesterol and neuronal activity-dependent nanodomains. Nat Commun 8:740
Huff RA, Vaughan RA, Kuhar MJ, Uhl GR (1997) Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem 68:225–232
Zhu SJ, Kavanaugh MP, Sonders MS, Amara SG, Zahniser NR (1997) Activation of protein kinase C inhibits uptake, currents and binding associated with the human dopamine transporter expressed in Xenopus oocytes. J Pharmacol Exp Ther 282:1358–1365
Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, Niznik HB (1998) Protein kinase-mediated bidirectional trafficking and functional regulation of the human dopamine transporter. Synapse 30:79–87
Melikian HE, Buckley KM (1999) Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci 19:7699–7710
Daniels GM, Amara SG (1999) Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem 274:35794–35801
Bermingham DP, Blakely RD (2016) Kinase-dependent regulation of monoamine neurotransmitter transporters. Pharmacol Rev 68:888–953
Fagan RR, Kearney PJ, Sweeney CG, Luthi D, Uiterkamp FS, Schicker K, O’Connor LC, Alejandro BS, Sitte HH, Melikian HE. Dopamine transporter trafficking and Rit2 GTPase: in vivo impact and mechanism of action. J Biol Chem (in press)
Sweeney CG, Kearney PJ, Fagan RR, Smith LA, Bolden NC, Zhao-Shea R, Rivera IV, Kolpakova J, Xie J, Gao G, Tapper AR, Martin GE, Melikian HE (2020) Conditional, inducible gene silencing in dopamine neurons reveals a sex-specific role for Rit2 GTPase in acute cocaine response and striatal function. Neuropsychopharmacology 45:384–393
Steinberg SF (2008) Structural basis of protein kinase C isoform function. Physiol Rev 88:1341–1378
Gorentla BK, Vaughan RA (2005) Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology 49:759–768
Boudanova E, Navaroli DM, Melikian HE (2008) Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology 54:605–612
Granas C, Ferrer J, Loland CJ, Javitch JA, Gether U (2003) N-terminal truncation of the dopamine transporter abolishes phorbol ester- and substance P receptor-stimulated phosphorylation without impairing transporter internalization. J Biol Chem 278:4990–5000
Page G, Peeters M, Najimi M, Maloteaux JM, Hermans E (2001) Modulation of the neuronal dopamine transporter activity by the metabotropic glutamate receptor mGluR5 in rat striatal synaptosomes through phosphorylation mediated processes. J Neurochem 76:1282–1290
Bendor J, Lizardi-Ortiz JE, Westphalen RI, Brandstetter M, Hemmings HC Jr, Sulzer D, Flajolet M, Greengard P (2010) AGAP1/AP-3-dependent endocytic recycling of M5 muscarinic receptors promotes dopamine release. EMBO J 29:2813–2826
Foster DJ, Gentry PR, Lizardi-Ortiz JE, Bridges TM, Wood MR, Niswender CM, Sulzer D, Lindsley CW, Xiang Z, Conn PJ (2014) M5 receptor activation produces opposing physiological outcomes in dopamine neurons depending on the receptor's location. J Neurosci 34:3253–3262
Shin JH, Adrover MF, Wess J, Alvarez VA (2015) Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc Natl Acad Sci USA 112:8124–8129
Fischer JF, Cho AK (1979) Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther 208:203–209
Kantor L, Gnegy ME (1998) Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther 284:592–598
Kahlig KM, Galli A (2003) Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol 479:153–158
Khoshbouei H, Wang H, Lechleiter JD, Javitch JA, Galli A (2003) Amphetamine-induced dopamine efflux. A voltage-sensitive and intracellular Na+-dependent mechanism. J Biol Chem 278:12070–12077
Kahlig KM, Binda F, Khoshbouei H, Blakely RD, McMahon DG, Javitch JA, Galli A (2005) Amphetamine induces dopamine efflux through a dopamine transporter channel. Proc Natl Acad Sci USA 102:3495–3500
Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, Galli A (2000) Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci USA 97:6850–6855
Sandoval V, Riddle EL, Ugarte YV, Hanson GR, Fleckenstein AE (2001) Methamphetamine-induced rapid and reversible changes in dopamine transporter function: an in vitro model. J Neurosci 21:1413–1419
Gulley JM, Doolen S, Zahniser NR (2002) Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J Neurochem 83:400–411
Johnson LA, Furman CA, Zhang M, Guptaroy B, Gnegy ME (2005) Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology 49:750–758
Kahlig KM, Lute BJ, Wei Y, Loland CJ, Gether U, Javitch JA, Galli A (2006) Regulation of dopamine transporter trafficking by intracellular amphetamine. Mol Pharmacol 70:542–548
Wheeler DS, Underhill SM, Stolz DB, Murdoch GH, Thiels E, Romero G, Amara SG (2015) Amphetamine activates Rho GTPase signaling to mediate dopamine transporter internalization and acute behavioral effects of amphetamine. Proc Natl Acad Sci USA 112:E7138–E7147
Underhill SM, Hullihen PD, Chen J, Fenollar-Ferrer C, Rizzo MA, Ingram SL, Amara SG (2019) Amphetamines signal through intracellular TAAR1 receptors coupled to Galpha13 and GalphaS in discrete subcellular domains. Mol Psychiatry. https://doi.org/10.1038/s41380-019-0469-2
Simon JR, Bare DJ, Ghetti B, Richter JA (1997) A possible role for tyrosine kinases in the regulation of the neuronal dopamine transporter in mouse striatum. Neurosci Lett 224:201–205
Doolen S, Zahniser NR (2001) Protein tyrosine kinase inhibitors alter human dopamine transporter activity in Xenopus oocytes. J Pharmacol Exp Ther 296:931–938
Carvelli L, Moron JA, Kahlig KM, Ferrer JV, Sen N, Lechleiter JD, Leeb-Lundberg LM, Merrill G, Lafer EM, Ballou LM, Shippenberg TS, Javitch JA, Lin RZ, Galli A (2002) PI 3-kinase regulation of dopamine uptake. J Neurochem 81:859–869
Garcia BG, Wei Y, Moron JA, Lin RZ, Javitch JA, Galli A (2005) Akt is essential for insulin modulation of amphetamine-induced human dopamine transporter cell-surface redistribution. Mol Pharmacol 68:102–109
Hoover BR, Everett CV, Sorkin A, Zahniser NR (2007) Rapid regulation of dopamine transporters by tyrosine kinases in rat neuronal preparations. J Neurochem 101:1258–1271
Zhu S, Zhao C, Wu Y, Yang Q, Shao A, Wang T, Wu J, Yin Y, Li Y, Hou J, Zhang X, Zhou G, Gu X, Wang X, Bustelo XR, Zhou J (2015) Identification of a Vav2-dependent mechanism for GDNF/Ret control of mesolimbic DAT trafficking. Nat Neurosci 18:1084–1093
Williams JM, Owens WA, Turner GH, Saunders C, Dipace C, Blakely RD, France CP, Gore JC, Daws LC, Avison MJ, Galli A (2007) Hypoinsulinemia regulates amphetamine-induced reverse transport of dopamine. PLoS Biol 5:e274
Speed N, Saunders C, Davis AR, Owens WA, Matthies HJ, Saadat S, Kennedy JP, Vaughan RA, Neve RL, Lindsley CW, Russo SJ, Daws LC, Niswender KD, Galli A (2011) Impaired striatal Akt signaling disrupts dopamine homeostasis and increases feeding. PLoS ONE 6:e25169
Littrell OM, Pomerleau F, Huettl P, Surgener S, McGinty JF, Middaugh LD, Granholm AC, Gerhardt GA, Boger HA (2012) Enhanced dopamine transporter activity in middle-aged Gdnf heterozygous mice. Neurobiol Aging 33:427.e1-14
Author information
Authors and Affiliations
Corresponding author
Additional information
Special issue in honor of Professor Michael Robinson.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fagan, R.R., Kearney, P.J. & Melikian, H.E. In Situ Regulated Dopamine Transporter Trafficking: There’s No Place Like Home. Neurochem Res 45, 1335–1343 (2020). https://doi.org/10.1007/s11064-020-03001-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03001-6